Back to Journals » Journal of Inflammation Research » Volume 15
Association of Serum Complement C1q and C3 Level with Age-Related Macular Degeneration in Women
Authors Ma Y , Ding X , Shao M, Qiu Y, Li S , Cao W, Xu G
Received 10 November 2021
Accepted for publication 25 December 2021
Published 14 January 2022 Volume 2022:15 Pages 285—294
DOI https://doi.org/10.2147/JIR.S348539
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Yingbo Ma,1,* Xueqing Ding,1,* Mingxi Shao,1 Yichao Qiu,1 Shengjie Li,1 Wenjun Cao,1,2 Gezhi Xu2– 4
1Department of Clinical Laboratory, Eye and ENT Hospital of Fudan University, Shanghai, People’s Republic of China; 2Department of Ophthalmology, Eye and ENT Hospital of Fudan University, Shanghai, People’s Republic of China; 3Shanghai Key Laboratory of Visual Impairment and Restoration, Fudan University, Shanghai, People’s Republic of China; 4NHC Key Laboratory of Myopia, Fudan University, Shanghai, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Shengjie Li; Wenjun Cao
Department of Clinical Laboratory, Eye and ENT Hospital of Fudan University, Fenyang Road 83th, Xuhui District, Shanghai, People’s Republic of China
Tel +86-21-64377134
Email [email protected]; [email protected]
Purpose: To investigate the association between serum complement components and age-related macular degeneration (AMD).
Patients and Methods: A total of 118 AMD patients and age- and sex-matched 106 control subjects were included. Demographic data and the level of serum complement component (C)1q, C3 and C4 were evaluated. Based on sex, the subjects were stratified into male and female subgroups.
Results: The level of C1q (226.31± 45.33mg/dL) was significantly higher and C3 (121.14± 15.76mg/dL) was significantly lower than that in control group (200.03± 38.54mg/dL) (128.42± 19.81mg/dL) in the female AMD patients (p = 0.005, p = 0.045). Logistic regression showed that increased C1q (OR = 1.132, p = 0.016) and decreased C3 (OR = 0.960, p = 0.048) were independent risk factors for female AMD patients. No statistical significance was observed in the male.
Conclusion: Increased C1q and decreased C3 were associated with increased risk of AMD, suggesting that the complement classical pathway probably be involved in AMD, especially in female.
Keywords: macular degeneration, complement classical pathway, complement component, peripheral blood, case-control study
Introduction
Age-related macular degeneration (AMD) is one of the leading cause of irreversible visual impairment and disability among the elderly worldwide.1,2 AMD is mainly characterized with the accumulation of drusen, retinal atrophy and the development of abnormal choroidal neovascularization. The pathogenesis of AMD is still not entirely clear, accumulating advances in the last decades have highlighted the contribution of dysregulation of the complement system to the pathology of AMD.3
Complement is a kind of protein existing in human serum, tissue fluid and cell surface, and after activation of the enzyme activity, can mediate immune reaction to eliminate pathogens.4 It is mainly activated by three independent but overlapping pathways, namely the classical pathway, the bypass pathway and the lectin pathway and all pathways lead to cleavage of complement C3, which leads to the formation of the membrane attack complex made up by C5b-C9. Overactivation of the complement system produces an immune inflammatory response that can result in tissue destruction.5
A large number of studies have shown that the activation of complement system is deeply involved in AMD.6 The increased expression of complement components including C3, CFB, CFH, CFI, and C1qA was found in atrophic regions of AMD patients.7 Whitcup et al found C3 and C3a and other immune substances accumulated under the retina and caused the activation of complement system and forming of drusen, which was associated with degeneration of retinal pigment epithelial (RPE) cells.8 The activators and regulatory proteins of complement have also been discovered to be constituents of drusen.9
Moreover, several scholars have proposed that the activation of the systemic complement system may make a contribution to AMD. Lechner et al found the increased systemic complement activation with increased C3a, C4a and C5a in AMD patients.10 Lynch et al discovered the elevated plasma levels of C3a and sC5b-9 were independent risk factors for wet AMD.11 As shown, the complement activation occurs in peripheral blood of AMD patients; however, the contribution of each complement pathway to C3 activation is still unknown. Notably, accumulating research has suggested that C1q, which is the initiator of classical pathway of complement system is related to aging-related disease.12
It has been reported that C1q not only facilitates apoptotic debris removal but also gets involved in the damage of vascular endothelial cells and nerve cells.13,14 Naito et al proved that the level of C1q in both serum and tissues increased with age and C1q could activate canonical Wnt signaling and promote aging-related phenotypes.15 In the study of Stasi et al, C1q expression was found to be upregulated in the retina of glaucomatous mice, monkey and human eyes, which preceded the time of extensive retinal ganglion cell death.16 In addition, Webster et al discovered that increased C1q was associated with amyloid-β accumulation in Alzheimer’s Disease.17
To date, the serum level of C1q has not been reported in AMD patients. Therefore, we aimed to detect serum complement mainly C1q and C3 to study the association of C1q and C3 with AMD to explore the contribution of the classical complement pathway to disease.
