Back to Journals » Neuropsychiatric Disease and Treatment » Volume 17
Association of Serum Dystroglycan, MMP-2/9 and AQP-4 with Haematoma Expansion in Patients with Intracerebral Haemorrhage
Authors Shi Y, Fan X, Li G, Zhong D, Zhang X
Received 21 September 2020
Accepted for publication 23 November 2020
Published 6 January 2021 Volume 2021:17 Pages 11—18
DOI https://doi.org/10.2147/NDT.S283016
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yuping Ning
Yue Shi,1 Xuehui Fan,1 Guozhong Li,1 Di Zhong,1 Xin Zhang2
1Department of Neurology, The First Affiliated Hospital of Harbin Medical University, Harbin 150001, Heilongjiang Province, People’s Republic of China; 2Department of Neurology, Liuzhou People’s Hospital, Liuzhou, Guangxi, 545006, People’s Republic of China
Correspondence: Xin Zhang
Department of Neurology, Liuzhou People’s Hospital, 8 Wenchang Road, Liuzhou, Guangxi 545006, People’s Republic of China
Tel +86-13946027603
Email [email protected]
Objective: The purpose of this study was to explore association of serum dystroglycan (DG), matrix metalloproteinase-2/matrix metalloproteinase-9 (MMP-2/9), and aquaporin-4 (AQP-4) expression and haematoma expansion in patients with intracerebral haemorrhage (ICH), which are proteins involved in maintaining the integrity of the blood-brain barrier.
Methods: We included patients older than 18 years old with ICH who had undergone baseline CT within 6 hours after intracerebral haemorrhage symptom onset in our hospital between April 2018 and December 2018. Two readers independently assessed haematoma volume and other imaging information upon admission and again within 24 hours. All patients underwent 5 mL of venous blood collection 6 and 24 hours after admission. Serum expression levels of dystroglycan, matrix metalloproteinase-2/matrix metalloproteinase-9 and aquaporin-4 were determined by quantitative enzyme-linked immunosorbent assay (ELISA). Repeated analysis of variance was used to determine whether expression of the four proteins in patients with cerebral haemorrhage changed within 24 hours and whether there were differences between the haematoma enlargement and non-haematoma enlargement groups over time. Univariate and multivariate logistic regression analyses were used to compare the correlation among expression of the four proteins, clinical characteristics of patients and haematoma enlargement.
Results: Expression levels of serum matrix metalloproteinase-2/matrix metalloproteinases-9 and aquaporin-4 gradually increased within 24 hours in patients with cerebral haemorrhage (P< 0.001), while expression levels of dystroglycan gradually decreased (P< 0.01). Expression of serum matrix metalloproteinases-9 6 hours after onset was independently correlated with the expansion of cerebral haemorrhage. The ROC curve (AUC=0.778, 95% Cl: 0.661– 0.894, P< 0.001) exhibited high sensitivity (0.900) and low specificity (0.642).
Conclusion: These data support that expression of MMP-9 in peripheral blood is independently correlated with the enlargement of haematoma in patients with intracerebral haemorrhage 6 hours after onset and can be used as an independent predictor of haematoma enlargement in patients with intracerebral haemorrhage. However, although the expression of MMP-2, AQP-4 and DG exhibited some changes within 6 and 24 hours after onset, they were not independently correlated with early haematoma enlargement in patients with intracerebral haemorrhage. Further multi-time point exploration and expansion of the sample size is necessary in future studies.
Keywords: intracerebral hemorrhage, ICH, hematoma enlargement, HE, dystroglycan, DG, matrix metalloproteinase-2/9, MMP-2/9, aquaporin-4, AQP-4
Intracerebral haemorrhage (ICH) is a type of devastating stroke for which there is currently no clear or effective treatment. Haematoma expansion (HE) is an important risk factor for early neurological deterioration and long-term clinical outcome in patients with cerebral haemorrhage.1 Therefore, controlling early haematoma expansion in cerebral haemorrhage is a key treatment direction for ICH.2 The blood-brain barrier (BBB) is a functional unit composed of vascular endothelial cells, astrocytes, pericytes, and extracellular matrix. Its primary functions are to prevent blood components from infiltrating into brain tissue and to protect nerve cell function. The BBB is a dynamic complex, and structural changes occur under pathological conditions. BBB destruction is the pathological basis of haematoma expansion.3 Dystroglycan (DG) is a highly glycosylated transmembrane protein that is essential for maintaining structural integrity of the basement membrane by binding to ECM proteins.4–6 However, under pathological conditions, such as tumours, inflammation, and autoimmune diseases, expression of DG often changes, disabling it from closely associating with the ECM components. Destruction of the BBB after cerebral haemorrhage is related to the cleavage of β-DG by matrix metalloproteinase (MMP)-2/9.5,6 In addition, abnormal cleave of DG after ICH is also related to the polarity change of aquaporin (AQP)-4.7 Destruction of the BBB is closely related to HE. DG, MMP-2/9 and AQP-4 are important proteins that affect the integrity and function of the BBB. Therefore, this study explored the correlation among changes in DG, MMP-2/9, and AQP-4 in peripheral blood of patients with cerebral haemorrhage and haematoma expansion, attempting to identify biological markers to predict HE.
