Back to Journals » International Journal of General Medicine » Volume 15
Association of Red Blood Cell Distribution Width with Stroke Prognosis Among Patients with Small Artery Occlusion: A Hospital-Based Prospective Follow-Up Study
Authors Guan Y, Zuo W, Jia K, Yu C, Liu F, Lv Z, Wang D, Shi FD, Wang X
Received 5 July 2022
Accepted for publication 7 September 2022
Published 22 September 2022 Volume 2022:15 Pages 7449—7457
DOI https://doi.org/10.2147/IJGM.S381160
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Yalin Guan,1,2,* Wenchao Zuo,1,2,* Kun Jia,1,2,* Changshen Yu,2 Feng Liu,2 Zhaoyang Lv,2 Dong Wang,3 Fu-Dong Shi,1 Xinping Wang2
1Department of Neurology, Tianjin Medical University General Hospital, Tianjin, 300052, People’s Republic of China; 2Department of Neurology, Tianjin Huanhu Hospital, Tianjin, 300350, People’s Republic of China; 3Department of Neurosurgery, Tianjin Medical University General Hospital, Tianjin, 300052, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xinping Wang, Department of Neurology, Tianjin Huanhu Hospital, 6 Jizhao Road, Jinnan District, Tianjin, 300350, People’s Republic of China, Tel +86-22-59065906, Fax +86-22-59065662, Email [email protected] Fu-Dong Shi, Department of Neurology, Tianjin Medical University General Hospital, 154 Anshan Road, Heping District, Tianjin, 300052, People’s Republic of China, Tel +86-22-60362255, Fax +86-22-60362400, Email [email protected]
Objective: Stroke is the leading cause of mortality and disability worldwide. However, there is no study on the relationship between red blood cell distribution width and the prognosis of small artery occlusion, which is a stroke subtype. This study aimed to assess the association of red blood cell distribution width at admission with outcomes among patients with small artery occlusion.
Methods: In this hospital-based follow-up study, all included patients were diagnosed with small artery occlusion. Outcomes included death, recurrence, and dependency at 3, 12, and 36 months after stroke onset. Multivariate analysis was performed to explore the association of red blood cell distribution width with stroke outcomes.
Results: This study included 1576 patients with small artery occlusion who were followed up at 3, 12, and 36 months. For every unit increase in red blood cell distribution width, the risk of stroke recurrence and dependency increased by 5.1% (95% CI 1.002– 1.102, P=0.039) at 3 months after stroke onset. At the 12-month follow-up, for every unit increase in red blood cell distribution width, the risk of stroke recurrence increased by 3.4% (95% CI 1.000– 1.069, P=0.047). However, the relationship between red blood cell distribution width and mortality rate was not significant at 36 months after stroke onset after adjustment of covariates.
Conclusion: Red blood cell distribution width is an important hematological index of small artery occlusion. It may be used to predict the recurrence of acute ischemic stroke in small artery occlusion. Therefore, patients with higher baseline values of red blood cell distribution width may need more risk factor control to reduce recurrence and dependency.
Keywords: red blood cell distribution width, stroke prognosis, risk factors, small artery occlusion, prospective follow-up study
Introduction
According to the Global Burden of Diseases, Injuries, and Risk Factors Study, stroke is the leading cause of mortality and disability worldwide.1,2 The situation is even more serious in China, with over 2 million new cases annually.3 Small artery occlusion (SAO) is an ischemic stroke subtype according to the Trial of ORG10172 in Acute Stroke Treatment (TOAST) classification.4 A study showed that SAO accounted for approximately 22% of all stroke types worldwide.5 A recent study indicated that small-vessel-disease stroke increased from 15.5% to 39.6% over 10 years in China,6 and this increase was higher than noted globally. Progressive neurological decline occurs after SAO, including cognitive impairment, vascular dementia, gait disorder, urinary incontinence, and affective disorders. Some patients have early neurological deterioration and a poor prognosis.7 Therefore, a simple and effective biomarker for predicting the prognosis of SAO is necessary.
