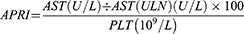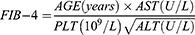Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 16
Association of Non-Invasive Markers with Significant Fibrosis in Patients with Nonalcoholic Fatty Liver Disease: A Cross-Sectional Study
Authors Zhang F , Han Y, Zheng L , Liu J, Wu Y, Bao Z, Liu L, Li W
Received 20 April 2023
Accepted for publication 25 July 2023
Published 31 July 2023 Volume 2023:16 Pages 2255—2268
DOI https://doi.org/10.2147/DMSO.S417754
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Prof. Dr. Juei-Tang Cheng
Fan Zhang,1,2 Yan Han,1,2 Liming Zheng,3 Jianhong Liu,4 Yunfei Wu,4 Zuowei Bao,5 Longgen Liu,6 Wenjian Li7
1Department of Endocrinology, Changzhou Third People’s Hospital, Changzhou Medical Center, Nanjing Medical University, Changzhou, Jiangsu, People’s Republic of China; 2Department of Clinical Nutrition, Changzhou Third People’s Hospital, Changzhou Medical Center, Nanjing Medical University, Changzhou, Jiangsu, People’s Republic of China; 3Clinical Laboratory, Changzhou Third People’s Hospital, Changzhou Medical Center, Nanjing Medical University, Changzhou, Jiangsu, People’s Republic of China; 4Department of Pathology, Changzhou Third People’s Hospital, Changzhou Medical Center, Nanjing Medical University, Changzhou, Jiangsu, People’s Republic of China; 5Department of Ultrasonography, Changzhou Third People’s Hospital, Changzhou Medical Center, Nanjing Medical University, Changzhou, Jiangsu, People’s Republic of China; 6Department of Liver Diseases, Changzhou Third People’s Hospital, Changzhou Medical Center, Nanjing Medical University, Changzhou, Jiangsu, People’s Republic of China; 7Department of Urology, Changzhou Third People’s Hospital, Changzhou Medical Center, Nanjing Medical University, Changzhou, Jiangsu, People’s Republic of China
Correspondence: Longgen Liu, Department of Liver Diseases, Changzhou Third People’s Hospital, Changzhou Medical Center, Nanjing Medical University, 300 Lanling North Road, Changzhou, Jiangsu, 213001, People’s Republic of China, Tel +86-0519-82009057, Email [email protected] Wenjian Li, Department of Urology, Changzhou Third People’s Hospital, Changzhou Medical Center, Nanjing Medical University, 300 Lanling North Road, Changzhou, Jiangsu, 213001, People’s Republic of China, Tel +86-0519-82009011, Email [email protected]
Purpose: The identification of significant fibrosis is critical for predicting the prognosis of non-alcoholic fatty liver disease (NAFLD). This study aimed to compare the predictive value of chitinase-3-like protein 1 (CHl3L1) and other non-invasive biomarkers, as well as to establish a novel non-invasive diagnostic model for assessing the risk of significant fibrosis in NAFLD.
Patients and Methods: A total of 71 patients with confirmed NAFLD based on liver biopsy were included in this study. Serum CHI3L1 levels and other non-invasive fibrosis assessment measures were determined. The aspartate aminotransferase-to-platelet ratio index (APRI) and Fibrosis-4 Index (FIB-4) were calculated to assess the diagnostic superiority of serum CHI3L1 compared to other non-invasive fibrosis assessment measures. Multivariate logistic regression analysis was conducted to identify relevant variables for constructing a diagnostic model. Receiver operating characteristic (ROC) curve analysis was performed to evaluate the diagnostic accuracy of each index, including the area under ROC curve (AUC), sensitivity, and specificity. A nomogram was established based on the logistic regression model.
Results: Serum CHI3LI levels were found to be higher in NAFLD patients with significant fibrosis compared to those without significant fibrosis. Multivariate logistic regression analysis revealed that aspartate aminotransferase (AST), type IV collagen (IV-C), CHI3L1, and liver stiffness measurement (LSM) were identified as potential independent risk factors associated with significant fibrosis in patients. The AUC of CHI3L1 for diagnosing significant liver fibrosis was 0.716 (0.596,0.836), with the optimal cut-off point of 125.315. The nomogram incorporating CHI3LI, AST, IV-C, and LSM further improved the potential predictive value, with an AUC for diagnosing significant fibrosis of 0.864 (0.766,0.962). This was superior to IV-C, CHI3L1, LSM, and APRI (all p < 0.05).
Conclusion: The diagnostic model constructed by CHI3L1 combined with the existing non-invasive markers AST, IV-C, and LSM can help assess the risk of significant liver fibrosis in NAFLD.
