Back to Journals » Journal of Inflammation Research » Volume 16
Association of sTREM‐1 and Neutrophil-to-Lymphocyte Ratio as Prognostic Markers in COVID-19 Short- and Long-Term Mortality
Authors Turgunova L, Mekhantseva I , Akhmaltdinova L , Kostinov M, Zhumadilova Z , Turmukhambetova A
Received 10 October 2023
Accepted for publication 21 November 2023
Published 4 December 2023 Volume 2023:16 Pages 5807—5817
DOI https://doi.org/10.2147/JIR.S435305
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Dr Tara Strutt
Lyudmila Turgunova,1 Irina Mekhantseva,1 Lyudmila Akhmaltdinova,1 Mikhail Kostinov,2,3 Zhibek Zhumadilova,1 Anar Turmukhambetova1
1Karaganda Medical University, Department of Internal Medicine, Karaganda, Kazakhstan; 2I.I. Mechnikov Research Institute of Vaccines and Sera, Moscow, Russia; 3Sechenov First Moscow State Medical University, Department of Epidemiology and Modern Vaccination Technologies, Moscow, Russia
Correspondence: Irina Mekhantseva, Email [email protected]
Aim: Current problem related to COVID-19 is various complications after disease, especially long-term mortality after COVID-19. Routine blood tests presented their effectiveness in the diagnosis, prognosis and mortality of COVID-19. The neutrophil-to-lymphocyte ratio (NLR) is an important marker of systemic inflammation. Soluble Trigger receptor expressed on myeloid cells-1 (sTREM-1) is considered an intrinsic enhancer of inflammatory signals. This study examined the predictive value of these markers in COVID-19 mortality.
Methods: A prospective study was conducted involving patients with COVID-19 in Karaganda, Kazakhstan. The neutrophil–to-lymphocyte ratio (NLR) was calculated as the absolute number of neutrophils divided by the absolute number of lymphocytes. The level of sTREM-1 in the blood serum was evaluated by ELISA.
Results: Plasma sTREM-1 concentration greater than 59.08 pg/mL and an NLR greater than 2.29 had an increased risk of early mortality (hazard ratio = 8.07; 95% CI: 1.03– 62.17 and 9.24; 95% CI: 1.202– 71.08, respectively); for long-term mortality of sTREM-1 greater than 47.34 pg/mL (hazard ratio = 7.96; 95% CI: 1.072– 59.18) and NLR greater than 2.10 (hazard ratio = 11.52; 95% CI: 1.551– 85.52).
Conclusion: This study suggests that early levels of sTREM-1 and NLR are associated with the risk of 6-month mortality after experiencing COVID-19.
Keywords: COVID-19, mortality, neutrophil-to-lymphocyte ratio, sTREM‐1, prognostic markers
Introduction
The COVID-19 epidemic caused by SARS-CoV-2 has become a worldwide challenge for the global health system.1 Current problem related to COVID-19 is various complications after COVID-19, especially long-term mortality after COVID-19. According to studies, mortality during the next year is relatively higher in people who have had COVID-19 compared to those who remained uninfected, especially elder people and people with chronic diseases.2,3
Previous studies have focused on the search for universal markers of mortality prognosis in the early period of COVID-19. Authors demonstrated that the comorbidity and severity of COVID-19 contribute more to mortality,4 either an independent predictor of cardiovascular complications5,6 kidney complications7 or other complications.8
However, nowadays the stratification of COVID-19 patients in the early stages and the forthcoming period remains not fully understood, and the search for the most accurate biomarkers to predict the mortality of patients with COVID-19 continues. It was demonstrated the importance of routine blood test, which are effective in the diagnosis, prognosis and mortality of COVID-19.9,10
According to recent studies, the Neutrophil-to-Lymphocyte ratio (NLR) has predictive value in diagnosing and predicting severity and mortality in patients in the early period of COVID-19, which may help in risk stratification models to predict severe and fatal outcomes.11,12 Generally, the NLR test is a quantitative ratio between the innate and adaptive immune systems that expresses the basic balance of the immune system which associate with the outcome of infectious or inflammatory diseases and complications.13,14
Trigger receptor expressed on myeloid cells-1 (TREM-1) is an immune receptor expressed by neutrophils, macrophages, and mature monocytes that can be activated in inflammatory diseases as an enhancer of pro-inflammatory innate immune responses in response to bacterial flora and were first described as a promising marker of sepsis.15 Soluble TREM-1, often referred to as sTREM-1, represents its cleaved and circulating soluble counterpart. sTREM-1 is a preferential biomarker of febrile mortality compared to classical inflammatory markers.16,17 Also, there are several attempts to analyze TREM-1 in non-communicable diseases.18,19 Moreover, sTREM-1 is now known to be a predictor of severity and mortality in the acute phase of COVID-19.20–22 However, TREM-1 alone as a biomarker is not enough to predict mortality in the acute period of COVID-19.
