Back to Journals » Cancer Management and Research » Volume 14
Association of Programmed Death-Ligand 1 Expression with Aggressive Histological Types of Thyroid Carcinoma
Authors Harahap AS , Lay FK, Kodariah R, Wongkar FJ , Ham MF
Received 6 October 2022
Accepted for publication 10 December 2022
Published 23 December 2022 Volume 2022:14 Pages 3539—3550
DOI https://doi.org/10.2147/CMAR.S392475
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sanjeev K. Srivastava
Agnes Stephanie Harahap,1,2 Fanny Kamarudy Lay,1 Ria Kodariah,1 Fresia Juwitasari Wongkar,1 Maria Francisca Ham1,2
1Department of Anatomical Pathology, Faculty of Medicine Universitas Indonesia/Dr. Cipto Mangunkusumo Hospital, Jakarta, Indonesia; 2Human Cancer Research Center - Indonesian Medical Education and Research Institute, Faculty of Medicine Universitas Indonesia, Jakarta, Indonesia
Correspondence: Maria Francisca Ham, Email [email protected]
Introduction: Immunohistochemical expression of programmed death-ligand 1 (PD-L1) has become a biomarker to predict the usefulness of cancer immunotherapy using PD-1/PD-L1 blockade in a variety of advanced-stage tumours. This emerging biomarker may serve to generate novel therapies for aggressive thyroid carcinoma (TC), which has not shown optimal results with existing treatments.
Methods: The present study investigated the relevance of PD-L1 expression in aggressive histological types of TC compared with that found in less aggressive types. Surgically resected specimens were investigated, including 52 cases of TC consisting of 26 cases of aggressive histological types and 26 cases of less aggressive histological types. Immunohistochemical examinations were carried out on paraffin blocks of both groups using a mouse monoclonal primary antibody against PD-L1 (clone 22C3). PD-L1 expression was evaluated by calculating the tumour proportion score (TPS) in both groups.
Results: The results revealed a significant difference in the median TPS value of PD-L1 expression between the two groups. The TPS values were found to be higher in the group of aggressive histological types of TC compared with those in the group of less aggressive histological types. A significant difference in TPS value was also found for the extrathyroidal extension variable.
Discussion: In conclusion, the present study found a significant association between PD-L1 expression and the aggressive histological type of TC. In addition, a potential association between PD-L1 expression and the presence of extrathyroidal extension of TC was observed. These findings provide novel approaches for immunotherapy as a potential new treatment modality in patients with aggressive histological types of TC.
Keywords: thyroid carcinoma, histological type, aggressive, programmed death-ligand 1
Introduction
Thyroid carcinoma (TC) is the most common malignant endocrine neoplasm and can originate from follicular or parafollicular cells.1 TCs arising from follicular cells are classified into well-differentiated TC (WDTC), poorly differentiated TC (PDTC) and anaplastic TC (ATC).1 Another type of TC that arises from parafollicular cells is medullary TC (MTC).1,2 The incidence of TC significantly increased over the last three decades.3 The highest increase has occurred in papillary TC (PTC),3 which is included in the WDTC group along with follicular TC (FTC) and Hurthle cell carcinoma (HCC).1,4 PTC is also the most common type of TC, which accounts for 65–90% of cases in adults and children, with an incidence rate of 13.5 cases per 100,000 individuals per year.1,3,5 The second most common type is FTC, which accounts for 6–10% of TC cases.1 Other less common types of TC, including PDTC, ATC and MTC, only account for 2–3% of all TC cases.1,2,5
TC has generally a good prognosis, with a 10-year survival rate of 90–95% in the WDTC group or non-aggressive TC, particularly for PTC and FTC.1 Several factors can worsen patient’s prognosis, including advanced age, male sex, increased tumour size, aggressive histological types, presence of extrathyroidal extension, and lymph node or distant metastasis.5,6 The worst prognosis is found in ATC, with a disease-specific mortality rate of almost 100%.1
Therapeutic modalities that can be used in the treatment of TC include surgery, I-131 radiotherapy and systemic medication.7 Surgery remains the main treatment for patients with well differentiated, but its role as a curative measure may have limited benefit for patients with more aggressive TCs, such as ATC, PDTC, MTC and several aggressive variants of PTC.6 Aggressive TCs usually show an infiltrative appearance, extrathyroidal extension and lymph node or distant metastasis.4,5 A number of these aggressive TC types also tend to show resistance to I-131 radiotherapy.1,6 These treatment problems may deteriorate the patient’s condition and worsen the prognosis. Therefore, it is necessary to identify other therapeutic modalities to increase the life expectancy of patients.