Recent studies have shown that a higher red blood cell distribution width (RDW) is associated with cardio-cerebrovascular disease, cancer, and mortality.8–11 Moreover, RDW was a potential predictor of mortality in patients with first stroke.12–14 An RDW study of the short-term prognosis after stroke showed that higher RDW was associated with poor function at discharge or 3 months later and that RDW had a dose-dependent relationship with 3-month mortality.15–18 There have also been studies on the relationship between RDW and the prognosis of patients with large artery cerebral infarction. These found that RDW is associated with 1-month mortality in patients with malignant middle cerebral artery infarction19 and that high RDW is a poor prognostic factor.20 However, to our knowledge, there is no study on the relationship between RDW and the prognosis of the stroke subtype—SAO. Moreover, previous studies investigated the prognostic indicators of mortality and poor function, but there were no studies related to recurrence.
Therefore, in this hospital-based prospective study, we attempted to determine the correlation of RDW with the prognosis of SAO and whether RDW is a predictor of recurrence in AIS caused by SAO.
Methods
Participants and Study Design
This was a hospital-based follow-up study conducted in the stroke unit of Tianjin Huanhu Hospital, China. The study design has been previously described.21 Briefly, all patients diagnosed with SAO according to the TOAST classification were recruited in this study. Patients diagnosed with transient ischemic attacks were excluded.
The study was approved by the medical research ethics committee at Tianjin Huanhu Hospital; written informed consent was obtained from each participant during recruitment.
SAO Diagnostic Criteria
All patients with AIS diagnosed with SAO met the TOAST classification criteria as follows:4 (1) presence of a traditional clinical lacunar syndrome without cerebral cortex dysfunction; (2) infarct located under the cortex or brainstem and with a diameter of < 1.5 cm as shown by computed tomography/magnetic resonance imaging within 24 h of admission; (3) history of diabetes or hypertension supporting the clinical diagnosis; (4) absence of potential cardiac embolism; and (5) presence of extracranial ipsilateral large artery stenosis < 50%. No patients in this study had received reperfusion therapy.
RDW Measurement
Blood samples were collected from peripheral veins with EDTA tubes within 24 h of admission and analyzed with an automatic blood cell analyzer (Sysmex, Kobe, Japan). Two RDW parameters were calculated: standard deviation (SD) and coefficient of variation (CV). RDW-CV has been widely studied and is calculated using the following formula: RDW-CV = (red blood cell volume/SD of average red blood cell volume) × 100. The reference range of RDW-CV is 11.5–14.5%.16
Clinical Features and Risk Factors
Clinical features included stroke severity, stroke risk factors (hypertension, diabetes mellitus, atrial fibrillation, obesity, smoking, and alcohol consumption), neurological function score, and laboratory values (fasting plasma glucose, total cholesterol, triglyceride, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, C-reactive protein, and homocysteine).
Patients were categorized into three age groups: < 45, 45–65, and ≥ 75 years. The severity of stroke was divided into three groups according to the National Institutes of Health Stroke Scale (NIHSS) score: mild (≤ 7 points), moderate (8–16 points), and severe (≥ 17 points).
Outcome Assessments and Follow-Up
In this study, outcomes were defined as death, recurrence, and dependence within 3 years after stroke onset. Death was defined as all-cause death occurring within the 3-year follow-up period. Recurrence was defined as all new-onset vascular events, including stroke, myocardial infarction, and venous thrombosis. Dependency was defined as a modified Rankin scale (mRS) score of > 2.22
Follow-up assessments were conducted by trained senior neurologists, and all patients were followed up using face-to-face interviews at 3, 12, and 36 months, except for those who required telephone follow-up because they underwent re-examination at another hospital.
Statistical Analysis
Continuous variables are presented as means and SDs; differences between groups were compared with the Student’s t-test and analysis of variance for two groups and more than two groups, respectively. Categorical variables are presented as numbers with frequencies and were compared with the chi-square test. Factors associated with death, recurrence, and dependency during the follow-up periods were assessed using logistic regression analyses. The results of the multivariate analyses are presented as adjusted relative risks and 95% confidence intervals (CIs) after adjusting for covariates that were statistically significant in the univariate analysis. Stroke outcomes were analyzed as dependent variables, while RDW and other variables were analyzed as independent variables in the logistic regression model. This study analyzed the relationship between RDW and stroke prognosis at 3, 12, and 36 months, with no longitudinal comparisons between the three time points. Statistical significance was set at P < 0.05. SPSS for Windows (version 22.0; SPSS, Chicago, IL, USA) was used for the statistical analyses.