Keywords: nonalcoholic fatty liver disease, fibrosis, chitinase 3-like protein 1, liver biopsy, nomogram
Introduction
Nonalcoholic fatty liver disease (NAFLD) is currently recognized as the prevailing chronic liver disease globally.1 NAFLD can potentially progress to fibrosis, cirrhosis, and hepatocellular carcinoma (HCC).2 A meta-analysis was conducted to comprehensively assess the incidence rate of HCC in different subpopulations of NAFLD patients, with a specific emphasis on the association between NAFLD and liver fibrosis. NAFLD is characterized by the excessive accumulation of fat in the liver and can progress to advanced stages of fibrosis. Moreover, it has been recognized as a potential precursor for the development of HCC. In studies specifically including patients with cirrhosis, the incidence rate of HCC was reported as 3.78 cases per 100 person-years (95% confidence interval, 2.47–5.78).3 Liver fibrosis is a reparative response of the liver to chronic inflammatory stimulation, characterized by excessive deposition of connective tissue and tissue remodeling. It serves as a critical stage in the progression of NAFLD towards cirrhosis. Notably, liver fibrosis can manifest in both nonalcoholic steatohepatitis (NASH) and non-NASH cases. Among various liver histological indices, the stage of liver fibrosis stands out as the sole independent predictor of long-term prognosis in NAFLD patients. Individuals with significant liver fibrosis (F2-F4) are at a considerably higher risk of developing severe liver ailments such as cirrhosis and hepatocellular carcinoma, with an onset occurring approximately a decade years earlier than those with F0-F1 liver fibrosis.4 Hence, there is an urgent clinical imperative to accurately identify patients with significant liver fibrosis (F2-F4), enabling prompt intervention and the formulation of tailored treatment and follow-up plans aimed at halting or even reversing disease progression.
Liver biopsy remains the gold standard for diagnosing liver fibrosis, but its invasive nature limits patient acceptance and hampers reproducibility during follow-up assessments. In contrast, non-invasive techniques like vibration-controlled transient elastography (VCTE) based on FibroScan offer a faster assessment of liver stiffness. However, VCTE is susceptible to interference from factors such as obesity, ascites and liver inflammation.5 Additionally, there is ongoing debate regarding the optimal cut-off value for diagnosing significant fibrosis. Several non-invasive serum markers have shown promise in diagnosing liver fibrosis, including hyaluronic acid (HA), laminin (LN), procollagen type III (PC III) and collagen type IV (IV-C), the AST-to-platelet ratio index (APRI), and fibrosis score-4 (FIB-4). These markers offer advantages in terms of simplicity, speed and reproducibility in clinical practice. They are suitable for screening and monitoring fibrosis in patients with chronic liver disease. Although these parameters exhibit a high negative predictive value (NPV) in distinguishing F0-F1 from F2 fibrosis, their positive predictive value (PPV) remains suboptimal. Consequently, the search for non-invasive markers for liver fibrosis diagnosis continues to be a major research focus.
Chitinase 3-like protein 1 (CHI3L1) is a glycoprotein that binds to heparin and chitin and is primarily produced by hepatic macrophages and hepatic stellate cells. Its expression in liver diseases surpasses that in other tissues, suggesting its involvement in tissue remodeling.6 Previous studies have demonstrated an association between CHI3L1 levels and the degree of liver fibrosis in patients with chronic liver disease.7–9 Immunofluorescence staining of liver specimens from NAFLD patients conducted by Kumagai et al revealed the expression of CHI3L1 in liver tissue macrophages, indicating its potential as a biomarker for predicting liver fibrosis in this patient population.10 Our previous investigation, albeit not differentiating the etiology of liver disease, evaluated serum CHI3L1 in 165 patients with chronic liver disease based on liver biopsy staging and found it to be an accurate diagnostic tool for significant fibrosis and cirrhosis. Notably, a fibrosis diagnostic model incorporating CHI3L1, along with IV-C and FIB-4, exhibited improved diagnostic efficiency.11 Moreover, serum ferritin has been considered as an independent predictor of the severity and progression of liver fibrosis in NAFLD patients.12 Ferritin serves as a marker for liver iron storage acts as a pro-inflammatory cytokine, potentially activating hepatic stellate cells and inducing fibrous tissue hyperplasia through the nuclear factor kappa-B (NF-κB) cascade. The relationship between serum iron markers and liver fibrosis in NAFLD patients, however, remains to be fully elucidated.
Given the significance of differentiating significant fibrosis in the diagnosis and management of NAFLD patients, our study aimed to compare the characteristics and predictive value of CHl3Ll, ferritin, and other widely utilized non-invasive biomarkers in NAFLD patients with varying fibrosis stages. The primary objective was to develop a novel non-invasive diagnostic model that can accurately identify significant fibrosis in patients with NAFLD.
Materials and Methods
Participants
In this single-center study, we enrolled patients who received an initial diagnosis of NAFLD and underwent liver biopsy at Changzhou Third People’s Hospital between July 2017 to March 2021. The inclusion criteria were as follows: (1) age > 18 years old (2) presence of fatty liver indicated by abdominal ultrasound, and (3) NAFLD duration > 6 months. Exclusion criteria were as follows: (1) weekly alcohol consumption exceeding 140 g of ethanol in males or 70 g in female (2) co-infection with hepatitis viruses, human immunodeficiency virus (HIV), cytomegalovirus (CMV), Epstein-Barr virus (EBV), etc. (3) presence of autoimmune diseases, severe infections, biliary tract diseases, chronic kidney disease, cardiovascular system, tumors in various organs (4) drug-induced hepatitis (5) blood disorders or other systemic conditions affecting serum iron and ferritin metabolism (6) incomplete data.
All participants signed the informed consent form and submitted it to the Ethics Committee of Changzhou Third People’s Hospital for approval.