In the past decade, numerous clinical trials have been conducted on programmed death-1 (PD-1)/PD ligand-1 (PD-L1) inhibitors as immunotherapy agents in patients with lung carcinoma, melanoma, urothelial carcinoma, renal cell carcinoma and Hodgkin’s lymphoma.8 These studies have led to the development of several types of drugs that have been approved by the Food and Drug Administration as PD-1 or PD-L1 inhibitors. These inhibitors are used as immunotherapy in patients with cancer who exhibit positive immunohistochemical PD-L1 expression.8
Several studies on the role of PD-L1 in TC have been conducted in recent years.9–20 These studies are mostly based on immunohistochemical examination and also evaluations at the mRNA level. The results of these studies generally indicate a role of PD-L1 in the diagnosis, prognosis and identification of patients with TC who may benefit from immunotherapy using PD-1 or PD-L1 inhibitors.9 Positive correlations between PD-L1 and certain histological types, clinical aggressiveness, recurrence and advanced stage of TC have been described in several studies.10,14,15,18–22 However, there are limited studies on immunohistochemical PD-L1 expression in aggressive histological types of TC.
The present study aimed to investigate the immunohistochemical expression profile of PD-L1 in TCs and the association of PD-L1 with aggressive histological types vs less aggressive types. The results are expected to serve as a basis for novel immunotherapy approaches for patients with aggressive TCs, which are generally difficult to treat.
Materials and Methods
Clinicopathological Features
In order to reach a power of 80% and significance level of 5% in this study, the minimum number of necessary samples is 23 in each group to statistically detect a significant difference of PD-L1 expression between 2 groups of TC (group of aggressive histological types and another group of less aggressive histological types).23 Based on the available funds for this study, the highest number of samples that could be obtained was 26 for each group (52 in total) which has passed the minimum sample size. The present study retrospectively evaluated the clinicopathological data of 52 patients in two groups of histological types of TC: i) A group of aggressive histological types; and ii) another group of less aggressive histological types. Each group contained 26 samples randomly selected from all patients who had undergone total or partial thyroidectomy for TC at Dr. Cipto Mangunkusumo National Central Public Hospital (Jakarta, Indonesia), in the period January 2015-December 2019. Each case should have an adequate paraffin block of the tumour tissue for additional immunohistochemical examination in order to be enrolled in the current study. TCs with lymphocytic thyroiditis background were excluded from the study. The patients’ clinical data were obtained from electronic health records. The original haematoxylin and eosin slides from all cases were reviewed by two pathologists (FKL and ASH), and a paraffin block containing sufficient representative tumour and adjacent/normal tissue of each case was selected for additional immunohistochemical examination. The histological types of TC were classified according to the criteria of the latest World Health Organization thyroid tumour classification.1 TCs with histological diagnosis of ATC, PDTC or MDTC as well as various aggressive variants of PTC (tall cell, columnar, solid and hobnail variant) were categorized as a group of aggressive histological types, whereas other types (including FTC, HCC and the remaining variants of PTC) were categorized as a group of less aggressive histological types. The present study was conducted according to the Declaration of Helsinki and was approved (approval no. KET-1295/UN2.F1/ETIK/PPM.00.02/2019) by the Ethics Committee of the Faculty of Medicine, Universitas Indonesia (Jakarta, Indonesia).