Patients and Methods
Study Population
This retrospective study was performed by the Eye and ENT Hospital of Fudan University in Shanghai and implemented according to the principles of Helsinki Declaration. In this project, patients diagnosed with AMD were collected from Ophthalmology Department of Eye and ENT Hospital of Fudan University from January 2019 to December 2020. During the study period, normal individuals were collected from a population that attended annual health screenings. At the same time, informed consent was obtained from AMD patients and normal controls. A structured questionnaire was used to determine their medical history, including current history of diabetes, hypertension, smoking and alcohol intake. Subjects were considered to have a smoking history if they were current or previous smokers ≥1 cigarette per day for ≥6 months. History of alcohol intake was considered if current or previous alcohol consumption was ≥3 times per week and the interval was ≥6 months.
Inclusion and Exclusion Criteria
Each subject underwent standardized ophthalmic examinations, including an assessment of refractive status and slit-lamp biomicroscopy. Patients with AMD underwent fundus fluorescein angiography and/or choroidal indocyanine green angiography and diagnosed by experienced ophthalmologist.
Inclusion Criteria of AMD Group
(1) ≥18 years old;
(2) non-pregnant women;
(3) had macular damage in one or both eyes: including fundus manifestation of vitreous warts, choroidal neovascularization, subretinal hemorrhage and disc-like fiber scar.
Exclusion Criteria of AMD Group
(1) had other diseases causing neovascularization and hemorrhage in macular area;
(2) had glaucoma, diabetic retinopathy, uveitis and other ocular disease;
(3) had tumor, heart disease, liver disease, nephropathy, nervous system disease, autoimmune diseases, hematonosis, severe infectious diseases and other systemic diseases;
(4) had missing data.
Inclusion Criteria of Control Group
(1) ≥18 years old;
(2) non-pregnant women;
(3) had no clinical manifestations of AMD or other eye diseases were confirmed by ophthalmic examination.
Exclusion Criteria of Control Group
(1) had tumor, heart disease, liver disease, nephropathy, nervous system disease, autoimmune diseases, hematonosis, severe infectious diseases and other systemic diseases;
(2) had missing data.
According to inclusion and exclusion criteria, as shown in Figure 1, a total of 418 AMD patients and 180 control subjects were collected. Three hundred AMD patients and 74 control subjects were excluded, and 118 AMD patients and 106 control subjects were eventually included in this study.
 |
Figure 1 The study population flowchart. |
Sample Collection and Testing
After fasting for 8 hours, 4.5mL blood samples were collected in the morning using standard anterior cubital fossa vein puncture (anterior cubital vein) and yellow cap vacuum vascularization (including coagulant). Centrifugation at 1700g for 10 min to separate the serum. The levels of C1q, C3 and C4 were determined by immunoturbidimetry with Cobas 8000 automatic biochemical analyzer (Roche Cobas, Basel, Switzerland). Normalized values for C1q are 159 to 233mg/dL, for C3 components are 90 to 180mg/dL and for C4 are 10 to 40mg/dL. The coefficient of variation for indoor quality control was 3% to 7% on a daily basis during the study period, with no significant change in values.
Data Analysis
Sample Evaluation
This study was a case-control study, with AMD patients in the case group, and healthy subjects in the control group, and C1q was considered as the main observation factor. The matching principle was that the age difference between the subjects in the case and control group was within ±3 years, and the matching ratio was 1:1. PASS 15 software was used to calculate the sample size. According to reports in the previous literature, we set the OR = 2.7, p = 0.2, α= 0.05, β= 0.10 and obtained the sample size of each group was 115. After strictly excluding, our research basically met the requirements.
Calculating
Statistical software (SPSS 25.0 version; SPSS, Inc., NewYork City, NY, USA). Independent sample t test, Mann–Whitney U-test and Pearson’s chi-square test were used to analyze differences in basic characteristics between AMD group and normal control group. Binary logistic regression analysis was used to evaluate the correlation between C1q, C3, C4 and AMD after adjusting for age, gender, history of diabetes, hypertension, smoking and alcohol consumption. P < 0.05 was considered statistically significant.