Materials and Methods
All enrolled subjects were patients with ICH treated in the neurosurgical department of our hospital from April 2018 to December 2018. The inclusion criteria were the following: (1) age >18 years; (2) patients with non-contrast CT within 6 hours of onset and confirmed spontaneous intracranial haemorrhage; and (3) informed consent provided by family. The exclusion criteria were as follows: (1) patients with arteriovenous malformations, ruptured intracranial aneurysms, brain trauma, brain tumours, haemorrhagic infarction, or intracranial haemorrhage after thrombolysis; (2) patients with primary indoor haemorrhage or brainstem haemorrhage; (3) patients taking oral anticoagulants; and (4) 24 h review of patients who underwent surgery to remove haematoma or who died before blood collection. For this study, we defined haematoma growth as a 33% increase in haematoma volume or >6 mL at the time of follow-up CT scan according to previous definitions.8–11
Patients presenting with ICH were enrolled in this observational cohort study that prospectively collected name, sex, age, hypertension/diabetes/smoking history/drinking history status; serology, including blood glucose at admission (mmol/L), white blood cell count, and neutrophil count. The same neurologist assessed the degree of neurological deficit according to the NIHSS score at the time of admission and judged the degree of coma upon admission according to the GCS score. The following imaging indexes were collected at the time of admission: time of head CT at admission (h), haemorrhage site, volume of bleeding at admission (mL), whether accompanied by intraventricular haemorrhage, whether accompanied by midline displacement, and review of bleeding volume at CT (mL). In addition, we also performed non-contrast CT scan again within 24 hours to obtain haematoma volume.
Protein determination within 6 h and 24 h after admission, 5 mL of venous blood was collected from each patient, centrifuged at 3500 rpm for 10 min, and the upper serum layer was taken and stored at −80°C for future batch testing. Quantitative enzyme-linked immunosorbent assay (ELISA) was used to determine plasma MMP-2/9, DG, and AQP-4 concentration; the kit was a product of MEIMIAN (Nanjing Jian Cheng Bioengineering Factory, China), and kit instructions were carefully followed.
Statistical Analysis
In this study, SPSS 24.0 software was used for statistical analysis. Measurement data that followed a normal distribution is represented by (X±s). Data that disobeyed normal distribution is expressed by the median (interquartile range), and count data is expressed as a percentage. Differences between groups were analysed by variance of independent sample data. If the variances were uneven, rank sum test was used. Count data rate and composition ratio were compared using χ2 test or Fisher’s exact test. P<0.05 indicates that the difference is statistically significant. Patients were divided into groups according to whether the haematoma had enlarged within 24 hours and was analysed by single factor. The variables with statistical significance in univariate logistic regression analysis were included in multivariate logistic regression analysis, and statistically significant influencing factors were identified. Repeated measurement analysis of variance was used to assess the relationship between haematoma expansion and protein expression at two different time points in the two groups. ROC curves were created for proteins that were meaningful in multiple logistic regression analysis.
Results
Analysis of Clinical Baseline Related to Hemorrhage Expansion
We included 73 patients with intracerebral hemorrhage and divided them into two groups according to whether the hematoma was enlarged or not. There were 20 patients in the enlarged hematoma group and 53 patients in the non-enlarged hematoma group. The baseline characteristics of the patients related to hematoma expansion are shown in Table 1: there was no difference in baseline data of the patients.