Immunohistochemical Examination
Paraffin-embedded tumour samples were sliced at a thickness of 3–5 µm, placed on poly-L-lysine coated slides and dried on a slide warmer at 60°C for 60 min. All slides were subjected to deparaffinization in three serial xylene incubations, followed by rehydration in gradually decreasing alcohol concentration, and then eventually washed in running water for 3 min in room temperature. Blocking of endogenous peroxidase was carried out using a solution of 5% H2O2 in methanol for 45 min. A Tris EDTA-based solution (pH 9) was used under high pressure to retrieve the antigen. Immunohistochemical staining using a mouse monoclonal primary anti-PD-L1 antibody (clone 22C3; Dako; Agilent Technologies, Inc.) was performed at a dilution of 1:75 overnight in room temperature. Subsequently, the slides were rinsed in PBS (pH 7.4) and incubated for 15 min with Novolink Polymer Detection System as a secondary antibody (Novocastra (Leica Microsystems, Inc.). A solution of 3’3-diaminobenzidine was used as the chromogen to visualize the antigen as dark brown colour. Non-cancerous tonsil tissue block taken from the archives of the Department of Anatomical Pathology of Universitas Indonesia/Dr. Cipto Mangunkusumo National Central General Hospital was used as a positive control and was prepared for each batch of slides in the immunostaining process. The negative control was generated by omitting the above primary antibody during immunohistochemical staining and was prepared for each tumour slide.
Immunohistochemical expression of PD-L1 was determined by using the tumour proportion score (TPS), which is defined as the percentage of PD-L1-stained tumour cell count over the total number of viable tumour cells.24 Tumour cells that were considered PD-L1 positive were any viable tumour cells that had total or partial linear membrane staining from weak to strong intensity, which was perceived as distinct from cytoplasmic staining and therefore was included in the scoring. TPS used for the analysis was assessed by the pathologists. The number of PD-L1 staining tumour cells was explored throughout the entire slide area and total number of viable tumour cells in the entire tumour field counted manually with the help of a cell counter, using ImageJ software version 1.53f (National Institutes of Health). A cut-off score ≥1% was used to define PD-L1 positivity in addition to numerical TPS data. The TPS of cases with combined morphological features, such as PTC with certain components of ATC or PDTC, was calculated based on the whole viable tumour area throughout the entire slide regardless of the morphological type.
Assessment of PD-L1 immunohistochemistry was accomplished independently by two investigators (FKL and ASH) to determine TPS. Cohen’s κ coefficient and Bland Altman analysis were employed to evaluate the reliability of the examination conducted by the above two investigators, both categorically and numerically.
Statistical Analysis
Mann–Whitney U-test was used to evaluate the statistical significance of differences in numerical TPS data, which were not normally distributed, between two groups. Data are presented as the median and range. P<0.05 was considered to indicate a statistically significant difference. All statistical analyses were performed using SPSS 20.0 software (IBM Corp.).
Results
From 2015 to 2019, among 800 patients who had diagnoses of TC, 568 patients underwent partial or total thyroidectomy, and the remaining were subjected to biopsies only. Aggressive histological type of TC was found in 272 cases, and less aggressive histological type was found in 296 cases of all the subjects who underwent surgery.
The present study evaluated a total of 52 samples of patients with TC. There were 40 females (76.9%) and 12 males (23.1%), with a predominant female-to-male ratio of 3.3:1. The age range of patients was 18–70 years, with a median age of 48.5 years. In total, 32 patients (61.5%) were ≥45 years of age. The tumour size exhibited a range of 0.5–20.0 cm, with a median size of 3.3 cm, and 31 patients (59.6%) had a tumour size ≤4 cm. There were 19 patients (36.5%) with lymph node metastases, 13 (25%) with distant organ metastases and 24 (46.2%) with tumour expansion to surrounding tissues outside the thyroid gland at the time of surgery. Complete data regarding the demographic and pathological characteristics of the present cohort are shown in Table 1.