Liver Biopsy
Liver tissues were obtained through a percutaneous approach using ultrasound guidance. Prior to the biopsy, the patient’s skin was cleaned and sterilized. Local anesthesia was administered to numb the area of the liver where the biopsy was to be performed. A small incision was made in the skin, and a biopsy needle was inserted through the incision and guided towards the targeted area of the liver under real-time ultrasound imaging. Once the needle reached the desired location, multiple tissue samples were obtained by puncturing the liver parenchyma. The needle was carefully retracted after each sample was collected to minimize bleeding and potential complications. The collected liver tissue samples were immediately placed in a container filled with 10% formaldehyde to fix the tissue. After fixation, the liver samples were subjected to a series of standard histological processing steps. This included dehydration, where the samples were gradually exposed to increasing concentrations of alcohol to remove water from the tissues. Subsequently, the dehydrated samples were embedded in paraffin wax to provide structural support. The paraffin-embedded liver tissue blocks were then sectioned into thin slices using a microtome. These slices were mounted onto glass slides, and the sections were stained with hematoxylin and eosin (H&E). The H&E staining allowed for visualization and examination of the cellular and structural features of the liver tissue. Histopathological examination was then performed by two liver pathologists, who were blinded to clinical data, to obtain a NAS score which was the sum of scores for steatosis (0–3), lobular inflammation (0–1), and hepatocyte ballooning (0–2).13 Liver fibrosis was subsequently divided into four stages (F0-F4), and ≥ 2 was defined as significant fibrosis.14 F0-F1: No fibrosis or minimal fibrosis. F2-F4: Increasing levels of fibrosis, with F2 indicating moderate fibrosis, F3 indicating advanced fibrosis without cirrhosis, and F4 representing cirrhosis (the most severe stage of fibrosis).
Measurement of LSM and CAP
Liver stiffness measurement (LSM) was determined non-invasively with the FibroScan-502 probe (Echosens, France) to evaluate liver fibrosis. The patient fasted for more than 8 hours, and the test was performed by lying flat, with the right arm fully exposed to the skin of the liver region at maximum abduction. The detection area was the horizontal line of the xiphoid process, the right midaxillary line, and the line connecting the lower edge of the ribs. The probe was vertical and close to the skin of the detection area, while the detection position was selected in the intercostal space for measurement. For effective LSM and controlled attenuation parameter (CAP) detection, the procedure success rate was set at ≥ 60% and the interquartile range (IQR)/median (IQR/M) ≤ 0.3 were required. The median of ten successful detections was the final value.
Body Measurements and Laboratory Test
The height and weight of the patients were measured routinely, and the body mass index (BMI) = weight/height2(kg/m2) was calculated accordingly. Patients also fasted for 8–10 hours, before drawing early morning fasting venous blood from them. In addition, ALT, AST, alkaline phosphatase (ALP), gamma-glutamyl transferase (GGT), total bilirubin (TBIL), direct bilirubin (DBIL), indirect bilirubin (IBIL), albumin (ALB), C-reactive protein (CRP), Creatinine, uric acid (UA), total cholesterol (TC), and triglyceride (TG) were detected by the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) method using Photopure Pharmaceutical Co., LTD reagent [These biochemical markers are analyzed in serum]. Meikang Biological Technology Co., Ltd. reagents were purchased to detect fasting plasma-glucose (FPG) using the dry chemical method before using Hitachi LABOSPECT008AS to detect the above biochemical indicators. Blood routine was determined by SYSMEX XN3000 complete blood cell analyzer.
Non-Invasive Diagnosis of Biomarkers for Liver Fibrosis
Detection of CHI3L1 in serum samples was performed using the CHI3L1 detection kit double-antibody sandwich enzyme-linked immunosorbent assay (ELISA) kit (Proprium Biotech Company Limited, Hangzhou, China), while serum ferritin was detected by the chemiluminescence method using the matching ferritin determination kit of American BECKMAN company, alongside the detection equipment from BECKMAN COULTERDxI 800. The levels of HA, LN, PC III and IV-C were detected by Snibe MAGLUMI 2000 PlLus automatic chemiluminescence immunoassay analyzer. APRI is calculated using the formula: (AST level [U/L]/ upper limit of normal AST range [U/L])/ platelet count [109/L] × 100. FIB-4 is calculated using the formula: (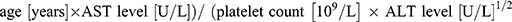 ).
).
Nomogram Usage
Identify variables: Observe the nomogram and identify the variables or factors listed on the axes or scales.
Identify patient values: Assess the patient’s specific values for each variable. For example, if the patient has an AST of 20, a CHI3L1 of 40, etc., you will need to identify the corresponding number of points on each axis or scale.
Calculate the total score: Once you have determined the patient’s values on each axis, add the scores together to obtain the total score.
Determine the probability: Find the total score on the total score scale of the nomogram. Draw a vertical line from the total score to the probability table to determine the corresponding predicted probability of the patient’s significant fibrosis.
For example, based on the patient’s total score, you can determine the estimated probability of their significant fibrosis in NAFLD by finding the intersection point on the nomogram scale.
Statistical Analysis
Continuous variables were presented as means ± standard deviations or median (25th/75th percentile), while categorical variables were presented as numbers (%). Differences in normally distributed continuous variables were assessed using independent samples t-test, while non-normally distributed variables were analyzed using the Mann–Whitney U-test. The chi-squared test was employed to compare categorical variables between groups. Univariate and multivariate logistic regression analyses were performed to examine the association between independent factors and incidence rate of significant fibrosis. Odds ratio (OR) and 95% confidence interval (CI) were estimated using logistic regression models. A nomogram was developed based on the logistic regression model to provide estimations of the probability of significant fibrosis. The calibration of the nomogram was graphically assessed using a bootstrap resampling approach with relocations. Diagnostic accuracy was evaluated using the area under the receiver operator characteristic curve (AUC). Additionally, false negative and false positive rates of the single test, as well as sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated. Statistical significance was set at p < 0.05 (two-tailed significance). All statistical analyses were conducted using SPSS, version 23.0 (SPSS, IBM, Corp., Armonk, NY, USA).