Nowadays, all studies have focused on the predictive value of these markers on short-term mortality in patients with COVID-19. However, there are still no studies that examine these markers to predict long-term mortality after COVID-19. It is hypothesized that immune-mediated markers such as TREM-1 and NLR can predict early and long-term mortality after COVID-19. Our study aims to investigate the association of immune-mediated markers TREM-1 and NLR as stratification predictors that can predict early and forthcoming mortality during COVID-19.
Materials and Methods
Study Setting and Participant Enrollment
A prospective study was performed to enroll patients with COVID-19 who were hospitalized between May and August 2021 in the infectious diseases clinic of Karaganda regional clinical hospital and Karaganda Medical University Hospital (Kazakhstan). Inclusion Criteria: (1) Adults over 18 years of age, (2) PCR-positive for COVID-19 nasopharyngeal swab. We excluded pregnant or lactating women, immunocompromised patients (human immunodeficiency virus infection, active treatment for malignancies). A total of 283 patients were included in the final analysis (Figure S1).
We collected all patients’ data from electronic medical records on the course of the disease, comorbidity, anthropometric parameters, blood pressure (BP), heart rate (HR), oxygen saturation, laboratory tests, lung damage, intensive care unit (ICU) stay, and mechanical ventilation support. The study only involved patients with lung tissue damage. To better determine the severity of COVID-pneumonia in predicting mortality, we utilized chest computer tomography (CT) data (% damage). This method is easy to access and provides a simple way to assess damage to lung tissue. CT data were visually classified by radiological severity according to the degree of pulmonary involvement using the RAD-Covid score.23 All patients were calculated with body mass index, and Charlson comorbidity index.24 All investigations were carried out on the first day of hospitalization. Discharged patients were monitored by phone and by collecting information in the medical information system, where all cases of treatment and/or death of patients are recorded. As the endpoint was taken all deaths from all non-specific and specific causes, taking into account the etiology and date of death. The follow-up period was 180 days from hospital admission: 30 days post-hospital to assess early mortality and additional 5 months follow-up of surviving patients to report late mortality. Patients who were still alive 180 days after the hospitalization were defined as surviving.
The study was approved by the Bioethics Committee of Karaganda Medical University No. 18, dated 14 April 2021. Written informed consent was obtained from the participants.
Immunological Analysis
Blood samples were collected in tubes with EDTA on the first day after admission to the hospital. Serum aliquots were stored in a freezer at −80°C until analysis. sTREM-1 level was measured using a human sTREM-1 ELISA Kit (Abcam) following the manufacturer’s instructions. The blood test was performed on a Mindray hematological analyzer. The neutrophil–to-lymphocyte ratio (NLR) was calculated as the absolute number of neutrophils divided by the absolute number of lymphocytes.
Statistical Analysis
Statistical analysis was performed using Statistical Package for the Social Sciences (SPSS) 21.0 (IBM SPSS Statistics). Data presented in tables and graphs were performed using Prism Version 7 software (Graph-Pad, La Jolla CA). Normal distribution was carried out using the Kolmogorov–Smirnov test. Quantitative indicators, given the non-normal distribution, are described using the median (Me) and the interquartile range. Qualitative features are described using percentages. Differences between the groups were evaluated by Kruskal–Wallis, Mann–Whitney U-tests with Bonferroni correction for multiple comparisons, or chi-square test, as appropriate. Factors associated with the development of mortality within 30 and 180 days were analyzed using univariate and multivariate regression. Predictor accuracy was determined by the area under the curve (AUC) of the receiver operating characteristic (ROC). AUCs with 95% confidence intervals were calculated to estimate the diagnostic value of NLR and sTREM-1. The classification threshold or the cut-off value was determined using ROC analysis, corresponding to the point on the ROC curve with the highest sensitivity index and the lowest value of false positive results. The specified point on the curve was determined using the calculation of the Youden’s index. Hazard ratios were based on Log rank tests (Cox regression). The p-values are given in the tables, and p < 0.05 is considered statistically significant.