 |
Table 1 Patient Characteristics |
Evaluation was carried out by two observers independently with a good level of conformity based on the Cohen’s κ coefficient (0.86) and Bland Altman analysis [limit of agreement, −3.66 to 4.68; 95% confidence interval (−0.67–1.69)] for the categorical (PD-L1 positivity) and numerical (TPS) results, respectively. In the majority of cases, PD-L1 staining revealed the presence of partial or total linear membrane staining, varying from a few tumour cells to almost all tumour cells as shown in Figure 1. The smallest TPS percentage value obtained was 0.01% which was found in 4 cases (2 cases in each group). The largest TPS percentage value obtained was >90%, which was found in 3 cases (91.17, 91.36 and 95.00%) in aggressive histological-type group. Research samples with no PD-L1 positivity at all (TPS=0.00%) were also found in 10 cases, of which 3 cases belonged to the aggressive histological-type group and 7 to the less aggressive histological-type group. TPS values in each group of TC type are shown in Table 2.
 |  |  |
Table 2 TPS Values in Groups of Aggressive and Less Aggressive Histological Types of Thyroid Carcinoma with Demographic and Pathologic Characteristics |
A bivariate statistical test, the Mann–Whitney U-test, was performed to compare the non-normally distributed TPS data between two groups, namely the aggressive histological type and the less aggressive histological type. The results revealed significant differences (P=0.01), as shown in Table 3. This suggested that there was a significant difference in TPS values between the two groups of TC, where the aggressive histological type exhibited higher PD-L1 immunoexpression positivity than the less aggressive histological type.
 |
Table 3 Comparison of TPS Values in Aggressive and Less Aggressive Histological Types of TC |
Additional analyses were also performed on all other demographic and pathological characteristics to evaluate their potential association with PD-L1 immunoexpression as expressed in TPS values (Table 4). Only the extrathyroidal extension variable showed a significant difference (P=0.02), where higher TPS values were found in cases with extrathyroidal extension.
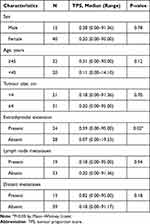 |
Table 4 Comparison of TPS Values Based on Demographic and Pathological Characteristics |
Discussion
The present study revealed a total of 52 TC cases with a predominant ratio of female-to-male ratio of 3.3:1, and 61.5% (32/52) of patients were aged ≥45 years old. This is consistent with the incidence rate of TC, which is 3-fold more commonly found in women than in men, and usually patients are >50 years old.1 The most frequent tumour size found in the present study was ≤4 cm, since the most common type of carcinoma is PTC, which usually measures 2–3 cm in diameter. Larger tumour sizes are generally found in PDTC and ATC, which tend to have a lower incidence than PTC.1
The majority of TC cases in the present study exhibited no metastasis in distant organs at the time of surgery; however, almost 50% showed tumour expansion into surrounding tissues outside the thyroid gland. These findings are in line with several previous studies, which frequently found stage I–II of TC cases without lymph node or distant metastases. Tumour extension out of the thyroid gland was commonly found in almost 50% of cases in various studies, although a number of studies reported a lower or higher percentage.11,12,14,15
Previous studies on the role of PD-L1 on TC commonly classified the PD-L1 staining results into positive or negative, based on the TPS and/or combined positive score (CPS) values with different cut-off points. There is currently no agreement on the TPS and/or CPS PD-L1 cut-off points for TC, contrarily to other carcinomas such as lung, breast and urothelial carcinoma. This leads to variations in the cut-off value used when evaluating the TPS value for TC. Previous studies on PD-L1 immunoexpression in TC mainly use a TPS cut-off point of 1 and/or 5% to determine PD-L1 positivity,11–13,15,25 while a study by Cantara et al18 used a higher TPS cut-off point (25%) to assess PD-L1 immunoexpression in ATC.
The present study on the role of PD-L1 in TC used a TPS value with a cut-off point of ≥1% positivity. The TPS values were quantitatively compared between the aggressive histological type and less aggressive histological-type groups. The use of TPS instead of CPS to assess PD-L1 immunoexpression in the present study was carried out to avoid the influence of lymphocytes, which can increase PD-L1 immunoexpression positivity, for example in cases of TC accompanied by lymphocytic thyroiditis. A previous study that supported this finding, which was carried out by Fadia et al,19 revealed that PTCs with lymphocytic thyroiditis showed significantly higher PD-L1 immunoexpression (39.1%) than PTC without lymphocytic thyroiditis (6.1%). A meta-analysis conducted by Girolami et al20 also reported the same findings.