Result
Demographic and Clinical Features in Patients with SAO at Baseline
This study included 1576 SAO patients (male, n=1050, 66.6%; female, n=526, 33.4%), who were followed up at 3, 12, and 36 months. The average age of the patients was 63.21±11.39 years, including 787 (49.9%) aged 45–64 years and 1312 (83.4%) with mild symptoms. The prevalence rates of hypertension, diabetes, atrial fibrillation, arteriosclerotic stenosis, smoking history, and drinking history were 74.9%, 32.0%, 2.2%, 14.3%, 37.3%, and 18.1%, respectively (Table 1). Moreover, the baseline characterization of patients according to distribution of red cell distribution width in quartiles was showed in Supplementary Table 1.
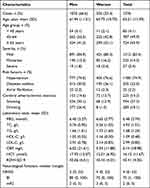 |
Table 1 Baseline Characterization of Patients According to Distribution of Red Cell Distribution Width |
Association of RDW with SAO Outcomes During the Follow-Up Period: Univariate Analysis
The average RDW-SD was higher in the recurrence group than in the non-recurrence group (P=0.005). The average RDW-SD was greater in the dependency group than in the non-dependency group at 3 months after stroke onset (P=0.005). The average RDW-SD was higher in those with death, recurrence, and dependency at 12 months after stroke onset than in those without these outcomes. RDW-SD was higher among dead patients than among survivors (44.53 vs 43.19, P=0.015) during the 36-month follow-up (Table 2).
 |
Table 2 The Average Levels of RDW in Patients with the Different Outcomes During Following-Up Periods |
Predictive Value of RDW for SAO Outcomes During the Follow-Up Period: Multivariate Analysis
The multivariate analysis results showed that the RDW-SD value associated with the stroke outcomes after adjusted the covariates which were significant statistical in the univariate analysis (Supplementary Tables 2.1–2.3). For every unit increase in RDW-SD, the risk of SAO recurrence increased by 5.1% (95% CI 1.002–1.102, P=0.039), and the risk of dependency increased by 5.1% (95% CI 1.002–1.102, P=0.039) at the 3-month follow-up. At the 12-month follow-up, for every unit increase in RDW-SD, the risk of SAO recurrence increased by 3.4% (95% CI 1.000–1.069, P=0.047; Table 3).
 |
Table 3 Association of the Average Levels of RDW in Patients with Outcomes During Following-Up Periods in the Multivariate Analysis |
Discussion
This hospital-based prospective study assessed the association of RDW with the prognosis of SAO. Patients with high RDW-SD had a poor prognosis, including a higher risk of death, recurrence, and dependency. High RDW-SD was associated with an increased risk of recurrence at 3 and 12 months and an increased risk of dependency at 3 months in patients with SAO.
RDW is considered a prognostic marker of vascular diseases, but the relationship between RDW and ischemic stroke outcomes is controversial. Some studies have shown that RDW is an independent predictor of stroke.8 A population-based study showed that high RDW was associated with an increased incidence of cerebral infarction.23 All the above studies observed a relationship between RDW and the first onset of ischemic stroke. However, to our knowledge, no study has observed the relationship between RDW and the recurrence of ischemic stroke, especially the recurrence of SAO. Our study noted that SAO patients with high RDW had a higher risk of recurrence. Increased RDW-SD was associated with an increased risk of recurrence at 3 and 12 months and was an independent predictor of recurrence at both time points.