Results
Demographic Data
As shown in Table 1, there were no significant differences in age, gender and diabetes history between the control group and the AMD group (p > 0.05). The age distribution of AMD and control group is shown in Supplementary Figure 1. There were significant differences in history of hypertension (p < 0.001), smoking (p < 0.001) and alcohol intake (p < 0.001) between the control group and the AMD group.
 |
Table 1 Demographic and Clinical Characteristics of AMD Patients and Controls |
Comparison of Complement Level Between Control Group and AMD Group
As shown in Table 1 and Figure 2, the level of C1q in AMD group (209.52±43.87mg/dL) was significantly higher than that in control group (190.63±44.18mg/dL) (p = 0.001). The level of C3 (120.30±16.17mg/dL) in AMD group was not significant lower than that in control group (C3: 122.84±20.44mg/dL) (p = 0.304). The level of C4 in AMD group (27.68±7.33mg/dL) was not significantly lower than that in control group (C4: 29.89±20.68mg /dL) (p = 0.279).
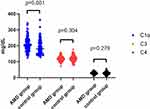 |
Figure 2 Comparison of complement levels between AMD patients and control group. |
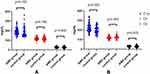
Subgroup Analysis of Complement Components in Control Group and AMD Group
A total of 118 patients were in AMD group, including 58 males and 60 females. A total of 106 subjects were in the normal control group, including 59 males and 47 females. As Table 2 and Figure 3 displays, there were no significant differences in C1q (p = 0.193), C3 (p = 0.756) and C4 (p = 0.409) levels between male AMD patients and control subjects. C1q in female AMD patients (226.31±45.33mg/dL) was significantly higher than that in the control group (200.03±38.54mg/dL) (p = 0.005). C3 in female AMD group (121.14±15.76mg/dL) was significantly lower than that in control group (128.42±19.81mg/dL) (p = 0.045).
 |
Table 2 Comparison of Complement Levels Between Male and Female AMD Patients and Controls |
Male and female AMD patients and the control subjects were divided into four subgroups according to the age quartile of AMD patients: Q1≤56, 56<Q2≤65, 65<Q3≤71, Q4>71 retrospectively. As Table 3 shown, there was no difference in the C1q, C3 and C4 levels between male AMD patients and control subjects in the four subgroups. As Table 4 shows, the level of C1q in Q1 (218.83±33.48mg/dL), Q2 (228.96±39.72mg/dL), Q3 (221.55±41.48mg/dL) and Q4 (220.40±44.60mg/dL) in female AMD patients was significantly higher than that in the control group (Q1:159.31±19.37mg/dL, Q2:186.88±38.67mg/dL, Q3:205.74±35.83mg/dL, Q4: 210.61±39.49mg/dL) (p < 0.001, p = 0.006, p = 0.041, p = 0.028; retrospectively). The level of C3 in Q1 (114.96±15.01mg/dL), Q2 (119.44±9.64mg/dL) and Q3 (119.44±10.64mg/dL) in female AMD patients was significantly lower than that in the control group (Q1:124.88±21.66mg/dL, Q2:129.39±18.40mg/dL, Q3:128.39±12.40mg/dL) (p = 0.045, p = 0.039, p = 0.036; retrospectively).
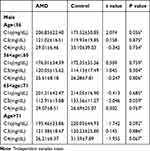 |
Table 3 Comparison of Complement Levels in Age Subgroups of Male AMD Patients and Controls |
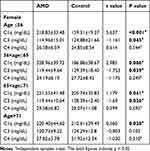 |
Table 4 Comparison of Complement Levels in Age Subgroups of Female AMD Patients and Controls |
Furthermore, AMD patients and control subjects were divided into subgroups according to the mean of C1q and C3—Q1 (lower C1q group, C1q≤200.43mg/dL), Q2 (higher C1q group, C1q>200.43mg/dL), Q3 (lower C3 group, C3≤121.51mg/dL), and Q4 (higher C3 group, C3>121.51mg/dL). The number of AMD patients and control subjects each group was counted and compared between Q1 and Q2, Q3 and Q4. As shown in Figure 4, the proportion of AMD patients in the Q2 group was significantly higher than that in Q1 (p = 0.004). The proportion of AMD patients in the Q3 group was not significantly different from that in Q4 (p = 0.535).