 |
Table 1 Correlation Analysis Between Baseline Data and Hematoma Enlargement in Patients with Cerebral Hemorrhage (X±s或 n (%)) |
Univariate and Multivariate Logistic Regression Analysis to Predict Factors Related to Haematoma Expansion
Univariate analysis was used to analyse factors related to haematoma enlargement (Table 3). Levels of serum MMP-9 (6 h), MMP-2 (24 h), haematoma volume (mL), white blood cell count and neutrophil count (6 and 24 h) after onset were statistically significant. To predict haematoma enlargement, we included statistically significant variables in univariate analysis into the multivariate logistic regression analysis (to predict haematoma enlargement, only 6 h statistically significant proteins were included). Multivariate logistic regression analysis revealed that there was still a statistically significant correlation between levels of MMP-9 6 h after onset and haematoma enlargement (OR=1.006, 95% CI: 1.002–1.009, P=0.001).
 |
Table 2 Univariate and Multivariate Logistic Regression Analysis to Predict Factors Related to Hematoma Expansion |
 |
Table 3 Univariate Logistic Regression Analysis of Hematoma Expansion in Patients with Cerebral Hemorrhage |
Evaluation of the Predictive Ability of the Factors Affecting Haematoma Enlargement
The ability of serum MMP-9 expression levels 6 hours after onset to predict haematoma enlargement was evaluated by receiver working characteristic curve (receiver operating characteristic curve, ROC). As shown in Table 5 and Figure 1, the area under the curve (AUC=0.778) and its 95% confidence interval (0.661–0.894, P ≤ 0.001) exhibited good predictive ability, high sensitivity and low specificity (0.90, 0.642).
 |
Table 4 Multivariate Logistic Regression Analysis of Hematoma Expansion in Patients with Cerebral Hemorrhage |
 |
Table 5 Evaluation of the Ability of MMP-9 to Predict Hematoma Enlargement in Patients with ICH |
 |
Figure 1 ROC curve showed (AUC=0.778, sensitivity 0.900, specificity 0.642 cut-off value 1120.96, P <0.001). |
The Trend of Protein Expression in Repeated Measurement Analysis of Variance and Its Relationship with Haematoma Enlargement
The results of repeated measurement analysis of variance are shown in Table 6. Changes in protein expression within 24 hours after the onset of cerebral haemorrhage are as follows: (1) Expression of DG decreased over time (F=20.178, P<0.001), but there was no significant difference in DG expression between the two groups. Furthermore, there was no significant difference in the degree of decrease in DG expression between the two groups. (2) Expression of MMP-2 gradually increased over time (F=24.249, P<0.001). The average level of MMP-2 expression in the haematoma enlargement group was significantly higher compared to the haematoma stable group (F=6.111, P=0.016), but there was no significant difference in the degree of MMP-2 increase between the two groups. (3) Over time, expression of MMP-9 significantly increased (F=43.766, P<0.001), and the average levels in the group with enlarged haematoma were significantly higher compared to levels in the group with stable haematoma (F=16.257, P<0.001). The degree of MMP-9 increase over time in the haematoma enlargement group was also higher compared to the haematoma stable group (F=4.226, P=0.043) (4) AQP-4 increased significantly over time (F =41.805, P<0.001), but there was no significant difference in the average level of AQP-4 expression between the two groups. In addition, there was no difference in the increase in AQP-4 between the two groups (Table 6).
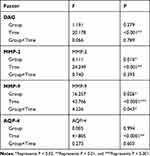 |
Table 6 Relationship Between Protein Expression and Hematoma Enlargement by Repeated Measurement and Analysis of Variance |
Discussion
ICH is the most severe type of stroke, and haematoma expansion is a predictor of poor prognosis.1 However, there are currently no effective biomarkers to predict haematoma enlargement. Destruction of the BBB during ICH is the pathophysiological basis of haematoma enlargement. The proteins (DG, MMP-2/9, and AQP-4) involved in the destruction and maintenance of BBB stability might have utility as predictors of haematoma enlargement, thus guiding clinical practice.1–3
Among the 73 patients with ICH (Table 1), 20 patients developed enlarged haematoma, and 53 patients maintained stable haematoma. We studied patient sex, age, previous hypertension/diabetes/smoking/drinking history, blood pressure, blood glucose and GCS, NIHSS score at admission, location of bleeding, time of initial wardrobe CT, whether there was intraventricular haemorrhage and midline shift, white blood cell count and neutrophil count at admission. The baseline characteristics of the patients related to hematoma expansion are shown in Table 1: there was no difference in baseline data of the patients.