Results
Demographics, Clinical and Laboratory Characteristics of Patients
There were analyzed Data from 283 patients with COVID-19 in an infectious diseases hospital. The clinical characteristics of patients are presented in Table 1. 23 people died: over a 30-day period - 13 people (4.6%), the median was 12 days; for another 6 months - 10 people (3.5%), the median of 56 days. The mean age of deceased patients at 30 and 180 days was higher than that of surviving patients (p=0.0001). There were no gender differences between the deceased and the survivors. The Charlson comorbidity index did not differ between survivors and deceased over a 30-day period, while patients who died within 6 months of illness had a higher comorbidity index than survivors (p=0.002). The percentage of lung damage according to CT was higher in the group of the deceased (p=0.007): in patients with 30-day mortality - 45 (28–60), in the deceased group within 180 days - 38 (17–50), among the survivor’s group lung damage was 25 (10–37). Oxygen saturation levels in 30- and 180-days deceased patients were lower (p=0.047), and respiratory rate (p=0.0001) were also higher than in the survivor’s group. Moreover, patients with a lethal outcome were more often transferred to the ICU (69.2% and 40%) and received respiratory support (69.2% and 30%). The mean length of hospital stays for patients with COVID-19 did not differ between groups.
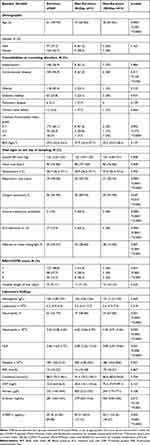 |
Table 1 Clinical and Demographic Data of Patients with COVID-19 Included in the Study |
The differences in laboratory markers between groups were detected: neutrophils count, D-dimer levels, and acute phase proteins (ferritin, CRP) were higher in the group of patients with early and late lethal outcomes (Table 1).
The etiology of early mortality was: cardiopulmonary insufficiency (77%) and cerebral edema (23%); in the etiology of late mortality: cardiopulmonary insufficiency also prevailed (80%), cerebral edema was less common (10%), death from other causes was 10%.
Prognostic Utility of sTREM-1 and Neutrophil-to-Lymphocyte Ratio on Mortality
Higher plasma concentrations of sTREM-1 in deceased patients at 30 days compared with survivors were 87.41 (63.9–117.12) pg/mL versus 59.18 (41.83–84.48) pg/mL, p=0.003 (Figure 1A). Similarly, deceased patients at 6 months had higher sTREM-1 plasma concentrations compared to survivors of 93.11 (52.26–140.27) pg/mL versus 59.18 (41.83–84.48) pg/mL, p=0.0001.
In comparison with NLR between groups of survivors and deceased, the following statistical differences were found (Figure 1B): NLR in the group of those who died within a month - 3.89 (2.50–15.53), within 6 months - 3.89 (2.81–9.06), which is significantly higher than in the group of survivors - 2.46 (1.62–3.73).
The predictive value of sTREM-1 and NLR in patients with COVID-19 was assessed by ROC curves. The ROC curve for survivors and deceased groups over a 30-day based on sTREM-1 concentrations and NLR levels is presented in Figure 1C. The characteristics of the ROC assay are presented in Table 2. The cut-off for sTREM-1 was calculated at 59.08 pg/mL with 92% sensitivity and 49% specificity, and the cut-off for NLR was 2.29 (92% sensitivity and 49% specificity). AUC for sTREM-1 was 0.744 (95% CI: 0.632–0.856), and for NLR 0.717 (0.589–0.844), indicating moderate discrimination between survivors and deceased groups.
 |
Table 2 Significance of sTREM-1 and NLR at Admission in Predicting Short- and Long-Term Mortality in Patients with COVID-19 |
The ROC curve of sTREM-1 and NLR levels for survivors and deceased groups over a 180-day is shown in Figure 1D. The cut-off of NLR was >2.10 and TREM-1 >47.34 have AUC values of 0.737 (95% CI 0.637–0.836) and 0.726 (95% CI 0.623–0.828), respectively (Table 2).