Ritin et al analyzed the relationship between RDW and the severity of ischemic stroke. The results showed that the higher the RDW, the greater the severity of ischemic stroke. Thus, RDW may be an important prognostic index in patients with ischemic stroke.11 Gianni et al used the mRS score to evaluate the prognosis of patients with AIS. Multivariate analysis showed that RDW was an important predictor of 3-month functional decline (mRS > 2) in patients with AIS, and each unit increase in RDW resulted in an increase in poor outcomes by 20.8%.24 Another study assessed the association between RDW and neurological scoring systems (GCS, CNS, and NIHSS) in AIS patients.24 This study showed that for stroke patients who have symptoms for < 24 h, RDW may be useful in predicting the severity and functional results of the stroke (area under the receiver operator characteristic curve = 0.760, 95% CI 0.676–0.844).25 Consistent with the above findings, our results also showed that RDW-SD was associated with an increased risk of dependency at 3 months in patients with SAO. In contrast, the results of Kavous et al showed that RDW could not predict the severity and final outcome of stroke in patients receiving intravenous tissue plasminogen activator.26
The relationship between RDW and the prognosis of ischemic stroke is controversial. Pinho et al showed that RDW was an independent predictor of 1-year survival in patients with ischemic stroke treated with intravenous thrombolysis. Their study also revealed that RDW was not related to the severity of stroke at admission.14 Another study showed that higher RDW was associated with a poorer prognosis in patients with ischemic stroke and with a greater risk of death in 1 year.27 However, our results showed that the baseline RDW was not associated with the risk of death at 3, 12, and 36 months after onset. This may be because of the differences in patients because our study only focused on SAO and not all types of ischemic stroke. The cause of these differences needs to be explored in future studies.
Elevated RDW can predict the incidence and prognosis of ischemic stroke, but the specific pathophysiological mechanisms remain unclear. Increased oxidative stress leads to damage to the vascular wall and increases the risk of stroke. Red blood cells have a strong antioxidant capacity and are vulnerable to oxidative stress. However, other studies suggest that ischemia-induced inflammation may be the mechanism underlying increased RDW during stroke. Our study only analyzed the relationship between baseline RDW and SAO prognosis and did not analyze the relationship between RDW and ischemia or blood flow recovery.28–32
This study showed that SAO patients with higher RDW were at higher risk of death, relapse, and dependence. The mechanism may be related to inflammatory response, oxidative stress, and microcirculation disturbance. RDW is a hallmark of systemic inflammation and oxidative stress;33,34 inflammatory cytokines may contribute to elevated RDW levels by inhibiting erythrocyte maturation and releasing new and large reticulocytes into the circulation.35 Inflammatory mechanisms are central to the pathogenesis and progression of atherosclerosis, plaque rupture,36 thrombosis,37 and stroke.38 Inflammation is associated with an increased risk of stroke and may be an important determinant of outcome.39
Oxidative stress can lead to increased RDW by inhibiting erythropoiesis, increasing erythrocyte size imbalance, and altering the deformability of cell membranes.40 Increased RDW levels lead to acute hypoxia, induction of EPO-driven erythropoiesis, and a rapid increase in erythrocyte size variability.41 Hypoxia can also increase blood-brain barrier permeability, leading to uncontrolled vasogenic edema, microvascular ischemia, or hemorrhagic transformation.42 Xie et al found that the RDW of stroke patients was higher than that of patients with transient ischemic attack, and the antioxidant capacity of stroke patients with higher RDW was lower.43
Oxidative stress injury and antioxidant levels have been shown to be associated with neuronal damage/protection during cerebral ischemia and reperfusion.44 An imbalance between antioxidants and oxidants can lead to oxidative damage, which can lead to stroke. Stroke patients with high RDW have higher oxidative stress levels, have a lower antioxidant capacity, and are more prone to oxidative damage, which may lead to worse stroke prognosis and higher recurrence rates.
RDW is a parameter that reflects the heterogeneity and variability of peripheral blood erythrocyte volume. Increased RDW indicates an uneven size and reduced deformability of erythrocytes, which may lead to increased microcirculation resistance and interrupted microcirculation through narrowed capillaries in ischemic tissue,45 thereby mediating an increase in ischemic vascular events.46
This study has some limitations. First, it was a single-center study, and the number of patients was relatively small. Second, RDW levels are affected by many factors and diseases, such as pregnancy, anemia, iron, vitamin B12, and folic acid deficiency. Iron, vitamin B12, and folic acid levels were not examined in this study. However, no patient in our study had pregnancy, severe anemia, or complex complications. Finally, this study did not collect patient-specific medication data, but all patients were administered secondary stroke prevention therapy and had good compliance.