The early release of ICH-related chemokines leads to neutrophil and macrophage infiltration, as well as increased expression of MMP-2/MMP-9 around the haematoma, promoting the degradation of basement membrane components and exacerbating destruction of the BBB.12 In this study, it was found that expression of serum MMP-2 and MMP-9 gradually increased within 24 hours after the onset of cerebral haemorrhage (Tables 2 and 3). Changes in the expression of MMP-2/9 in ICH have distinct patterns in different models of ICH. Studies in a mouse ICH model showed that levels of MMP-2/9 increased during the early stage and peaked at 2–3 days. Activation of MMP-2/9 might be closely related to brain oedema, inflammation and destruction of the BBB.12 In patients with cerebral haemorrhage, Castellazzi et al13 found that the levels of serum MMP-9 increased the first day after haemorrhage, significantly increased on the second day, and continued to rise on the seventh day.12 Levels of MMP-2 at 48 hours and 7 days were significantly lower compared to 24 hours. Although the peaks in MMP-2 and MMP-9 expression were different in these studies, they were consistent with the increases in both factors within 24 hours observed in this study. However, there was no multi-point measurement in this study, because the experimental time was not sufficient, the degree of cooperation of patients was not high, and it is difficult to collect multi-time point blood samples. Expression of MMP-2 and MMP-9 is very low in basal physiological states, but their expression levels increase during ICH. Previous studies have shown that this might be related to the inflammatory reaction. In the multiple logistic regression analysis in this study (Table 4), expression levels of MMP-9 6 hours after onset were independently correlated with enlargement of haematoma. The ROC curve (Table 5 and Figure 1) shows that MMP-9 was an independent predictor of haematoma enlargement after ICH. The predictive effect was good (AUC=0.778), and its 95% confidence interval (0.661–0.894, P<0.001) was more sensitive (0.900). However, the specificity was not strong (0.642). Determination of MMP-9 levels during the acute stage is helpful for identifying high-risk patients with haematoma enlargement to guide clinical treatment. This is consistent with the study of Senn et al.14 In addition, we found that the increase in MMP-9 in the enlarged haematoma group was significantly higher compared to the stable haematoma group (Table 6). This might be due to greater destruction of the BBB and increased MMP-9 in the enlarged haematoma group, but further quantitative measurement of the destruction of the BBB is still needed to confirm our conjecture.15 In this study, we found that MMP-2 gradually increased over time, and the average levels of MMP-2 from 6 to 24 hours were different between the two groups. Although expression of MMP-2 6 hours after onset was not related to the expansion of haematoma in ICH, in univariate logical regression analysis, we found that 24 hours after onset, MMP-2 was related to haematoma enlargement (Table 3). Therefore, we speculated that MMP-2 might be involved in/accelerate destruction of the BBB in a time-dependent manner. In addition, studies have shown that MMP-2 not only participates in destruction of the BBB but also promotes peripheral haematoma nerve inflammation and tissue repair protection, showing its dual role in ICH.13 Therefore, MMP-2 may not indicate haematoma expansion. Currently, there are few studies on the relationship between MMP-2 and haematoma enlargement in ICH. Our study provides clues that MMP-2 may predict early haematoma enlargement. In the future, we will expand the sample size and perform multi-time point dynamic measurements.
This study demonstrated that expression of DG decreased within 24 hours in patients with ICH. In the nervous system, DG is expressed in the foot process of astrocytes at the junction of glial boundary membranes and cerebral vessels and in the endothelial cells of the BBB, representing an important protein for maintaining the integrity of the BBB.4,9 This study revealed that expression of DG decreased within 24 hours in patients with ICH. In the nervous system, DG was expressed in the foot process of astrocytes at the junction of glial boundary membrane and cerebral vessels and in the endothelial cells of the BBB, which is an important protein to maintain the integrity of the BBB.9,16,17
Our previous study18 demonstrated that the MMP-2/MMP-9-mediated β-DG cleavage 30 kD fragment was increased in rat haemorrhage model. This effect was antagonized by administration of an MMP-2/9 inhibitor. Therefore, the increased abnormal cleavage of DG by MMP-2/MMP-9 in ICH leads to decreased expression of DG, which is an important mechanism of BBB destruction in ICH. Therefore, we assume that the cleavage fragment of DG will be detected in the peripheral blood of patients with cerebral haemorrhage. However, due to the limitations of the ELISA kit, we only detected the core DG protein. Expression of the DG core protein in peripheral blood has not been reported. In this study, expression of the DG core protein decreased within 24 hours, which might be related to its abnormal cleavage. The statistical results indicated that there was no correlation between expression of the DG core protein in peripheral blood and haematoma enlargement after ICH. In the future, we will further explore expression of DG cleavage products in peripheral blood and/or cerebrospinal fluid, as well as the correlation between their expression and haematoma enlargement in ICH.