Binary Logistic Regression Analysis
As shown in Table 5, after adjusting for age, history of hypertension, diabetes, smoking and alcohol consumption, binary logistic regression analysis showed the OR of C1q was 1.120 (95% CI: 1.009–1.132; p = 0.001). In male, there was no correlation between C1q (OR = 1.011), C3 (OR = 0.990), C4 (OR=0.952), and AMD (p > 0.05). In female, the OR of C1q was 1.132 (95% CI: 1.106–1.159, p = 0.016) and the OR of C3 was 0.960 (95% CI: 0.923–1.000; p = 0.048). The increased C1q and decreased C3 were risk factors for AMD.
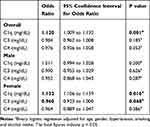 |
Table 5 Logistic Regression Models Evaluating the Risk Factors for AMD |
Discussion
In this study, we found a significant increased C1q and decreased C3 level in serum in the female AMD patients compared with the control subjects. In contrast, there was no significant difference between male AMD patients and control subjects in the level of C1q and C3. Moreover, we found increased C1q and decreased C3 were risk factors for developing AMD in females, which to our knowledge has not been reported previously.
Several authors demonstrated elevated concentrations of numerous complement compounds, for example C3a, C3d, C5a, Ba, Bb, factor D and the complex C5b-9 in peripheral blood of patients with AMD, suggesting the activation of systemic complement system, which was consistent with our findings.3,18,19 Recent studies also showed a significant correlation between the SERPING-1 gene responsible for expression of C1 inhibitor factor and AMD, which may strongly indicate the activation of the classical complement pathway in the pathogenesis of AMD.20 In our study, C1q was found to be elevated and was accompanied by decreased C3, which suggested the classical pathway in peripheral blood may be activated, leading to more activation and cleavage of C3, and hence a decrease in its level. Therefore, we speculated that classical pathway of systemic complement system may be activated and probably contribute to the development of AMD.
It is well noteworthy that there is accumulating evidence indicating the upregulation of C1q in tissue in AMD patients.21–23 Jiao et al discovered that subretinal macrophages could produce classical complement activator C1q leading to the progression of focal retinal degeneration.22 Demirs et al demonstrated that in macular RPE/choroid tissue, complement mRNA and protein expression levels are about 15–100 times higher than in normal neuroretinas, and C1qA was one of the components detectable in atrophic areas.7
Moreover, some scholars found that there was some correlation between AMD and several disease related to systemic complement system. Nitsch et al performed a case-control study including 18,007 AMD patients and 81,869 normal controls.23 They reported that AMD patients were more likely to have systemic lupus erythematosus, glomerulonephritis, and there was a strong and independent association between diabetic nephropathy and AMD after excluding the effect of diabetes. Acosta et al found that the progression from diabetes to diabetic nephropathy may be in part mediated by disorders of the complement system24 and a rat model with arterial hypertension and albuminuria proved that the expression of C1q was found to be significantly higher glomerular than controls.25 Combining with the above reports, it was reasonable to give a speculation that the upregulation of C1q was not only located in retina, but also involved in whole system in AMD patients.
How this may contribute to the development of AMD is currently not well understood. Firstly, circulating complement C1q may be recruited to the macular in AMD. Previous studies showed that C1q and classical pathway were involved in the drusen formation which was the most important pathological feature of AMD.26,27 Johnson et al established a human RPE cell-culture model that mimicked several key aspects of early-stage AMD.26 They found drusen formation involved an activation of the complement cascade, which appeared to be mediated via the classical pathway by the binding of C1q to ligands in APOE-rich deposits, triggering the deposition of terminal complement complexes and inflammatory response. Another study performed by Doyle et al indicated that C1q could activate the inflammasome NLRP3, leading to lysosome rupture and eventual release into the extracellular matrix and damage of surrounding RPE cells.9 Furthermore, C1q was suggested to be involved in the progressive photoreceptor loss and neuroinflammation in retinal degenerations, facilitating the progression of late-stage retinal atrophy.22
Secondly, accumulated complement activated products such as C3a, C5a, and membrane attack complex was found to be involved in the formation of drusen and may also be related to the new blood vessels as reported.7,11 The following question is what is the cause of complement activation. A growing body of evidence indicates that AMD pathogenesis involves ocular autoimmunity.28 It has been reported that 94% of patients with early-stage AMD and 83% of patients with late-stage AMD displayed elevated levels of retinal autoantibodies.29 Moreover, the reactivity pattern of serum IgG antibodies of AMD patients with retinal tissue was relatively correlated with the disease stage.30 Since the Fc segment of IgG binds to C1q to activate the complement system, activation of autoantibodies in AMD patients may lead to depletion of C34.