In agreement with our working hypothesis, PD-L1 immunoexpression in the present study was found to be more abundant, and exhibited higher TPS values in the aggressive histological type of TC compared with those in the less aggressive histological type (P<0.05). Based on the cut-off point of TPS ≥1% in determining PD-L1 positivity status, the number of positive PD-L1 cases was 46.2% (12 of 26 cases) in the aggressive histological type of TC group and 15% (4 of 26 cases) in the less aggressive histological type of TC group. TPS values in the aggressive histological type were also found to be significantly higher than those in the less aggressive histological type. These findings are consistent with a previous study conducted by Chowdhury et al21 using an anti-PD-L1 antibody (clone E1L3N), which demonstrated a significant difference in the number of positive PD-L1 cases between the aggressive variant (53/74 cases) and the non-aggressive variant (21/111 cases) of TC. The aggressive variants involved in that study were ATC, PDTC and the tall cell variant of PTC. The authors also found a greater rate of recurrence in TCs with positive PD-L1 status.
The highest PD-L1 immunoexpression found in the present study (TPS >50.00%) was detected in 4 cases of aggressive histological type of TC. Of them, 3 cases were ATC (TPS=84.87, 91.17 and 91.36%) and 1 case was a mixed variant of PTC (solid and tall cell) with certain anaplastic components (TPS=95%). Other cases of aggressive histological type that also showed positive PD-L1 status included 1 case of hobnail variant of PTC (TPS=3.07%), 2 cases of tall cell variant of PTC (TPS=5.57 and 21.14%), 2 cases of mixed variant of PTC (solid and tall cell) with anaplastic components (TPS=9.81 and 36.60%) and 3 cases of ATC (TPS=1.15, 4.12 and 21.72%). The present study did not find PD-L1 immunoexpression in any of the MTC cases (TPS=0.00%). In the less aggressive histological type, positive PD-L1 immunoexpression with TPS ≥1% was found in 4 cases, including 2 cases of the follicular variant of PTC (TPS=3.31 and 14.10%), 1 case of the classic variant of PTC (TPS=8.94%) and 1 case of HCC with minimal invasion (TPS=19.53%). The difference in TPS values in various types of TC found in the present study was in line with the findings of a previous study conducted by Ahn et al,12 which showed differences in PD-L1 immunoexpression among different histological types of TC using tissue microarray samples with anti-PD-L1 antibody clone SP142 and a TPS cut-off point of ≥1% to define PD-L1 positivity. The authors found positive PD-L1 immunoexpression status in 6.1% (20/326) of PTC cases, 7.6% (5/66) of FTC cases and 22.2% (2/9) of ATC cases. All the ATC cases in their study showed much higher TPS values (80–90%) than other types of TC. Positivity of PD-L1 immunoexpression in MTC was not found in the current study. This may be due to the small sample number of MTC in the present study, since only 1 sample was included, and the frequency of PD-L1 positivity in this type of carcinoma is low according to the results of previous studies.15,26 One of those studies was conducted by Kemal et al,22 who used an anti-PD-L1 antibody (clone SP263) with a TPS cut-off point of ≥1% to explore the PD-L1 positivity in 41 MTC cases and found PD-L1 positivity in only 5 cases.
A prospective observational study is the best method to evaluate the risk of recurrence of TC and its association with PD-L1 immunoexpression; however, this methodology requires extensive resources and time. The simple but reasonable approach of evaluating the presence or absence of tumour extension outside the thyroid gland can be used as an alternative method to predict the recurrence of TC, as suggested by Bai et al.27 Tumour expansion outside the thyroid gland is one of the clinicopathological factors assessed in the present study. Additional analyses performed found a significant association between PD-L1 immunoexpression and extrathyroidal extension. This finding is in line with the results of several other studies, including that by Aghajani et al,13 who found that 11 of 15 cases of PTC with positive PD-L1 immunoexpression (TPS ≥1%) showed tumour expansion outside the thyroid gland. Based on this previous study, TPS values were expected to be higher in cases with extrathyroidal extension. A similar result was also reported in a study conducted by Shi et al,25 who found extrathyroidal extensions of the thyroid tumour in 60 of 136 cases of PTC with positive PD-L1 status. The association between PD-L1 immunoexpression and the presence of extrathyroidal extension found in the present and previous studies may suggest an association between PD-L1 immunoexpression and the risk of recurrence, which could affect the patient’s prognosis, as suggested by Chowdhury et al.18 Further studies with a more suitable research design should be considered in the future to validate this potential association.