Regression analysis to predict mortality and significance of sTREM-1 and NLR at admission in predicting short-term and long-term mortality in patients with COVID-19.
The multivariate Cox regression evaluating the relationship of markers with early mortality is shown in Table 3. After adjusting for age, comorbidity index, and percentage of lung damage, NLR and sTREM-1 scores retained a statistically significant effect on the risk of patient mortality over a 30-day period.
 |
Table 3 The Univariate and Multivariate Cox Regression Analysis to Assess Risk Factors for Early Death in Patients with COVID-19 |
Stratified analysis of long-term mortality demonstrated that sTREM-1 (HR 1.011 (CI 1.002–1.023); p=0.049) and NLR (HR 1.062 (CI 1.004–1.124); p=0.035) retained predictive power after model adjustment for age, number of comorbidities, and assessment of lung damage (Table 4).
 |
Table 4 The Univariate and Multivariate Cox Regression Analysis to Assess Risk Factors for Long-Term Death in Patients with COVID-19 |
Analysis of early patient survival, stratified for the association of sTREM-1 and NLR, indicates that patients with a plasma sTREM-1 concentration greater than 59.08 pg/mL and an NLR greater than 2.29 had an increased risk of early mortality (hazard ratio = 8.07; 95% CI: 1.03–62.17 and 9.24; 95% CI: 1.202–71.08, respectively); for long-term lethality of sTREM-1 greater than 47.34 pg/mL (hazard ratio = 7.96; 95% CI: 1.072–59.18) and NLR greater than 2.10 (hazard ratio = 11.52; 95% CI: 1.551–85.52).
Discussion
Data from several independent epidemiological studies have shown that people who suffer with COVID-19 have a three times greater risk of death within the next 6 months after the disease.25 However, the mechanisms underlying the development of increased mortality after COVID-19 are not fully understood. One of the predicted mechanisms is the influence of inflammation in the acute period of COVID-19 on mortality after the patient’s recovery.
A hyperinflammatory response involving cytokines underlies acute COVID-19 infection.26 Increasing evidence suggests that subsequent persistent low-grade chronic inflammation may lead to a persistent catabolic state and contribute to the development of long-term COVID-19.27 Elevated D-dimer, CRP, and ferritin levels were more common in deceased and in patients with long-COVID than in fully recovered patients,28 which was also confirmed in this study. It was demonstrated that significant inflammatory responses persist in patients even 40 to 60 days after an acute COVID-19 infection.29 A. Mainous et al showed that an elevated CRP level, which is one of the indicators of severe COVID-19 in the acute period, is associated with an increased risk of mortality at 12 months of follow-up.30 According Morrow et al post-COVID syndrome is associated with activation of the hemostasis pathway and systemic inflammation during recovery stage.31 On the other hand, some studies have shown no correlation between pro-inflammatory biomarkers and long-COVID syndrome.32–34
NLR and TREM-1 are complementary markers, reflecting quantitative and functional activity of the myeloid component of innate immunity. NLR, which appears to be more sensitive than absolute neutrophil or lymphocyte count alone in both bacterial and viral pneumonia, is a known marker of the systemic inflammatory response since the COVID-19 pandemic, the predictive value of NLR in the development of severe adverse outcomes in patients hospitalized with COVID-19 pneumonia.11–13 Furthermore, sTREM-1 level in patients with COVID-19 correlates with severe disease, risk of intubation, and in-hospital mortality.20,22,35 Significantly elevated plasma levels of sTREM-1 in patients with COVID-19 can indicate an excessive inflammatory response and may contribute to severe illness or even death.