In addition, gene polymorphisms of complement components including C3 may also cause the activation of complement.31,32 Yates et al reported the common functional polymorphism rs2230199 (Arg80Gly) in the C3 gene resulting in overactivation of complement system, was strongly associated with age-related macular degeneration.33 Interestingly, complement variants also confer susceptibility to invasive infection with several bacteria in AMD patients.34 Javed et al considered that periodontal diseases which was mainly caused by the periodontal infection may be a plausible risk factor for AMD and may have a potential role in the earlier stages of this eye disease.35 Robman et al found that AMD patients with high antibody titers of C. pneumoniae were also found to have a 2- to 3-fold increased risk of disease progression.36 In our study, AMD subjects with acute infectious diseases were excluded; however, chronic subclinical inflammation in AMD subjects was usually difficult to detect through medical screenings. Therefore, we speculated that AMD patients who had lower levels of C3 were likely to be more susceptible to infection as well which can in turn cause a further decrease in C3 levels.
In our study, we found the difference in serum of C1q and C3 concentrations between female but not male AMD patients and control subjects. The influence of gender on developing AMD is not fully understood.37 Several studies concluded that AMD had no significant relationship with gender, but a meta-analysis of gender of AMD showed that the incidence of exudative AMD was higher in women than in men, despite the fact that women tend to live longer and pay more attention to health care.38 A survey in Japanese cohort demonstrated the differential associations of early AMD with both systemic and genetic factors by sex, which suggested that disease process of early AMD could be different by sex.39
As reported by Nuzzi et al, the risk of AMD was increased in women with early menopause.40 Our team previously investigated the relationship between complement system and primary angle-closure glaucoma (PACG) and also found the sex difference in terms of the level of C3 in elder female PACG patients.41,42 According to related study, the differences may be due to the hormone levels especially estrogen and menopausal status between men and women.43,44 In our study, most of the female subjects were aged more than 50 years old so most of them were in postmenopausal with lower levels of sex hormones.
Many mechanisms have been proposed for the role of female sex hormones in AMD.45 Estrogen is a multifunctional hormone that regulates the inflammatory process. Premenopausal women are thought to be more protected from inflammation than men due to higher estrogen concentrations.46 Shepherd et al concluded that a drop of menopausal estrogen level was associated with increased concentrations of pro-inflammatory cytokines and chemokines such as IL-1β, IL-6, IL-8 and TNF-α, which were related to the damage of RPE cells.3,47 Additionally, the decrease of estrogen was associated with the increase of reactive oxygen species and lipid peroxidation end products, which was also correlated with decreased lysosomal activity, RPE damage and choroidal neovascularization.48–50
Meanwhile, estrogen levels may influence the complement system as well. Researchers discovered that postmenopausal women who received a sex hormone replacement therapy regimen had significantly higher levels of C3 than control subjects, suggesting plasma C3 context had a positive and strong correlation with sex hormone concentrations.51 Supporting to this, Yilmazer et al found that the mean values of C3 in healthy postmenopausal women with oral conjugated equine estrogen treatment was significantly higher than those with untreated.52
This study still has some limitations. Firstly, there were differences in the baseline data including hypertension smoking history although they were strictly adjusted for in the logistic regression. Secondly, AMD subtypes cannot be identified for further analysis based on available data. Thirdly, all participants were recruited from one location and the data only represent results from areas around Shanghai. Replication of the study findings in other locations and with a much improved larger population is necessary to confirm our results. Lastly, we did not show the downstream molecules of complement activation in the classical pathway. We will continue to conduct in-depth studies on this part in the future to better reveal the systemic activation of complement system in AMD.
Conclusion
In conclusion, our data suggested that increased C1q and decreased C3 were independent risk factors for female AMD patients, indicating the activation of the complement system in classical pathway may be involved in the pathology of AMD. To our best knowledge, it is the first time to report an elevated C1q and decreased C3 level in female AMD patients, which is helpful for more comprehensive understanding of the complement activation in peripheral blood of AMD. Large and multi-center studies are needed to explore whether complement components can be considered as novel biomarkers to diagnose and/or predict progression of AMD.53–55
Abbreviations
AMD, age-related macular degeneration; C, complement component; RPE, retinal pigment epithelial; PACG, primary angle-closure glaucoma.