In conclusion, the results of this study showed that RDW is an important hematological index of SAO patients. RDW may be related to the prognosis of SAO and should be used as a predictor of AIS recurrence in SAO. Therefore, patients with higher baseline RDW may need more risk factor control to reduce recurrence and dependency.
Abbreviations
DALYs, disability-adjusted life-years; SAO, small artery occlusion; TOAST, Trial of ORG10172 in Acute Stroke Treatment; RDW, red blood cell distribution width; RBC-SD, standard deviation of the red blood cell volume; MCV, mean corpuscular volume; AIS, acute ischemic stroke; CV, coefficient of variation; NIHSS, National Institutes of Health Stroke Scale; Cis, confidence intervals.
Data Sharing Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Informed Consent
The study was approved by the medical research ethics committee at Tianjin Huanhu Hospital and conducted in accordance with the Declaration of Helsinki. A written informed consent was obtained from each participant during recruitment.
Acknowledgments
We thank all participants in this study. Yalin Guan, Wenchao Zuo and Kun Jia are co-first authors for this study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research funded partly by Tianjin Key Medical Discipline(Specialty) Construction Project (No. TJYXZDXK-052B) and the National Natural Science Foundation of China (82171359).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet. 2020;396(10258):1204–1222. doi:10.1016/S0140-6736(20)30925-9
2. Johnson CO, Nguyen M, Roth GA; GBD 2016 Stroke Collaborators. Global, regional, and national burden of stroke, 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet Neurol. 2019;18(5):439–458. doi:10.1016/S1474-4422(19)30034-1
3. Wu S, Wu B, Liu M, et al. Stroke in China: advances and challenges in epidemiology, prevention, and management. Lancet Neurol. 2019;18(4):394–405. doi:10.1016/S1474-4422(18)30500-3
4. Adams HP Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in acute stroke treatment. Stroke. 1993;24(1):35–41. doi:10.1161/01.STR.24.1.35
5. Ornello R, Degan D, Tiseo C, et al. Distribution and temporal trends from 1993 to 2015 of Ischemic stroke subtypes: a systematic review and meta-analysis. Stroke. 2018;49(4):814–819. doi:10.1161/STROKEAHA.117.020031
6. Tian D, Yang Q, Dong Q, Li N, Yan B, Fan D. Trends in stroke subtypes and vascular risk factors in a stroke center in China over 10 years. Sci Rep. 2018;8(1):5037. doi:10.1038/s41598-018-23356-9
7. Petrone L, Nannoni S, Del Bene A, Palumbo V, Inzitari D. Branch atheromatous disease: a clinically meaningful, yet unproven concept. Cerebrovasc Dis. 2016;41(1–2):87–95. doi:10.1159/000442577
8. Pilling LC, Atkins JL, Kuchel GA, Ferrucci L, Melzer D. Red cell distribution width and common disease onsets in 240,477 healthy volunteers followed for up to 9 years. PLoS One. 2018;13(9):e0203504. doi:10.1371/journal.pone.0203504
9. Ramírez-Moreno JM, Gonzalez-Gomez M, Ollero-Ortiz A, Roa-Montero AM, Gómez-Baquero MJ, Constantino-Silva AB. Relation between red blood cell distribution width and ischemic stroke: a case-control study. Int J Stroke. 2013;8(6):E36. doi:10.1111/ijs.12091
10. Vayá A, Hernández V, Rivera L, et al. Red blood cell distribution width in patients with cryptogenic stroke. Clin Appl Thromb Hemost. 2015;21(3):241–245. doi:10.1177/1076029614547262
11. Mohindra R, Mishra U, Mathew R, Negi NS. Red Cell Distribution Width (RDW) index as a predictor of severity of acute ischemic stroke: a correlation study. Adv J Emerg Med. 2020;4(2):e24. doi:10.22114/ajem.v0i0.257