This study examined the association of NLR and sTREM-1 with the development of lethal cases after 6 months of follow-up after COVID-19 pneumonia. It was demonstrated that the association of NLR and sTREM-1 levels with an increased risk of death, confirmed in the acute period, persists despite the decrease in the threshold values of these indicators: in the acute period, the risk of mortality increased with a TREM cut-off of more than 59.08 pg/mL and NLR above 2.29, in the long term optimal cut-off TREM-1 was more than 47.34 pg/mL and NLR above 2.10.
sTREM-1 is a receptor expressed both on the surface of blood neutrophils and on mature monocytes/macrophages that enhances the pro-inflammatory innate immune response in synergy with Toll-like receptors that recognize a wide range of bacterial, fungal, and viral components.36 TREM-1 activation induces increased secretion of TNF-α, IL-6, IL-1, and IL-2 by monocytes, macrophages, and dendritic cells, which increases inflammation in infections caused by various pathogens.37–40 Moreover, it has been established that sTREM-1 levels in sepsis may reflect significant immune dysfunction, in which excessive TREM-1 cleavage may contribute to immunosuppression and death in severe infection.41 Along with the established role of the sTREM-1 level during acute infection, it has been shown that the increase in sTREM-1 is the subject of intensive research in various conditions, especially inflammation.42 Accordingly, sTREM-1 levels were significantly associated with the risks of all-cause mortality and major cardiovascular events at 2 years of follow-up in post-heart attack patients. The activity of matrix metalloproteinases, which are produced by myeloid cells, especially neutrophils, was associated with the risk of rupture and remodeling of the heart.43 Neutrophils are considered an important source of sTREM-1 in infectious processes.44 sTREM-1 levels significantly correlated with the expression of matrix metalloproteinase (MMP)-8 during the acute phase of COVID-19, which could release TREM-1 from the surface of peripheral blood cells.39 However, the role and mechanisms of TREM-1 involvement in the development of post-COVID mortality need further study.
Older age and comorbidity have been repeatedly reported to adversely affect COVID-19 outcomes.45 This study also points to an independent effect of age on COVID-19 mortality. The mean age of deceased patients within 30 and 180 days was higher than that of surviving patients: it was 74 years in the group of patients who died in the acute period (p=0.002) and 76 years in the group with long-term mortality (p=0.0001). The association effect of high sTREM-1 levels with risk of long-term mortality was present for both unadjusted (HR = 1.017; 95% CI 1.009–1.025) and adjusted analyzes for age, comorbidity index, and percentage of lung tissue involvement (HR = 1.011; 95% CI 1.002–1.023).
There are some limitations in our study. Firstly, our study is based on a study of patients from two hospitals located in the same region. In all regions of Kazakhstan, COVID-19 treatment was carried out according to a single protocol for diagnosis and treatment, and therefore regional features should not have a significant impact on inflammation indicators. Secondly, the study was observational; no analysis was made of the effect of therapy, which was more common, on the risk of developing mortality in the long-term period.46 There were no differences in the frequency of taking drugs with anti-inflammatory effects in the group of survivors and those who died in the long-term period. The study encountered limitations due to the absence of an analysis regarding the association between increased mortality from various causes and sTREM-1 levels. The examination of causes of death among 3704 patients revealed that those who had recovered from SARS-CoV-2 faced elevated risks of respiratory diseases (aHR 1.9, 95% CI 1.2–3.0), malignancies (aHR 1.5, 95% CI 1.2–1.9) over 12 months, and death from cardiovascular diseases (aHR 2.1, 95% CI 1.8–2.3).2 In our study cardiovascular failure and acute vascular events emerged as the most common causes of death in the post-COVID period. The study’s limitations included the relatively small sample size in each group, posing challenges for conducting a comprehensive analysis of TREM-1 levels based on the causes of mortality. To address this, the decision was made to merge the deceased group, considering the multiorgan damage after SARS-CoV-2, and recording death from any cause within a year.
Conclusion
Taken together, this study suggests that early levels of sTREM-1 and NLR are associated with the risk of 6-month mortality after experiencing COVID-19. This study expands our understanding of long-term outcomes by providing opportunities to identify a cohort of patients at increased risk of death after recovery from COVID-19. Further research is needed to clarify knowledge about long-COVID-19. The obtained data suggest a potential role of inflammatory markers in the development of severe complications after suffering COVID-19 and may be the basis for studying the effectiveness of anti-inflammatory therapy in terms of reducing the risk of developing severe manifestations of post-COVID-19 syndrome.
Data Sharing Statement
The data that support the results of this study are available from the corresponding author upon reasonable request.
Ethics Approval and Informed Consent
This study was conducted in accordance with the Declaration of Helsinki and approved by the Bioethics Committee of Karaganda Medical University No. 18, dated 14 April 2021. Written informed consent was obtained from the participants.