Acknowledgments
We would like to thank all the participants who have been part of the project and to the many members of the study teams at different study centers who have enabled this research. The sponsor or funding organization had no role in the design or conduct of this research.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Mitchell P, Liew G, Gopinath B, et al. Age-related macular degeneration. Lancet. 2018;392(10153):1147–1159. doi:10.1016/S0140-6736(18)31550-2
2. Wang W, Yan Y, Guo Z, et al. All around suboptimal health - a joint position paper of the Suboptimal Health Study Consortium and European Association for Predictive, Preventive and Personalised Medicine. EPMA J. 2021;1:31.
3. Kauppinen A, Paterno JJ, Blasiak J, et al. Inflammation and its role in age-related macular degeneration. Cell Mol Life Sci. 2016;73(9):1765–1786. doi:10.1007/s00018-016-2147-8
4. Merle NS, Church SE, Fremeaux-Bacchi V, et al. Complement system part I - molecular mechanisms of activation and regulation. Front Immunol. 2015;6:262. doi:10.3389/fimmu.2015.00262
5. Radu RA, Hu J, Yuan Q, et al. Complement system dysregulation and inflammation in the retinal pigment epithelium of a mouse model for Stargardt macular degeneration. J Biol Chem. 2011;286(21):18593–18601. doi:10.1074/jbc.M110.191866
6. Anderson DH, Radeke MJ, Gallo NB, et al. The pivotal role of the complement system in aging and age-related macular degeneration: hypothesis re-visited. Prog Retin Eye Res. 2010;29(2):95–112. doi:10.1016/j.preteyeres.2009.11.003
7. Demirs JT, Yang J, Crowley MA, et al. Differential and altered spatial distribution of complement expression in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2021;62(7):26. doi:10.1167/iovs.62.7.26
8. Whitcup SM, Sodhi A, Atkinson JP, et al. The role of the immune response in age-related macular degeneration. Int J Inflam. 2013;2013:348092. doi:10.1155/2013/348092
9. Doyle SL, Campbell M, Ozaki E, et al. NLRP3 has a protective role in age-related macular degeneration through the induction of IL-18 by drusen components. Nat Med. 2012;18(5):791–798. doi:10.1038/nm.2717
10. Lechner J, Chen M, Hogg RE, et al. Higher plasma levels of complement C3a, C4a and C5a increase the risk of subretinal fibrosis in neovascular age-related macular degeneration: complement activation in AMD. Immun Ageing. 2016;13(1):4. doi:10.1186/s12979-016-0060-5
11. Lynch AM, Mandava N, Patnaik JL, et al. Systemic activation of the complement system in patients with advanced age-related macular degeneration. Eur J Ophthalmol. 2020;30(5):1061–1068. doi:10.1177/1120672119857896
12. Lafon DC, Thiel S, Kim YI, et al. Classical and lectin complement pathways and markers of inflammation for investigation of susceptibility to infections among healthy older adults. Immun Ageing. 2020;17(1):18. doi:10.1186/s12979-020-00189-7
13. Horii N, Uchida M, Hasegawa N, et al. Resistance training prevents muscle fibrosis and atrophy via down-regulation of C1q-induced Wnt signaling in senescent mice. FASEB J. 2018;32:3547–3559. doi:10.1096/fj.201700772RRR
14. Shah D, Romero F, Zhu Y, et al. C1q deficiency promotes pulmonary vascular inflammation and enhances the susceptibility of the lung endothelium to injury. J Biol Chem. 2015;290(49):29642–29651. doi:10.1074/jbc.M115.690784
15. Naito AT, Sumida T, Nomura S, et al. Complement C1q activates canonical Wnt signaling and promotes aging-related phenotypes. Cell. 2012;149(6):1298–1313. doi:10.1016/j.cell.2012.03.047
16. Stasi K, Nagel D, Yang X, et al. Complement component 1Q (C1Q) upregulation in retina of murine, primate, and human glaucomatous eyes. Invest Ophthalmol Vis Sci. 2006;47(3):1024–1029. doi:10.1167/iovs.05-0830
17. Webster SD, Yang AJ, Margol L, et al. Complement component C1q modulates the phagocytosis of Abeta by microglia. Exp Neurol. 2000;161(1):127–138. doi:10.1006/exnr.1999.7260