Results
Clinical Characteristics
A total of 105 patients diagnosed with NAFLD and who underwent liver biopsy at Changzhou Third People’s Hospital from July 2017 to March 2021, were initially included in this study. After excluding 34 patients with conditions such as autoimmune hepatitis, drug-induced hepatitis, alcoholic fatty liver, and missing data, a final cohort of 71 patients was analyzed. Among these patients, 48 were males and 23 were females with an average age of 45.51±12.31 years. Based on the degree of liver fibrosis, the patients were divided into two groups. The first group comprised 48 patients with stage F0-F1 fibrosis, accounting for 67.61% of the total cohort. The second group consisted of 23 patients with stage F2-F4 fibrosis, representing 32.39% of the total cohort (Figure 1).
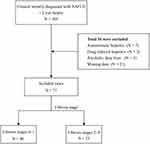 |
Figure 1 Study flowchart. |
Comparison of Non-Invasive Biomarker Levels Between the NAFLD Non-Significant Fibrosis Group and Significant Fibrosis Group
The serum levels of CHI3L1, ferritin, four markers of liver fibrosis (HA, LN, PCIII, and IV-C), LSM, CAP, AST, APRI, and FIB-4 in the NAFLD patients with significant fibrosis compared to those in the NAFLD patients without significant fibrosis (all P < 0.05). However, no significant differences were observed in the other indicators between the two groups (P > 0.05) (Table 1).
 |
Table 1 Clinical Features of Patients |
Univariate and Multivariate Logistic Regression Analysis of Risk Factors for Significant Fibrosis in NAFLD
Univariate Logistic regression analysis was used to analyze sex, age, BMI, PLT, ALT, AST, TBIL, DBIL, IBIL, FPG, TC, TG, CRP, HA, LN, PCIII, IV-C, CHI3L1, ferritin, Fe, CAP and LSM based on whether there was significant liver fibrosis. The results suggest that AST, IV-C, CHI3L1, ferritin, CAP, and LSM could be potentially independent risk factors for significant fibrosis in NAFLD. However, there was no direct correlation with patient gender, age, BMI, PLT, ALT, TBIL, DBIL, IBIL, FPG, TG, TC, CRP, HA, LN, PCIII, and Fe. Furthermore, multivariate logistic regression analysis was performed based on the presence or absence of significant liver fibrosis as well as on AST, IV-C, CHI3L1, ferritin, LSM, and CAP. The results suggest that AST, IV-C, CHI3L1, and LSM may be potentially independent risk factors for the occurrence of significant fibrosis in NAFLD (Table 2).
 |
Table 2 Results of Univariate and Multivariate Analysis for Prediction of Significant Fibrosis |
The Predictive Value of Serum CHI3L1 Level in NAFLD with Significant Fibrosis
The ROC curves were plotted for the evaluation of significant fibrosis prediction using IV-C, CHI3L1, LSM, APRI, and FIB-4 indices in NAFLD patients (Figure 2). The AUC for CHI3L1 in diagnosing significant liver fibrosis was 0.716 (95% CI:0.596,0.836), with a corresponding optimal cut-off value of 125.315. Moreover, IV-C, LSM, APRI and FIB-4 models also exhibited diagnostic potential for predicting significant fibrosis (all P < 0.05). The AUC values (95% CI) for these models were 0.738 (0.626,0.85), 0.737 (0.604,0.87), 0.673 (0.53,0.816), 0.726 (0.599,0.853), respectively. The optimal cut-off values for IV-C, LSM, APRI, and FIB-4 were 25.315, 7.450, 0.745 and 2.225 respectively (Table 3). Although the AUC value of CHI3L1 was slightly lower than that of IV-C, there were no statistically differences in AUC among IV-C, CHI3L1, LSM, and APRI were not statistically significant (P > 0.05) (Table 4).
 |
Table 3 Performance Assessment of Our Developed Nomogram Model and Other Variables (IV-C, CHI3L1, LSM, APRI and FIB-4) for the Prediction of Significant Fibrosis |
 |
Table 4 The Comparation of AUROCs of IV-C, CHI3L1, LSM, APRI, FIB-4, and Nomogram for the Detection of Significant Fibrosis |
The Predictive Value of Nomogram in Significant Fibrosis of NAFLD
The nomogram, incorporating AST, IV-C, CHI3L1, LSM, and four independent risk factors, was developed and presented in Figure 3. The ROC curve of the nomogram for predicting significant fibrosis in NAFLD was plotted, and the AUC was calculated to assess its sensitivity and specificity (Figure 2).
The nomogram demonstrated good diagnostic potential for predicting significant fibrosis in NAFLD, with an AUC (95% CI) of 0.864 (0.766,0.962) and a cut-off value of −0.157 (P < 0.05) (Table 3). Compared to IV-C, CHI3L1, LSM, and APRI, the AUC of the nomogram was higher than that of the above non-invasive fibrosis indicators, and the differences between the nomogram and IV-C, CHI3L1, LSM, and APRI were statistically significant (all P < 0.05). The model was then compared with FIB-4, and although its AUC 0.864 (0.766,0.962) of the nomogram was higher than that of FIB-4 0.726 (0.599,0.853), there were no statistical differences between them (P > 0.05) (Table 4). An ideal model would result in a plot where the actual and predicted probabilities fall along the 45°line. The nomogram calibration curve for predicting the risk of significant fibrosis in NAFLD patients showed relatively good agreement in this cohort (Figure 4).