Author Contributions
All authors have made significant contributions to the reported work, including contributions to the conception, study design, data acquisition, analysis, interpretation, and execution or in all these aspects. They have been involved in drafting, revising, and critically reviewing the manuscript. All authors have provided their final approval for the version to be published; have reached an agreement on the journal to which the article has been submitted; and have committed to being accountable for all aspects of the work.
Funding
This research was funded by the Ministry of Health of the Republic of Kazakhstan, Program No. BR11065386.
Disclosure
The authors declare no conflict of interest.
References
1. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in china, 2019. N Engl J Med. 2020;382(8):727–733. doi:10.1056/NEJMoa2001017
2. Uusküla A, Jürgenson T, Pisarev H, et al. Long-term mortality following SARS-CoV-2 infection: a national cohort study from Estonia. Lancet Region Health Europe. 2022;18:100394. doi:10.1016/j.lanepe.2022.100394
3. Mainous AG, Rooks BJ, Wu V, Orlando FA. COVID-19 post-acute sequelae among adults: 12 month mortality risk. Front Med. 2021;8. doi:10.3389/fmed.2021.778434
4. Vlachogiannis NI, Baker KF, Georgiopoulos G, et al. Clinical frailty, and not features of acute infection, is associated with late mortality in COVID‐19: a retrospective cohort study. J Cachexia, Sarcopenia Muscle. 2022;13(3):1502–1513. doi:10.1002/jcsm.12966
5. Wang W, Wang CY, Wang SI, Wei JCC. Long-term cardiovascular outcomes in COVID-19 survivors among non-vaccinated population: a retrospective cohort study from the TriNetX US collaborative networks. EClinicalMedicine. 2022;53:101619. doi:10.1016/j.eclinm.2022.101619
6. Satterfield BA, Bhatt DL, Gersh BJ. Cardiac involvement in the long-term implications of COVID-19. Nat Rev Cardiol. 2022;19(5):332–341. doi:10.1038/s41569-021-00631-3
7. Copur S, Berkkan M, Basile C, Tuttle K, Kanbay M. Post-acute COVID-19 syndrome and kidney diseases: what do we know? J Nephrol. 2022;35(3):795–805. doi:10.1007/s40620-022-01296-y
8. Oronsky B, Larson C, Hammond TC, et al. A Review of Persistent Post-COVID Syndrome (PPCS). Clin Rev Allergy Immunol. 2021;64(1):66–74. doi:10.1007/s12016-021-08848-3
9. Tahir Huyut M, Huyut Z, Ilkbahar F, Mertoğlu C. What is the impact and efficacy of routine immunological, biochemical and hematological biomarkers as predictors of COVID-19 mortality? Int Immunopharmacol. 2022;105:108542. doi:10.1016/j.intimp.2022.108542
10. Zhang J, Cao Y, Tan G, et al. Clinical, radiological, and laboratory characteristics and risk factors for severity and mortality of 289 hospitalized COVID‐19 patients. Allergy. 2021;76(2):533–550. doi:10.1111/all.14496
11. Alkhatip AAAMM, Kamel MG, Hamza MK, et al. The diagnostic and prognostic role of neutrophil-to-lymphocyte ratio in COVID-19: a systematic review and meta-analysis. Expert Rev Mol Diagn. 2021;21(5):505–514. doi:10.1080/14737159.2021.1915773
12. Parthasarathi A, Padukudru S, Arunachal S, et al. The role of neutrophil-to-lymphocyte ratio in risk stratification and prognostication of COVID-19: a systematic review and meta-analysis. Vaccines. 2022;10(8):1233. doi:10.3390/vaccines10081233
13. Jiang J, Liu R, Yu X, et al. The neutrophil-lymphocyte count ratio as a diagnostic marker for bacteraemia: a systematic review and meta-analysis. Am J Emerg Med. 2019;37(8):1482–1489. doi:10.1016/j.ajem.2018.10.057
14. Niu D, Huang Q, Yang F, et al. Serum biomarkers to differentiate gram-negative, gram-positive and fungal infection in febrile patients. J Med Microbiol. 2021;70(7). doi:10.1099/jmm.0.001360