18. Reynolds R, Hartnett ME, Atkinson JP, et al. Plasma complement components and activation fragments: associations with age-related macular degeneration genotypes and phenotypes. Invest Ophthalmol Vis Sci. 2009;50(12):5818–5827. doi:10.1167/iovs.09-3928
19. Sivaprasad S, Adewoyin T, Bailey TA, et al. Estimation of systemic complement C3 activity in age-related macular degeneration. Arch Ophthalmol. 2007;125(4):515–519. doi:10.1001/archopht.125.4.515
20. Gibson J, Hakobyan S, Cree AJ, et al. Variation in complement component C1 inhibitor in age-related macular degeneration. Immunobiology. 2012;217(2):251–255. doi:10.1016/j.imbio.2011.07.015
21. Yin C, Ackermann S, Ma Z, et al. ApoE attenuates unresolvable inflammation by complex formation with activated C1q. Nat Med. 2019;25(3):496–506. doi:10.1038/s41591-018-0336-8
22. Jiao H, Rutar M, Fernando N, et al. Subretinal macrophages produce classical complement activator C1q leading to the progression of focal retinal degeneration. Mol Neurodegener. 2018;13(1):45. doi:10.1186/s13024-018-0278-0
23. Nitsch D, Douglas I, Smeeth L, et al. Age-related macular degeneration and complement activation-related diseases: a population-based case-control study. Ophthalmology. 2008;115(11):1904–1910. doi:10.1016/j.ophtha.2008.06.035
24. Acosta J, Hettinga J, Flückiger R, et al. Molecular basis for a link between complement and the vascular complications of diabetes. Proc Natl Acad Sci U S A. 2000;97(10):5450–5455. doi:10.1073/pnas.97.10.5450
25. Kreutz R, Schulz A, Sietmann A, et al. Induction of C1q expression in glomerular endothelium in a rat model with arterial hypertension and albuminuria. J Hypertens. 2007;25(11):2308–2316. doi:10.1097/HJH.0b013e3282ef8611
26. Johnson LV, Forest DL, Banna CD, et al. Cell culture model that mimics drusen formation and triggers complement activation associated with age-related macular degeneration. Proc Natl Acad Sci USA. 2011;108(45):18277–18282. doi:10.1073/pnas.1109703108
27. Yang P, Skiba NP, Tewkesbury GM, et al. Complement-mediated regulation of apolipoprotein e in cultured human rpe cells. Invest Ophthalmol Vis Sci. 2017;58(7):3073–3085. doi:10.1167/iovs.16-20083
28. Chen M, Luo C, Zhao I, et al. Immune regulation in the aging retina. Prog Retin Eye Res. 2019;69:159–172. doi:10.1016/j.preteyeres.2018.10.003
29. Morohoshi K, Goodwin AM, Ohbayashi M, et al. Autoimmunity in retinal degeneration: autoimmune retinopathy and age-related macular degeneration. J Autoimmun. 2009;33(3–4):247–254. doi:10.1016/j.jaut.2009.09.003
30. Atel N, Ohbayashi M, Nugent AK, et al. Circulating anti-retinal antibodies as immune markers in age-related macular degeneration. Immunology. 2005;115(3):422–430. doi:10.1111/j.1365-2567.2005.02173.x
31. Matušková V, Zeman T, Ewerlingová L, et al. An association of neovascular age-related macular degeneration with polymorphisms of CFH, ARMS2, HTRA1 and C3 genes in Czech population. Acta Ophthalmol. 2020;98(6):e691–e699. doi:10.1111/aos.14357
32. Despriet DD, Van Duijn CM, Oostra BA, et al. Complement component C3 and risk of age-related macular degeneration. Ophthalmology. 2009;116(3):474–480. doi:10.1016/j.ophtha.2008.09.055
33. Yates JR, Sepp T, Matharu BK, et al. Complement C3 variant and the risk of age-related macular degeneration. N Engl J Med. 2007;357(6):553–561. doi:10.1056/NEJMoa072618
34. Ho E, Cheung C, Sim S, et al. Human pharyngeal microbiota in age-related macular degeneration. PLoS One. 2018;13(8):e201768. doi:10.1371/journal.pone.0201768
35. Javed F, Sculean A, Romanos GE. Association between age-related macular degeneration and periodontal and peri-implant diseases: a systematic review. Acta Ophthalmol. 2021;99(4):351–356. doi:10.1111/aos.14629
36. Robman L, Mahdi O, Mccarty C, et al. Exposure to Chlamydia pneumoniae infection and progression of age-related macular degeneration. Am J Epidemiol. 2005;161(11):1013–1019. doi:10.1093/aje/kwi130
37. Zetterberg M. Age-related eye disease and gender. Maturitas. 2016;83:19–26. doi:10.1016/j.maturitas.2015.10.005