Discussion
Based on the analysis of non-invasive markers for fibrosis in 71 NAFLD patients confirmed by liver biopsy in our hospital, it was found that: (1) The level of CHI3L1 in the significant liver fibrosis group was higher than that in the non-significant liver fibrosis group. (2) AST, IV-C, CHI3L1, and LSM were identified as potential independent risk factors associated with significant fibrosis. (3) The model constructed by CHI3LI, AST, IV-C, and LSM has a good diagnostic accuracy for significant fibrosis in patients with NAFLD.
The progression of fibrosis in NAFLD occurs gradually over several years, necessitating multiple follow-up visits and repeated assessments. It has been observed that liver fibrosis can develop even in the absence of inflammatory damage in NAFLD. Advanced fibrosis (F3-F4) is the histological feature that predicts the mortality of NAFLD.15 Existing non-invasive scoring systems such as FIB-4, APRI, and NFS, are reliable in predicting advanced liver fibrosis (F3-F4), but their accuracy in distinguishing between F0-F1 fibrosis and ≥F2 fibrosis remains controversial.16,17 Therefore, there is an ongoing search for new biomarkers in clinical practice to facilitate timely treatment and delay disease progression.18 CHI3L1, also known as YKL-40, is a glycoprotein that binds to chitin and is involved in tissue remodeling. Studies have shown that serum CHI3L1 levels are associated with liver fibrosis caused by various factors including hepatitis B virus, hepatitis C virus, and NAFLD.19–21 CHI3L1 has been found to be upregulated in liver fibrosis resulting from hepatic lipid accumulation and viral infections.22 Additionally, CHI3L1 has been shown to predict rapid fibrosis progression after liver transplantation.23 In this study, the serum level of CHI3L1 were found to be higher in NAFLD patients with significantly fibrosis compared to those with non-significant fibrosis. Consistent with the findings Kumagai et al.10 Logistic regression analysis indicated that CHI3L1 may serve as a potential independent risk factor for significant fibrosis in NAFLD. CHI3L1 exhibited good performance with an AUC of 0.716 (0.596–0.836), and the optimal cut-off value was determined to be 125.315 ng/mL. Although the AUC of CHI3L1 was lower than that of IV-C (0.738, 0.626–0.85) and LSM (0.737, 0.604–0.87), the difference was not statistically significant. Therefore, serum CHI3L1, along with the established fibrosis markers IV-C, LSM, FIB-4, or APRI, demonstrated similar diagnostic accuracy for significant fibrosis. Overall, these findings suggest that CHI3L1 can be a potential valuable biomarker for predicting significant fibrosis in NAFLD, exhibiting comparable diagnostic accuracy to recognized fibrosis markers such as IV-C, LSM, FIB-4, or APRI.
Higashiyama et al proposed that CHI3L1 exacerbates the progression of liver fibrosis by inhibiting apoptosis in hepatic macrophages.24 CHI3L1 is primarily derived from hepatic macrophages and hepatic stellate cells in the liver. Acting as a growth factor for fibroblasts and hepatic stellate cells, CHI3L1 promotes the activation and proliferation, and differentiation of stellate cells. This leads to increased expression of smooth muscle actin and cytoglobin, as well as the secretion of extracellular matrix proteins, including collagen fibers type I and type III, ultimately contributing to the development of liver fibrosis. In this context, He et al25 demonstrated that CHI3L1 binds to interleukin-13 receptor α2 (IL-13Rα2), activates macrophage mitogen-activated protein kinase (MAPK), protein kinase B/AKT, and Wnt/β-catenin signaling, and regulates TGF-β1 production through an IL-13Rα2-dependent mechanism. CHI3L1 participates in the process of liver fibrosis by participating in the inflammatory response, cell proliferation, and differentiation, and promoting extracellular matrix remodeling.26 However, the study of Lebensztejn et al suggested that CHI3L1could not be used as a useful prediction of liver fibrosis.27 This may be because their subjects are children. We further included CHI3L1 and other independent risk factors for the occurrence of significant liver fibrosis, AST, IV-C, and LSM, into a nomogram. The nomogram yielded an AUC of 0.864 (0.766,0.962), with a best critical point was −0.157. It demonstrated a sensitivity of 0.696%, and a specificity of 0.917% for predicting significant fibrosis in NAFLD. Compared to using IV-C, CHI3L1and LSM alone, the nomogram exhibited higher predictive value. Although the AUC of the nomogram (0.864, 0.766–0.962) was higher than that of FIB-4 (0.726, 0.599–0.853), there were no statistical differences between the two in terms of predictive accuracy. Therefore, the predictive value of the nomogram and FIB-4 in NAFLD patients with significant fibrosis was similar. Building upon previous studies, this research further elucidated the characteristics of CHI3L1 in liver fibrosis. We propose that combining CHI3L1 with other non-invasive markers can enhance the predictive value for significant fibrosis in NAFLD. Additionally, the results obtained from CHI3L1 measurements reflect the speed of liver fibrosis development. We observed higher average levels of CHI3L1 (105.34 ± 45.12) in the F0-F1 phase compared to some previous studies. This discrepancy could be attributed to patient concerns regarding liver biopsy. It is possible that the selected study participants, even though they underwent liver biopsy in the F0-F1 phase, had a longer disease duration with repeated episodes of liver dysfunction or prolonged disease course. These factors might have contributed to the relatively elevated average CHI3L1 levels in the F0-F1 phase. Even though they are currently in the early stage of fibrosis, or having normal hepatic function, it suggests that their fiber progression rate may still be rapid.