15. Siskind S, Brenner M, Wang P. TREM-1 modulation strategies for sepsis. Front Immunol. 2022;13. doi:10.3389/fimmu.2022.907387
16. Richard-Greenblatt M, Boillat-Blanco N, Zhong K, et al. Prognostic accuracy of soluble triggering receptor expressed on myeloid cells (sTREM-1)-based algorithms in febrile adults presenting to Tanzanian outpatient clinics. Clin Infect Dis. 2019. doi:10.1093/cid/ciz419
17. Su L, Liu D, Chai W, Liu D, Long Y. Role of sTREM-1 in predicting mortality of infection: a systematic review and meta-analysis. BMJ Open. 2016;6(5):e010314. doi:10.1136/bmjopen-2015-010314
18. Kouassi K, Gunasekar P, Agrawal D, Jadhav G. TREM-1; is it a pivotal target for cardiovascular diseases? J Cardiovasc Dev Dis. 2018;5(3):45. doi:10.3390/jcdd5030045
19. Tammaro A, Derive M, Gibot S, Leemans JC, Florquin S, Dessing MC. TREM-1 and its potential ligands in non-infectious diseases: from biology to clinical perspectives. Pharmacol Ther. 2017;177:81–95. doi:10.1016/j.pharmthera.2017.02.043
20. de Nooijer AH, Grondman I, Lambden S, et al. Increased sTREM-1 plasma concentrations are associated with poor clinical outcomes in patients with COVID-19. Biosci Rep. 2021;41(7). doi:10.1042/BSR20210940
21. de Sá Resende A, Matos de Oliveira YL, Rodrigues de Moura T, Martins-Filho PR. Potential role of triggering receptor expressed on myeloid cells-1 (TREM-1) in SARS-CoV-2 infection: first insights. EXCLI J. 2021;20:722–723. doi:10.17179/excli2021-3581
22. Van Singer M, Brahier T, Ngai M, et al. COVID-19 risk stratification algorithms based on sTREM-1 and IL-6 in emergency department. J Aller Clin Immunol. 2021;147(1):99–106.e4. doi:10.1016/j.jaci.2020.10.001
23. Ribeiro TFG, Rstom RA, Barbosa PNVP, et al. Tomographic score (RAD-Covid Score) to assess the clinical severity of the novel coronavirus infection. Braz J Infect Dis. 2021;25(4):101599. doi:10.1016/j.bjid.2021.101599
24. Radovanovic D, Seifert B, Urban P, et al. Validity of Charlson Comorbidity Index in patients hospitalised with acute coronary syndrome. Insights from the nationwide AMIS Plus registry 2002–2012. Heart. 2014;100(4):288–294. doi:10.1136/heartjnl-2013-304588
25. Davis HE, McCorkell L, Vogel JM, Topol EJ. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol. 2023;21(3):133–146. doi:10.1038/s41579-022-00846-2
26. Rabaan AA, Al-Ahmed SH, Muhammad J, et al. Role of inflammatory cytokines in COVID-19 patients: a review on molecular mechanisms, immune functions, immunopathology and immunomodulatory drugs to counter cytokine storm. Vaccines. 2021;9(5):436. doi:10.3390/vaccines9050436
27. Maamar M, Artime A, Pariente E, et al. Post-COVID-19 syndrome, low-grade inflammation and inflammatory markers: a cross-sectional study. Curr Med Res Opin. 2022;38(6):901–909. doi:10.1080/03007995.2022.2042991
28. Huyut MT, Huyut Z. Effect of ferritin, INR, and D-dimer immunological parameters levels as predictors of COVID-19 mortality: a strong prediction with the decision trees. Heliyon. 2023;9(3):e14015. doi:10.1016/j.heliyon.2023.e14015
29. Doykov I, Hällqvist J, Gilmour KC, Grandjean L, Mills K, Heywood WE. ‘The long tail of Covid-19’ - The detection of a prolonged inflammatory response after a SARS-CoV-2 infection in asymptomatic and mildly affected patients. F1000Res. 2020;9:1349. doi:10.12688/f1000research.27287.1
30. Mainous AG, Rooks BJ, Orlando FA. The impact of initial COVID-19 episode inflammation among adults on mortality within 12 months post-hospital discharge. Front Med. 2022;9. doi:10.3389/fmed.2022.891375