38. Rudnicka AR, Jarrar Z, Wormald R, et al. Age and gender variations in age-related macular degeneration prevalence in populations of European ancestry: a meta-analysis. Ophthalmology. 2012;119(3):571–580. doi:10.1016/j.ophtha.2011.09.027
39. Sasaki M, Harada S, Kawasaki Y, et al. Gender-specific association of early age-related macular degeneration with systemic and genetic factors in a Japanese population. Sci Rep. 2018;8(1):785. doi:10.1038/s41598-017-18487-4
40. Nuzzi R, Scalabrin S, Becco A, et al. Gonadal hormones and retinal disorders: a review. Front Endocrinol (Lausanne). 2018;9:66. doi:10.3389/fendo.2018.00066
41. Li S, Chen Y, Shao M, et al. Association of plasma complement c3 levels with primary angle-closure glaucoma in older women. Invest Ophthalmol Vis Sci. 2017;58(1):682–689. doi:10.1167/iovs.16-20675
42. Li S, Li D, Shao M, et al. Decreased serum levels of complement c3 reflect complement system dysregulation in patients with primary open-angle glaucoma: results from a pilot study. J Glaucoma. 2018;27(9):761–768. doi:10.1097/IJG.0000000000001014
43. Li S, Zhang H, Shao M, et al. Association between 17-β-estradiol and interleukin-8 and visual field progression in postmenopausal women with primary angle closure glaucoma. Am J Ophthalmol. 2020;217:55–67. doi:10.1016/j.ajo.2020.04.033
44. Cascio C, Deidda I, Russo D, et al. The estrogenic retina: the potential contribution to healthy aging and age-related neurodegenerative diseases of the retina. Steroids. 2015;103:31–41. doi:10.1016/j.steroids.2015.08.002
45. Kaarniranta K, Machalińska A, Veréb Z, et al. Estrogen signalling in the pathogenesis of age-related macular degeneration. Curr Eye Res. 2015;40(2):226–233. doi:10.3109/02713683.2014.925933
46. Monteiro R, Teixeira D, Calhau C. Estrogen signaling in metabolic inflammation. Mediators Inflamm. 2014;2014:615917. doi:10.1155/2014/615917
47. Shepherd R, Cheung AS, Pang K, et al. Sexual dimorphism in innate immunity: the role of sex hormones and epigenetics. Front Immunol. 2020;11:604000. doi:10.3389/fimmu.2020.604000
48. Zhou J, Jang YP, Kim SR, et al. Complement activation by photooxidation products of A2E, a lipofuscin constituent of the retinal pigment epithelium. Proc Natl Acad Sci U S A. 2006;103(44):16182–16187. doi:10.1073/pnas.0604255103
49. Du X, Yang L, Shujun Z, et al. Protective effects of estrogen on cardiovascular disease mediated by oxidative stress. Oxid Med Cell Longev. 2021;2021:5523516.
50. Kim SY, Kambhampati SP, Bhutto IA, McLeod DS, Lutty GA, Kannan RM. Evolution of oxidative stress, inflammation and neovascularization in the choroid and retina in a subretinal lipid induced age-related macular degeneration model. Exp Eye Res. 2021;203:108391. doi:10.1016/j.exer.2020.108391
51. Liu Y, Lv L. Effect of hormone replacement therapy on serum complement (C3, C4) and immunoglobulin (IgG, IgM) levels in post-menopausal women. J Huazhong Univ Sci Technolog Med Sci. 2008;28(1):102–103. doi:10.1007/s11596-008-0126-5
52. Yilmazer M, Fenkci V, Fenkci S, et al. Association of serum complement (C3, C4) and immunoglobulin (IgG, IgM) levels with hormone replacement therapy in healthy post-menopausal women. Hum Reprod. 2003;18(7):1531–1535. doi:10.1093/humrep/deg292
53. Golubnitschaja O, Liskova A, Koklesova L, et al. Caution, “normal” BMI: health risks associated with potentially masked individual underweight-EPMA Position Paper 2021. EPMA J. 2021;12(3):1–22. doi:10.1007/s13167-021-00251-4
54. Li S, Qiu Y, Yu J, et al. Association of systemic inflammation indices with visual field loss progression in patients with primary angle-closure glaucoma: potential biomarkers for 3P medical approaches. EPMA J. 2021;12(4):1–17.
55. Reddy V, Grogan D, Ahluwalia M, et al. Targeting the endocannabinoid system: a predictive, preventive, and personalized medicine-directed approach to the management of brain pathologies. EPMA J. 2020;11(2):217–250. doi:10.1007/s13167-020-00203-4
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.