Imaging-based liver stiffness assessment reflects an intrinsic physical property of liver tissue and has been widely utilized for evaluating liver fibrosis in various liver diseases,28 including hepatitis C, chronic hepatitis B and NAFLD.29,30 Vibration-controlled transient elastography (VCTE), such as FibroScan, has emerged as a reliable method for assessing liver fibrosis, particularly for identifying moderate to severe fibrosis. Given the high prevalence of NAFLD, liver biopsy as the gold standard for fibrosis assessment is challenging to implement on a routine basis. However, the use of transient elastography for liver fibrosis evaluation has generated some controversy due to the variability in its results.31,32 Despite this, it remains a valuable tool in predicting significant fibrosis in NAFLD. In our study, LSM demonstrated an AUC of 0.737 (0.604–0.87) for predicting significant fibrosis, with an optimal cut-off point of 7.450 kPa. These findings support the effectiveness of VCTE in distinguishing significant liver fibrosis, although it may not be the most robust diagnostic predictor in our analysis.
Furthermore, a nomogram was utilized to visually represent the logistic regression model, allowing for a more intuitive interpretation of the predictive factors for significant liver fibrosis. Based on the magnitude of the regression coefficient, each influencing factor in the model was assigned a score based on its impact on significant liver fibrosis. These individual scores were then summed to obtain the total score. Subsequently, the relationship between the total score and the probability of significant liver fibrosis was transformed using a mathematical function to calculate the predictive value in NAFLD patients. In our study, after conducting multivariate logistic regression analysis, the non-invasive biomarkers AST, IV-C, CHI3L1, and LSM were selected and incorporated into the nomogram. The model exhibited an AUC (95% CI) of 0.864 (0.766,0.962). And the closer it was to 1.0, the better the consistency between the predicted results and the actual results. Notably, complex fibrosis models like FIB-4 have been reported to be more accurate in detecting fibrosis compared to simple fibrosis models.33 We propose the nomogram combining direct serum markers (CHI3L1, AST, IV-C) and LSM to comprehensively evaluate the extent of liver fibrosis from multiple perspectives. Moreover, it serves as a supplement to existing indirect biomarkers, thus enhancing the diagnostic accuracy of non-invasive markers for NAFLD patients with significant fibrosis.
Serum ferritin is the most abundant iron-containing protein in the body and is present in high amounts in the liver, where it plays an important role in cellular iron metabolism. Serum ferritin is an indicator of the amount of iron in the body, and high levels of ferritin often indicate iron overload. An imbalance in iron homeostasis is also frequently observed in patients with NAFLD. Iron overload can increase the risk of NAFLD, and the oxidative stress, lipid peroxidation and liver iron accumulation resulting from the overload can further cause liver injury and advanced fibrosis, and other poor prognosis.34,35 Our study also confirmed that ferritin level was higher in significant liver fibrosis than in non-significant liver fibrosis. Patients with NAFLD have iron overload when the degree of fibrosis is high. Manousou et al36 showed that serum ferritin concentration and body mass index (BMI) were strongly associated with liver fibrosis, portal vein and lobular inflammation in patients with nonalcoholic fatty liver disease. Serum ferritin and BMI may be potential distinguishing markers for selecting patients for liver biopsy and correlate with inflammation and fibrosis. Another study believe that a low serum ferritin level may be one cost-effective option to exclude patients with advanced fibrosis from liver biopsy and elastography.37 This could be because the production of reactive oxygen species, as mediated by liver iron involvement stimulates the release of free radicals and hydrogen peroxide through the Fenton and Haber-Weiss reaction, promotes oxidative stress, and leads to the attack of cell membrane lipids, proteins and DNA, resulting in liver tissue damage.38 Moreover, the interplay between iron metabolism and oxidative stress may synergistically promote the “second shock” of NAFLD, leading to steatohepatitis and liver fibrosis.39 However, when further logistics regression analysis was performed to explore the relationship between ferritin and liver fibrosis, no correlation was found. In a similar way to the results of this study, Modares et al did not observe a significant correlation between ferritin levels and the degree of fibrosis in a prospective cross-sectional study of patients with confirmed NAFLD/NASH.40 In the future, the scope of the study needs to be expanded, and other members of the iron metabolism family, such as hepcidin and transferrin saturation, need to be further analyzed.
In this study, the NAFLD fibrosis diagnostic model incorporating CHI3L1 demonstrated a significant improvement in the diagnostic accuracy of non-invasive markers for significant liver fibrosis, particularly when combined with AST, IV-C, and LSM. The nomogram provided a more comprehensive reflection of fibrosis severity, potentially due to the inclusion of both direct serum markers and VCTE, which offer multiple perspectives on fibrosis status compared to models based solely on direct serum markers. However, the study did not assess CHI3L1 expression at the protein level in different stages of liver fibrosis. Additionally, the limited sample size and single-center nature of this study were inherent limitations. Therefore, further multicenter studies with larger sample sizes are planned to validate the proposed model.
Conclusion
Serum CHI3L1 demonstrates a potential association with significant liver fibrosis in NAFLD patients. The diagnostic model constructed by CHI3L1 combined with the existing non-invasive markers AST, IV-C, and LSM can help clinicians assess the risk of significant liver fibrosis in NAFLD. There is potential for expanded screening in the population and long-term follow-up evaluation.