31. Morrow AJ, Sykes R, McIntosh A, et al. A multisystem, cardio-renal investigation of post-COVID-19 illness. Nat Med. 2022;28(6):1303–1313. doi:10.1038/s41591-022-01837-9
32. Townsend L, Dyer AH, Jones K, et al. Persistent fatigue following SARS-CoV-2 infection is common and independent of severity of initial infection. PLoS One. 2020;15(11):e0240784. doi:10.1371/journal.pone.0240784
33. van den Borst B, Peters JB, Brink M, et al. Comprehensive Health assessment 3 months after recovery from acute coronavirus disease 2019 (COVID-19). Clin Infect Dis. 2021;73(5):e1089–e1098. doi:10.1093/cid/ciaa1750
34. Moreno-Pérez O, Merino E, Leon-Ramirez JM, et al. Post-acute COVID-19 syndrome. Incidence and risk factors: a Mediterranean cohort study. J Infect. 2021;82(3):378–383. doi:10.1016/j.jinf.2021.01.004
35. de Oliveira YLM, de Sá Resende A, Martins-Filho PR, de Moura TR. Role of triggering receptor expressed on myeloid cells-1 (TREM-1) in COVID-19 and other viral pneumonias: a systematic review and meta-analysis of clinical studies. Inflammopharmacology. 2022;30(3):1037–1045. doi:10.1007/s10787-022-00972-6
36. Owen AM, Luan L, Burelbach KR, et al. MyD88-dependent signaling drives toll-like receptor-induced trained immunity in macrophages. Front Immunol. 2022:13. doi:10.3389/fimmu.2022.1044662
37. Adukpo S, Gyan BA, Ofori MF, Dodoo D, Velavan TP, Meyer CG. Triggering receptor expressed on myeloid cells 1 (TREM-1) and cytokine gene variants in complicated and uncomplicated malaria. Tropical Medicine and International Health. 2016;21(12):1592–1601. doi:10.1111/tmi.12787
38. Hommes TJ, Dessing MC, Veer C, et al. Role of triggering receptor expressed on myeloid cells-1/3 in Klebsiella -derived pneumosepsis. Am J Respir Cell Mol Biol. 2015;53(5):647–655. doi:10.1165/rcmb.2014-0485OC
39. de Oliveira Matos A, Dos Santos Dantas PH, Figueira Marques Silva-Sales M, Sales-Campos H. The role of the triggering receptor expressed on myeloid cells-1 (TREM-1) in non-bacterial infections. Crit Rev Microbiol. 2020;46(3):237–252. doi:10.1080/1040841X.2020.1751060
40. Dantas PHDS, Matos ADO, da Silva Filho E, Silva-Sales M, Sales-Campos H. Triggering receptor expressed on myeloid cells-1 (TREM-1) as a therapeutic target in infectious and noninfectious disease: a critical review. Int Rev Immunol. 2020;39(4):188–202. doi:10.1080/08830185.2020.1762597
41. Hotchkiss RS, Monneret G, Payen D. Immunosuppression in sepsis: a novel understanding of the disorder and a new therapeutic approach. Lancet Infect Dis. 2013;13(3):260–268. doi:10.1016/S1473-3099(13)70001-X
42. da Silva-Neto PV, de Carvalho JCS, Pimentel VE, et al. sTREM-1 predicts disease severity and mortality in COVID-19 patients: involvement of peripheral blood leukocytes and MMP-8 activity. Viruses. 2021;13(12):2521. doi:10.3390/v13122521
43. Kempf T, Zarbock A, Widera C, et al. GDF-15 is an inhibitor of leukocyte integrin activation required for survival after myocardial infarction in mice. Nat Med. 2011;17(5):581–588. doi:10.1038/nm.2354
44. Klesney-Tait J, Turnbull IR, Colonna M. The TREM receptor family and signal integration. Nat Immunol. 2006;7(12):1266–1273. doi:10.1038/ni1411
45. Wan EYF, Zhang R, Mathur S, et al. Post-acute sequelae of COVID-19 in older persons: multi-organ complications and mortality. J Travel Med. 2023;30(5). doi:10.1093/jtm/taad082
46. Noreen S, Maqbool I, Madni A. Dexamethasone: therapeutic potential, risks, and future projection during COVID-19 pandemic. Eur J Pharmacol. 2021;894:173854. doi:10.1016/j.ejphar.2021.173854
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