In the present study, other demographic or pathological factors, such as sex, age, tumour size, lymph node metastases or distant metastases, did not show a significant association with PD-L1 immunoexpression based on the bivariate statistical tests performed in the additional analyses, using Mann–Whitney U-test. These findings were also reported by other studies that assessed the same clinicopathological factors.12,13,15 One of those studies was conducted by Ahn et al,12 and included a large sample size of TC (407 cases). The authors found no association between PD-L1 immunoexpression and clinicopathological variables such as age, sex, tumour size, multifocality or stage.12 Similarly, another study with a smaller sample size (110 cases of PTC stained with anti-PD-L1 antibody clone SP142) conducted by Bai et al16 did not reveal significant associations between PD-L1 immunoexpression and clinicopathological factors.13
Based on meta-analysis by Wan et al,28 they found that high expression of PD-L1 in patients with thyroid cancer was associated with poor DFS (disease free survival), tumour size ≥2 cm, tumour recurrence, extrathyroidal extension, concurrent thyroiditis, unifocal tumour, and absence of psammoma bodies. Some of this clinicopathological variables can be also found in our study, such as tumor size and extrathyroidal extension. The significant association between high PD-L1 expression and extrathyroidal extension was also found in our study. However, our study did not find any significant association between PD-L1 expression and tumour size. This may be caused by a higher cut-off (4 cm) to categorize the tumour size in our study. The novelty of our study, we did the direct comparison of PD-L1 expression quantitatively between the aggressive histological types and the less aggressive histological types of thyroid cancer. Our study also provides the numeric score of TPS values of each cases related to morphological types of thyroid cancer which is never found in any other previous studies. We also excluded cases with concurrent thyroiditis to avoid the effect of thyroiditis on PD-L1 expression in order to see the real PD-L1 expression in thyroid cancer. In addition, we also evaluated the status of distant metastases which is not provided in this meta-analysis.
This study has certain limitations related to the association between TPS value and distant metastases. The size of samples in one group (cases with distant metastases) is very different to that in another group (cases without distant metastases) and there is also such high variance in TPS scores in both groups. Both factors may lead to an insignificant P-value and obscure the real association between TPS value and distant metastases. We did not compare the cancerous versus adjacent/normal tissues in this study. The main goal of this study is to compare the PD-L1 expression (TPS value) of the aggressive histological types versus the less aggressive histological types of TC. All the tissue specimens used in this study are the tumour tissues. The absence of data in adjacent/normal tissue and the retrospective methods that we used in this study are the limitation of our study.
In conclusion, the present study found a clinically and statistically significant association between PD-L1 immunoexpression and aggressive histological types of TC. PD-L1 immunoexpression, as evaluated by the TPS value, was found to be significantly higher in the aggressive histological-type group compared with that in the less aggressive histological-type group. The histological types of TC that showed the highest PD-L1 positivity were ATC, tall cell variant of PTC and other aggressive histological types of TC with anaplastic components involved. Only a few TC cases from the less aggressive histological group showed PD-L1 immunoexpression (follicular variant of PTC, classic PTC and HCC with minimal invasion). Other demographic/pathological factor that also appeared to show a significant association with PD-L1 immunoexpression was extrathyroidal extension of TC. Other factors such as sex, age, tumor size, lymph node metastasis and/or distant metastasis did not show a significant association with PD-L1 immunoexpression. These findings provide novel approaches for immunotherapy as a potential new treatment modality in patients with aggressive histological types of TC.