Data Sharing Statement
The data used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of Changzhou Third People’s Hospital (decision No. 2020002 of 23 April 2020).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Acknowledgments
The authors thank all the study participants for their assistance.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research was funded by the Science and Technology Project of Changzhou (CJ20200059) and the Major Projects of Changzhou Health Commission (ZD202119).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Golabi P, Paik JM, AlQahtani S, Younossi Y, Tuncer G, Younossi ZM. Burden of non-alcoholic fatty liver disease in Asia, the Middle East and North Africa: data from global burden of disease 2009–2019. J Hepatol. 2021;75(4):795–809. doi:10.1016/j.jhep.2021.05.022
2. Gutiérrez-Cuevas J, Santos A, Armendariz-Borunda J. Pathophysiological molecular mechanisms of obesity: a link between MAFLD and NASH with cardiovascular diseases. Int J Mol Sci. 2021;22(21):11629. doi:10.3390/ijms222111629
3. Orci LA, Sanduzzi-Zamparelli M, Caballol B, et al. Incidence of hepatocellular carcinoma in patients with nonalcoholic fatty liver disease: a systematic review, meta-analysis, and meta-regression. Clin Gastroenterol Hepatol. 2022;20(2):283–292 e10. doi:10.1016/j.cgh.2021.05.002
4. Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the study of liver diseases. Hepatology. 2018;67(1):328–357. doi:10.1002/hep.29367
5. Nogami A, Yoneda M, Iwaki M, et al. Diagnostic comparison of vibration-controlled transient elastography and MRI techniques in overweight and obese patients with NAFLD. Sci Rep. 2022;12(1):21925. doi:10.1038/s41598-022-25843-6
6. Tao H, Yang JJ, Shi KH, et al. The significance of YKL-40 protein in liver fibrosis. Inflammat Res. 2014;63(4):249–254. doi:10.1007/s00011-013-0698-9
7. Jin X, Fu B, Wu ZJ, et al. Serum chitinase-3-like protein 1 is a biomarker of liver fibrosis in patients with chronic hepatitis B in China. Hepatobil Pancr Dis Int. 2020;19(4):384–389. doi:10.1016/j.hbpd.2020.05.009
8. Jiang Z, Wang S, Jin J, et al. The clinical significance of serum chitinase 3-like 1 in hepatitis B-related chronic liver diseases. J Clin Lab Anal. 2020;34(5):e23200. doi:10.1002/jcla.23200
9. Kang Q, Chen J, Luo H, et al. Decrease in chitinase 3-like protein 1 levels reflects improvement in liver fibrosis after HCV eradication. Dis Markers. 2020;2020:8539804. doi:10.1155/2020/8539804
10. Kumagai E, Mano Y, Yoshio S, et al. Serum YKL-40 as a marker of liver fibrosis in patients with non-alcoholic fatty liver disease. Sci Rep. 2016;6(1):35282. doi:10.1038/srep35282
11. Li H, Yan T, Zhu Z, et al. [Diagnostic value of serum chitinase 3-like protein 1 in chronic liver disease of significant fibrosis and cirrhosis]. Chin J Hepatol. 2018;26(5):337–341. Chinese. doi:10.3760/cma.j.issn.1007-3418.2018.05.005
12. Fargion S, Valenti L, Fracanzani AL. Beyond hereditary hemochromatosis: new insights into the relationship between iron overload and chronic liver diseases. Digest Liver Dis. 2011;43(2):89–95. doi:10.1016/j.dld.2010.07.006
13. Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41(6):1313–1321. doi:10.1002/hep.20701
14. Stal P. Liver fibrosis in non-alcoholic fatty liver disease - diagnostic challenge with prognostic significance. World J Gastroenterol. 2015;21(39):11077–11087. doi:10.3748/wjg.v21.i39.11077
15. Kugelmas M, Noureddin M, Gunn N, et al. The use of current knowledge and non-invasive testing modalities for predicting at-risk nonalcoholic steatohepatitis and assessing fibrosis. Liver Int. 2023;43(5):964–974. doi:10.1111/liv.15555
16. Cao Y, Guo S, Dong Y, Liu C, Zhu W. Comparison of liver fibrosis scores for predicting mortality and morbidity in heart failure with preserved ejection fraction. ESC Heart Fail. 2023;10(3):1771–1780. doi:10.1002/ehf2.14336
17. Salomone F, Micek A, Godos J. Simple scores of fibrosis and mortality in patients with NAFLD: a systematic review with meta-analysis. J Clin Med. 2018;7(8):219. doi:10.3390/jcm7080219
18. Xia F, Huang Z, Zhang Q, et al. Clinically significant portal hypertension (CSPH) on early-stage HCC following hepatectomy: what’s the impact? Eur J Surg Oncol. 2023;49(4):771–779. doi:10.1016/j.ejso.2022.11.005
19. Nishimura N, De Battista D, McGivern DR, et al. Chitinase 3-like 1 is a profibrogenic factor overexpressed in the aging liver and in patients with liver cirrhosis. Proc Natl Acad Sci U S A. 2021;118(17). doi:10.1073/pnas.2019633118
20. Zhang S, Sousa A, Lin M, et al. Role of chitinase 3-like 1 protein in the pathogenesis of hepatic insulin resistance in nonalcoholic fatty liver disease. Cells. 2021;10(2). doi:10.3390/cells10020201