Our study observed that expression of DG decreased, while expression of AQP-4 increased, within 24 hours after the onset of ICH (Table 6). AQP-4 is primarily expressed in ependymal cells and astrocytes. The concentration of AQP-4 on the foot process membrane of astrocytes is 10 times higher than that of the non-foot process membrane, reflecting the polarized expression of AQP-4. DG plays an important role in polarized distribution of AQP-4.19 Qiu et al20 found that during the abnormal cleavage of β-DG, the polarity of AQP-4 was lost in the peri-haematoma area after ICH, and expression of the AQP-4 protein reached its peak on the first day, subsequently gradually decreasing but remaining at high levels on the third day after ICH, consistent with the trends in AQP-4 expression within 24 hours observed in our study. In addition, Wang et al found that upregulation of the AQP-4 protein after ICH might be affected by hypoxia, thrombin and iron overload.20 Wu et al also found that AQP-4 mRNA was upregulated from 2 h in a mouse ICH model, increased continuously from 3 h to 6 h, and reached its peak at 12 h, which confirmed the upward trend of AQP-4 during the early stage of ICH at the transcriptional level. Some studies have shown that AQP-4 connects with the DDC complex through the PDZ domain at the C-terminal of α-syntrophin. Failure of this connection may have many implications, such as decreased glial polarity, disrupted water balance, and destruction of the BBB. Because β-DG is not directly connected with AQP-4, but rather connected through α-syntrophin, the molecular chain of β-DG from α-syntrophin and dystrophin to AQP-4 might be disrupted in ICH. In the area around the haematoma after ICH, AQP-4 might be separated from β-DG, resulting in upregulated expression of the AQP-4 protein.21,22
In this study, the expression levels of AQP-4 did not predict the expansion of ICH haematoma, and the reason for this is not clear. Recently, it has been reported that there is no significant change in the morphological characteristics or permeability of the BBB in AQP-4 deficient mice, suggesting that there is no direct causal relationship between AQP-4 and the destruction of BBB function. Furthermore, expression of AQP-4 does not change the integrity or morphological characteristics of BBB. Current data support the view that AQP-4 induces brain oedema through extracellular water channels rather than directly leading to destruction of the BBB.23 Therefore, we speculate that the increase in AQP-4 expression is primarily related to the increased peripheral oedema volume after ICH, but the relationship between increased AQP-4 expression and haematoma enlargement remains to be further explored. In the future, we will continue to study the correlation between perihaematomal oedema and peripheral blood AQP-4 expression in patients with ICH.
Conclusion
These data support that MMP-9 represents an independent predictor of haematoma enlargement in ICH. Inhibition of MMP-9 expression during acute phase ICH may represent an effective intervention for haematoma enlargement in ICH and provides necessary clues for future clinical work.
Acknowledgments
This work was supported by the Heilongjiang Postdoctoral Research Funding Project (No. Lbh-q15106)
Disclosure
The authors report no conflicts of interest in this work.
References
1. Morotti A, Boulouis G, Charidimou A, et al. Integration of computed tomographic angiography spot sign and noncontrast computed tomographic hypodensities to predict hematoma expansion. Stroke. 2018;49(9):2067–2073.
2. Sporns PB, Kemmling A, Schwake M, et al. Triage of 5 noncontrast computed tomography markers and spot sign for outcome prediction after intracerebral hemorrhage. Stroke. 2018;49(10):2317–2322. doi:10.1161/STROKEAHA.118.021625
3. Schlunk F, Greenberg SM. The pathophysiology of intracerebral hemorrhage formation and expansion. Transl Stroke Res. 2015;6(4):257–263. doi:10.1007/s12975-015-0410-1
4. Yan W, Zhao X, Chen H, et al. β-Dystroglycan cleavage by matrix metalloproteinase-2/-9 disturbs aquaporin-4 polarization and influences brain edema in acute cerebral ischemia. Neuroscience. 2016;326:141–157. doi:10.1016/j.neuroscience.2016.03.055
5. Yadav L, Puri N, Rastogi V, Satpute P, Ahmad R, Kaur G. Matrix metalloproteinases and cancer – roles in threat and therapy. Asian Pacific J Cancer Prevention. 2014;15(3):1085–1091. doi:10.7314/APJCP.2014.15.3.1085
6. Verslegers M, Lemmens K, Van Hove I, Moons L. Matrix metalloproteinase-2 and −9 as promising benefactors in development, plasticity and repair of the nervous system. Prog Neurobiol. 2013;105:60–78. doi:10.1016/j.pneurobio.2013.03.004
7. Qiu GP, Xu J, Zhuo F, et al. Loss of AQP4 polarized localization with loss of β-dystroglycan immunoreactivity may induce brain edema following intracerebral hemorrhage. Neurosci Lett. 2015;588:42–48. doi:10.1016/j.neulet.2014.12.053
8. Dowlatshahi D, Demchuk AM, Flaherty ML, Ali M, Lyden PL, Smith EE. Defining hematoma expansion in intracerebral hemorrhage: relationship with patient outcomes. Neurology. 2011;76(14):1238–1244. doi:10.1212/WNL.0b013e3182143317
9. Yang Q, Zhuang X, Peng F, Zheng W. Relationship of plasma matrix metalloproteinase-9 and hematoma expansion in acute hypertensive cerebral hemorrhage. Int J Neurosci. 2016;126(3):213–218.