Data Sharing Statement
All data are available from the corresponding author upon reasonable request.
Ethics Approval and Consent to Participate
This study was approved by the Faculty of Medicine, Universitas Indonesia Research Ethics Committees, with protocol number KET-1295/UN2.F1/ETIK/PPM.00.02/2019. All data in this study has been approved by the same ethics committee to be taken without direct consent. The policy of our IRB that direct informed consent form can be waived to study that meet several criteria, including research that uses existing collection of data, documents, pathological specimens, or other diagnostic specimens, in which the documents are managed in such a way that identity of each subject is protected and cannot be identified. Informed consent waiver ND-382/UN2.F1/ETIK/PPM.00.02/2022.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation; took part in drafting, revising and reviewing the article; gave final approval of the final manuscript to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The present study was funded by Universitas Indonesia through a Publikasi Terindeks Internasional (PUTI) grant with contract no. NKB-1298/UN2.RST/HKP.05.00/2020, Jakarta, Indonesia.
Disclosure
The authors declare that they have no competing interests.
References
1. Rosai J, Albores SJ, Asioli S, Baloch ZW, Bogdanova T, Chen H. Tumours of the thyroid gland. In: Lloyd RV, Osamura RY, Kloppel G, Rosai J, editors. WHO Classification of Tumours of Endocrine Organs.
2. Ceolin L, Duval M, Benini AF, Ferreira CV, Maia AL. Medullary thyroid carcinoma beyond surgery: advances, challenges, and perspectives. Endocr Relat Cancer. 2019;26:R499–R518. doi:10.1530/ERC-18-0574
3. Pellegriti G, Frasca F, Regalbuto C, Squatrito S, Vigneri R. Worldwide increasing incidence of thyroid cancer: update on epidemiology and risk factors. J Cancer Epidemiol. 2013;2013:1–10. doi:10.1155/2013/965212
4. Baloch ZW, LiVolsi VA. Special types of thyroid carcinoma. Histopathology. 2018;72:40–52. doi:10.1111/his.13348
5. Sipos JA, Mazzaferri EL. Thyroid cancer epidemiology and prognostic variables. Clin Oncol. 2010;22:395–404. doi:10.1016/j.clon.2010.05.004
6. Haddad RI, Nasr C, Bischoff L, et al. NCCN guidelines insights: thyroid carcinoma. J Natl Compr Canc Netw. 2018;16:1429–1440. doi:10.6004/jnccn.2018.0089
7. Paschke R, Lincke T, Muller SP, Kreissl MC, Dralle H, Fassnacht M. The treatment of well-differentiated thyroid carcinoma. Dtsch Arztebl Int. 2015;112:452–458. doi:10.3238/arztebl.2015.0452
8. Kythreotou A, Siddique A, Mauri FA, Bower M, Pinato DJ. PD-L1. J Clin Pathol. 2018;71:189–194. doi:10.1136/jclinpath-2017-204853
9. Ulisse S, Tuccilli C, Sorrenti S, et al. PD-1 ligand expression in epithelial thyroid cancers: potential clinical implications. Int J Mol Sci. 2019;20:1–15. doi:10.3390/ijms20061405
10. Zhang GQ, Wei WJ, Song HJ, et al. Programmed cell death-ligand 1 overexpression in thyroid cancer. Endocr Pract. 2019;25:279–286. doi:10.4158/EP-2018-0342
11. Aghajani MJ, Yang T, McCafferty CE, Graham S, Wu X, Niles N. Predictive relevance of programmed cell death protein 1 and tumor-infiltrating lymphocyte expression in papillary thyroid cancer. Surgery. 2018;163:130–136. doi:10.1016/j.surg.2017.04.033
12. Ahn S, Kim TH, Kim SW, et al. Comprehensive screening for PD-L1 expression in thyroid cancer. Endocr Relat Cancer. 2017;24:97–106. doi:10.1530/ERC-16-0421