12. Ye WY, Li J, Li X, et al. Predicting the one-year prognosis and mortality of patients with acute ischemic stroke using red blood cell distribution width before intravenous thrombolysis. Clin Interv Aging. 2020;15:255–263. doi:10.2147/CIA.S233701
13. Chen CM, Lee M, Yang YH, Huang SS, Lin CH. Association between clinical and laboratory markers and 5-year mortality among patients with stroke. Sci Rep. 2019;9(1):11521. doi:10.1038/s41598-019-47975-y
14. Pinho J, Marques SA, Freitas E, et al. Red cell distribution width as a predictor of 1-year survival in ischemic stroke patients treated with intravenous thrombolysis. Thromb Res. 2018;164:4–8. doi:10.1016/j.thromres.2018.02.002
15. Pinho J, Silva L, Quintas-Neves M, et al. Red cell distribution width is associated with 30-day mortality in patients with spontaneous intracerebral hemorrhage. Neurocrit Care. 2021;34(3):825–832. doi:10.1007/s12028-020-01103-1
16. Wang L, Wang C, Wu S, Li Y, Guo W, Liu M. Red blood cell distribution width is associated with mortality after acute ischemic stroke: a cohort study and systematic review. Ann Transl Med. 2020;8(4):81. doi:10.21037/atm.2019.12.142
17. Song SY, Hua C, Dornbors D, et al. Baseline red blood cell distribution width as a predictor of stroke occurrence and outcome: a comprehensive meta-analysis of 31 studies. Front Neurol. 2019;10:1237. doi:10.3389/fneur.2019.01237
18. Kim J, Kim YD, Song TJ, et al. Red blood cell distribution width is associated with poor clinical outcome in acute cerebral infarction. Thromb Haemost. 2012;108(2):349–356. doi:10.1160/TH12-03-0165
19. Lorente L, Martín MM, Abreu-González P, et al. Early mortality of brain infarction patients and red blood cell distribution width. Brain Sci. 2020;10:4. doi:10.3390/brainsci10040196
20. Güneş M. The correlation of routine hematological parameters with in-hospital mortality and length of hospital stay in patients with large middle cerebral artery infarction. Cureus. 2020;12(4):e7886. doi:10.7759/cureus.7886
21. Qiao Q, Hong Y, Zhao W, et al. Sex differences in outcomes and associated factors among stroke patients with small artery occlusion in China. Biol Sex Differ. 2018;9(1):35. doi:10.1186/s13293-018-0194-6
22. Banks JL, Marotta CA. Outcomes validity and reliability of the modified Rankin scale: implications for stroke clinical trials: a literature review and synthesis. Stroke. 2007;38(3):1091–1096. doi:10.1161/01.STR.0000258355.23810.c6
23. Söderholm M, Borné Y, Hedblad B, Persson M, Engström G. Red cell distribution width in relation to incidence of stroke and carotid atherosclerosis: a population-based cohort study. PLoS One. 2015;10(5):e0124957. doi:10.1371/journal.pone.0124957
24. Turcato G, Cervellin G, Cappellari M, et al. Early function decline after ischemic stroke can be predicted by a nomogram based on age, use of thrombolysis, RDW and NIHSS score at admission. J Thromb Thrombolysis. 2017;43(3):394–400. doi:10.1007/s11239-016-1456-y
25. Kara H, Degirmenci S, Bayir A, et al. Red cell distribution width and neurological scoring systems in acute stroke patients. Neuropsychiatr Dis Treat. 2015;11:733–739. doi:10.2147/NDT.S81525
26. Shahsavarinia K, Ghavam Laleh Y, Moharramzadeh P, et al. The predictive value of red cell distribution width for stroke severity and outcome. BMC Res Notes. 2020;13(1):288. doi:10.1186/s13104-020-05125-y
27. Turcato G, Cappellari M, Follador L, et al. Red blood cell distribution width is an independent predictor of outcome in patients undergoing thrombolysis for ischemic stroke. Semin Thromb Hemost. 2017;43(1):30–35. doi:10.1055/s-0036-1592165
28. Furer A, Finkelstein A, Halkin A, et al. High red blood cell distribution width and preclinical carotid atherosclerosis. Biomarkers. 2015;20(6–7):376–381. doi:10.3109/1354750X.2015.1096304