21. Fontana RJ, Litman HJ, Dienstag JL, et al. YKL-40 genetic polymorphisms and the risk of liver disease progression in patients with advanced fibrosis due to chronic hepatitis C. Liver Int. 2012;32(4):665–674. doi:10.1111/j.1478-3231.2011.02686.x
22. Zhao T, Su Z, Li Y, Zhang X, You Q. Chitinase-3 like-protein-1 function and its role in diseases. Sig Transd Target Therapy. 2020;5(1):201. doi:10.1038/s41392-020-00303-7
23. Pungpapong S, Nunes DP, Krishna M, et al. Serum fibrosis markers can predict rapid fibrosis progression after liver transplantation for hepatitis C. Liver Transplant. 2008;14(9):1294–1302. doi:10.1002/lt.21508
24. Higashiyama M, Tomita K, Sugihara N, et al. Chitinase 3-like 1 deficiency ameliorates liver fibrosis by promoting hepatic macrophage apoptosis. Hepatol Res. 2019;49(11):1316–1328. doi:10.1111/hepr.13396
25. He CH, Lee CG, Ma B, Kamle S, Choi AMK, Elias JA. N-glycosylation regulates chitinase 3-like-1 and IL-13 ligand binding to IL-13 receptor α2. Am J Respir Cell Mol Biol. 2020;63(3):386–395. doi:10.1165/rcmb.2019-0446OC
26. He CH, Lee CG, Dela Cruz CS, et al. Chitinase 3-like 1 regulates cellular and tissue responses via IL-13 receptor alpha2. Cell Rep. 2013;4(4):830–841. doi:10.1016/j.celrep.2013.07.032
27. Lebensztejn DM, Wierzbicka A, Socha P, et al. Cytokeratin-18 and hyaluronic acid levels predict liver fibrosis in children with non-alcoholic fatty liver disease. Acta biochimica Polonica. 2011;58(4):563–566. doi:10.18388/abp.2011_2225
28. European Association for Study of L, Asociacion Latinoamericana para el Estudio del H. EASL-ALEH clinical practice guidelines: non-invasive tests for evaluation of liver disease severity and prognosis. J Hepatol. 2015;63(1):237–264. doi:10.1016/j.jhep.2015.04.006
29. Johannessen A, Stockdale AJ, Henrion MYR, et al. Systematic review and individual-patient-data meta-analysis of non-invasive fibrosis markers for chronic hepatitis B in Africa. Nat Commun. 2023;14(1):45. doi:10.1038/s41467-022-35729-w
30. Cazac GD, Lacatusu CM, Mihai C, Grigorescu ED, Onofriescu A, Mihai BM. Ultrasound-based hepatic elastography in non-alcoholic fatty liver disease: focus on patients with type 2 diabetes. Biomedicines. 2022;10(10):2375. doi:10.3390/biomedicines10102375
31. Ferraioli G, Wong VW, Castera L, et al. Liver ultrasound elastography: an update to the world federation for ultrasound in medicine and biology guidelines and recommendations. Ultrasound Med Biol. 2018;44(12):2419–2440. doi:10.1016/j.ultrasmedbio.2018.07.008
32. Selvaraj EA, Mózes FE, Jayaswal ANA, et al. Diagnostic accuracy of elastography and magnetic resonance imaging in patients with NAFLD: a systematic review and meta-analysis. J Hepatol. 2021;75(4):770–785. doi:10.1016/j.jhep.2021.04.044
33. Adams LA, George J, Bugianesi E, et al. Complex non-invasive fibrosis models are more accurate than simple models in non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2011;26(10):1536–1543. doi:10.1111/j.1440-1746.2011.06774.x
34. Jézéquel C, Lainé F, Laviolle B, Kiani A, Bardou-Jacquet E, Deugnier Y. Both hepatic and body iron stores are increased in dysmetabolic iron overload syndrome. A case-control study. PLoS One. 2015;10(6):e0128530. doi:10.1371/journal.pone.0128530
35. Sumida Y, Nakashima T, Yoh T, et al. Serum thioredoxin levels as a predictor of steatohepatitis in patients with nonalcoholic fatty liver disease. J Hepatol. 2003;38(1):32–38. doi:10.1016/s0168-8278(02)00331-8
36. Manousou P, Kalambokis G, Grillo F, et al. Serum ferritin is a discriminant marker for both fibrosis and inflammation in histologically proven non-alcoholic fatty liver disease patients. Liver Int. 2011;31(5):730–739. doi:10.1111/j.1478-3231.2011.02488.x
37. Seyedian SS, Hajiani E, Hashemi SJ, et al. Relationship between serum ferritin level and transient elastography findings among patients with nonalcoholic fatty liver disease. J Fam Med Primary Care. 2017;6(4):750–754. doi:10.4103/jfmpc.jfmpc_158_17
38. Bessone F, Razori MV, Roma MG. Molecular pathways of nonalcoholic fatty liver disease development and progression. Cell Mol Life Sci. 2019;76(1):99–128. doi:10.1007/s00018-018-2947-0
39. Corradini E, Buzzetti E, Dongiovanni P, et al. Ceruloplasmin gene variants are associated with hyperferritinemia and increased liver iron in patients with NAFLD. J Hepatol. 2021;75(3):506–513. doi:10.1016/j.jhep.2021.03.014
40. Modares Mousavi SR, Geramizadeh B, Anushiravani A, Ejtehadi F, Anbardar MH, Moini M. Correlation between serum ferritin level and histopathological disease severity in non-alcoholic fatty liver disease. Middle East J Digest Dis. 2018;10(2):90–95. doi:10.15171/mejdd.2018.96
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.