13. Bai Y, Guo T, Huang X, et al. In papillary thyroid carcinoma, expression by immunohistochemistry of BRAF V600E, PD-L1, and PD-1 is closely related. Virchows Arch. 2018;472:779–787. doi:10.1007/s00428-018-2357-6
14. Bai Y, Niu D, Huang X, et al. PD-L1 and PD-1 expression are correlated with distinctive clinicopathological features in papillary thyroid carcinoma. Diagn Pathol. 2017;12:72. doi:10.1186/s13000-017-0662-z
15. Bi Y, Ren X, Bai X, et al. PD-1/PD-L1 expressions in medullary thyroid carcinoma: clinicopathologic and prognostic analysis of Chinese population. Eur J Surg Oncol. 2019;45:353–358. doi:10.1016/j.ejso.2018.10.060
16. Capdevila J, Wirth LJ, Ernst T, et al. PD-1 Blockade in Anaplastic Thyroid Carcinoma. J Clin Oncol. 2020;38:2620–2627. doi:10.1200/JCO.19.02727
17. Chintakuntlawar AV, Rumilla KM, Smith CY, et al. Expression of PD-1 and PD-L1 in anaplastic thyroid cancer patients treated with multimodal therapy: results from a retrospective study. J Clin Endocrinol Metab. 2017;102:1943–1950. doi:10.1210/jc.2016-3756
18. Chowdhury S, Veyhl J, Jessa F, et al. Programmed death-ligand 1 overexpression is a prognostic marker for aggressive papillary thyroid cancer and its variants. Oncotarget. 2016;7:32318–32328. doi:10.18632/oncotarget.8698
19. Fadia M, Fookeerah P, Ali S, Shadbolt B, Greenaway T, Perampalam S. PD-L1 expression in papillary thyroid cancer with and without lymphocytic thyroiditis: a cross sectional study. Pathology. 2020;52:318–322. doi:10.1016/j.pathol.2019.11.007
20. Girolami I, Pantanowitz L, Mete O, et al. Programmed Death-Ligand 1 (PD-L1) is a potential biomarker of disease-free survival in papillary thyroid carcinoma: a systematic review and meta-analysis of PD-L1 immunoexpression in follicular epithelial derived thyroid carcinoma. Endocr Pathol. 2020;31:291–300. doi:10.1007/s12022-020-09630-5
21. Wang H, Zhang Z, Yan Z, Ma S. PD-L1, PDK-1 and p-Akt are correlated in patients with papillary thyroid carcinoma. Adv Clin Exp Med. 2020;29:785–792. doi:10.17219/acem/121518
22. Shi RL, Qu N, Luo TX, et al. Programmed death-ligand 1 expression in papillary thyroid cancer and its correlation with clinicopathologic factors and recurrence. Thyroid. 2017;27:537–545. doi:10.1089/thy.2016.0228
23. Malone HE, Nicholl H, Coyne I. Fundamentals of estimating sample size. Nurse Res. 2016;23:21–25. doi:10.7748/nr.23.5.21.s5
24. Ilie M, Khambata-Ford S, Copie-Bergman C, et al. Use of the 22C3 anti-PD-L1 antibody to determine PD-L1 expression in multiple automated immunohistochemistry platforms. PLoS One. 2017;12:1–13.
25. Zwaenepoel K, Jacobs J, De Meulenaere A, et al. CD70 and PD-L1 in anaplastic thyroid cancer - promising targets for immunotherapy. Histopathology. 2017;71:357–365. doi:10.1111/his.13230
26. Kemal YY, Gün S, Çalışkan S, Kefeli M. 1926P PD-L1 expression in medullary thyroid carcinoma and its relevance with clinicopathological findings. Ann Oncol. 2020;31:S1090–S1. doi:10.1016/j.annonc.2020.08.1414
27. Bai Y, Kakudo K, Li Y, et al. Subclassification of non-solid-type papillary thyroid carcinoma identification of high-risk group in common type. Cancer Sci. 2008;99:1908–1915. doi:10.1111/j.1349-7006.2008.00908.x
28. Wan B, Deng P, Dai W, et al. Association between programmed cell death ligand 1 expression and thyroid cancer: a meta-analysis. Medicine. 2021;100(14):e25315. doi:10.1097/MD.0000000000025315
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