29. Kaya A, Isik T, Kaya Y, et al. Relationship between red cell distribution width and stroke in patients with stable chronic heart failure: a propensity score matching analysis. Clin Appl Thromb Hemost. 2015;21(2):160–165. doi:10.1177/1076029613493658
30. Semba RD, Patel KV, Ferrucci L, et al. Serum antioxidants and inflammation predict red cell distribution width in older women: the women’s health and aging study I. Clin Nutr. 2010;29(5):600–604. doi:10.1016/j.clnu.2010.03.001
31. Cherubini A, Polidori MC, Bregnocchi M, et al. Antioxidant profile and early outcome in stroke patients. Stroke. 2000;31(10):2295–2300. doi:10.1161/01.STR.31.10.2295
32. Macdougall IC, Cooper A. The inflammatory response and epoetin sensitivity. Nephrol Dial Transplant. 2002;17(Suppl 1):48–52. doi:10.1093/ndt/17.suppl_1.48
33. Patel KV, Ferrucci L, Ershler WB, Longo DL, Guralnik JM. Red blood cell distribution width and the risk of death in middle-aged and older adults. Arch Intern Med. 2009;169(5):515–523. doi:10.1001/archinternmed.2009.11
34. Förhécz Z, Gombos T, Borgulya G, Pozsonyi Z, Prohászka Z, Jánoskuti L. Red cell distribution width in heart failure: prediction of clinical events and relationship with markers of ineffective erythropoiesis, inflammation, renal function, and nutritional state. Am Heart J. 2009;158(4):659–666. doi:10.1016/j.ahj.2009.07.024
35. Ku NS, Kim HW, Oh HJ, et al. Red blood cell distribution width is an independent predictor of mortality in patients with gram-negative bacteremia. Shock. 2012;38(2):123–127. doi:10.1097/SHK.0b013e31825e2a85
36. Stoll G, Bendszus M. Inflammation and atherosclerosis: novel insights into plaque formation and destabilization. Stroke. 2006;37(7):1923–1932. doi:10.1161/01.STR.0000226901.34927.10
37. van der Spuy WJ, Pretorius E. Interrelation between inflammation, thrombosis, and neuroprotection in cerebral ischemia. Rev Neurosci. 2012;23(3):269–278. doi:10.1515/revneuro-2012-0028
38. Anrather J, Iadecola C. Inflammation and Stroke: an Overview. Neurotherapeutics. 2016;13(4):661–670. doi:10.1007/s13311-016-0483-x
39. Ramiro L, Simats A, García-Berrocoso T, Montaner J. Inflammatory molecules might become both biomarkers and therapeutic targets for stroke management. Ther Adv Neurol Disord. 2018;11:1756286418789340. doi:10.1177/1756286418789340
40. Diederich L, Suvorava T, Sansone R, et al. On the effects of reactive oxygen species and nitric oxide on red blood cell deformability. Front Physiol. 2018;9:332. doi:10.3389/fphys.2018.00332
41. Yčas JW, Horrow JC, Horne BD. Persistent increase in red cell size distribution width after acute diseases: a biomarker of hypoxemia. Clin Chim Acta. 2015;448:107–117. doi:10.1016/j.cca.2015.05.021
42. Kanazawa M, Takahashi T, Nishizawa M, Shimohata T. Therapeutic strategies to attenuate hemorrhagic transformation after tissue plasminogen activator treatment for acute ischemic stroke. J Atheroscler Thromb. 2017;24(3):240–253. doi:10.5551/jat.RV16006
43. Xie KH, Liu LL, Liang YR, et al. Red cell distribution width: a novel predictive biomarker for stroke risk after transient ischaemic attack. Ann Med. 2022;54(1):1167–1177. doi:10.1080/07853890.2022.2059558
44. Warner DS, Sheng H, Batinić-Haberle I. Oxidants, antioxidants and the ischemic brain. J Exp Biol. 2004;207(Pt 18):3221–3231. doi:10.1242/jeb.01022
45. Yedgar S, Koshkaryev A, Barshtein G. The red blood cell in vascular occlusion. Pathophysiol Haemost Thromb. 2002;32(5–6):263–268. doi:10.1159/000073578
46. Patel KV, Mohanty JG, Kanapuru B, Hesdorffer C, Ershler WB, Rifkind JM. Association of the red cell distribution width with red blood cell deformability. Adv Exp Med Biol. 2013;765:211–216.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