10. Li Q, Liu QJ, Yang WS, et al. Island sign: an imaging predictor for early hematoma expansion and poor outcome in patients with intracerebral hemorrhage. Stroke. 2017;48(11):3019–3025. doi:10.1161/STROKEAHA.117.017985
11. Li Q, Zhang G, Huang YJ, et al. Blend sign on computed tomography: novel and reliable predictor for early hematoma growth in patients with intracerebral hemorrhage. Stroke. 2015;46(8):2119–2123. doi:10.1161/STROKEAHA.115.009185
12. Zhang Z, Zhang Z, Lu H, Yang Q, Wu H, Wang J. Microglial polarization and inflammatory mediators after intracerebral hemorrhage. Mol Neurobiol. 2017;54(3):1874–1886. doi:10.1007/s12035-016-9785-6
13. Castellazzi M, Tamborino C, De Santis G, et al. Timing of serum active MMP-9 and MMP-2 levels in acute and subacute phases after spontaneous intracerebral hemorrhage. Acta Neurochir Suppl. 2010;106:137–140.
14. Senn R, Elkind MS, Montaner J, Christ-Crain M, Katan M. Potential role of blood biomarkers in the management of nontraumatic intracerebral hemorrhage. Cerebrovascular Diseases. 2014;38(6):395–409. doi:10.1159/000366470
15. Lin Z, Li Y, Su P, et al. Non-contrast MR imaging of blood-brain barrier permeability to water. Magnetic Resonance Med. 2018;80(4):1507–1520. doi:10.1002/mrm.27141
16. Sbardella D, Inzitari R, Iavarone F, et al. Enzymatic processing by MMP-2 and MMP-9 of wild-type and mutated mouse β-dystroglycan. IUBMB Life. 2012;64(12):988–994. doi:10.1002/iub.1095
17. Mahajan-Thakur S, Böhm A, Jedlitschky G, Schrör K, Rauch BH. Sphingosine-1-phosphate and its receptors: a mutual link between blood coagulation and inflammation. Mediators Inflamm. 2015;2015:831059. doi:10.1155/2015/831059
18. Zhang X, Gu Y, Li P, et al. Matrix metalloproteases-mediated cleavage on β-dystroglycan may play a key role in the blood-brain barrier after intracerebral hemorrhage in rats. Med Sci Monitor. 2019;25:794–800. doi:10.12659/MSM.908500
19. Liu H, Qiu G, Zhuo F, et al. Lost polarization of aquaporin4 and dystroglycan in the core lesion after traumatic brain injury suggests functional divergence in evolution. Biomed Res Int. 2015;2015:471631. doi:10.1155/2015/471631
20. Qing WG, Dong YQ, Ping TQ, et al. Brain edema after intracerebral hemorrhage in rats: the role of iron overload and aquaporin 4. J Neurosurg. 2009;110(3):462–468. doi:10.3171/2008.4.JNS17512
21. Wu H, Zhang Z, Hu X, et al. Dynamic changes of inflammatory markers in brain after hemorrhagic stroke in humans: a postmortem study. Brain Res. 2010;1342:111–117. doi:10.1016/j.brainres.2010.04.033
22. Broderick JP, Diringer MN, Hill MD, et al. Determinants of intracerebral hemorrhage growth: an exploratory analysis. Stroke. 2007;38(3):1072–1075. doi:10.1161/01.STR.0000258078.35316.30
23. McCourt R, Gould B, Kate M, et al. Blood-brain barrier compromise does not predict perihematoma edema growth in intracerebral hemorrhage. Stroke. 2015;46(4):954–960. doi:10.1161/STROKEAHA.114.007544
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
