Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 17
Association of MST1 in the First Trimester of Pregnancy with Gestational Diabetes Mellitus and Adverse Pregnancy Outcomes
Authors Gao BB, Zhang QQ , Dong SQ, Gao F, Liu X, Wei J, Lu Y
Received 21 September 2023
Accepted for publication 12 December 2023
Published 3 January 2024 Volume 2024:17 Pages 19—29
DOI https://doi.org/10.2147/DMSO.S436178
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Antonio Brunetti
Bai-Bing Gao,1,2,* Qing-qing Zhang,1,* Shu-qin Dong,1,2 Fei Gao,1,2 Xia Liu,3 Jing Wei,3 Yu Lu1
1Department of Endocrinology, The Affiliated Taizhou People’s Hospital of Nanjing Medical University, Taizhou School of Clinical Medicine, Nanjing Medical University, Taizhou, 225300, People’s Republic of China; 2Dalian Medical University, Dalian, Liaoning, 116044, People’s Republic of China; 3Department of Obstetrics and Gynecology, The Affiliated Taizhou People’s Hospital of Nanjing Medical University, Taizhou School of Clinical Medicine, Nanjing Medical University, Taizhou, 225300, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yu Lu; Jing Wei, Email [email protected]; [email protected]
Aim: We investigate the association of mammalian sterile line 20-like kinase 1 (MST1) in the first trimester with the risks of gestational diabetes mellitus (GDM) and adverse pregnancy outcomes.
Methods: Pregnancies were recruited during their first antenatal care visit between 8 and 12 gestational weeks. These pregnancies underwent an oral glucose tolerance test between 24 and 28 gestational weeks and were followed up until delivery. Serum MST1 levels at 8– 12 gestational weeks and 24– 28 gestational weeks were measured using an enzyme-linked immunosorbent assay (ELISA) kit. Logistic regression models were used to evaluate the association between MST1 levels in the first trimester and the risks of GDM and adverse pregnancy outcomes.
Results: This cohort study enrolled a total of 231 pregnancies. GDM was present in 42 (18.18%) women. Compared to the normal glucose tolerance (NGT) group, the GDM group had higher levels of FPG, HOMA-IR, and MST1 both in the first and second trimesters, but had lower HOMA-β levels only in the second trimester. Then participants were classified according to the median MST1 value in the first trimester. Incidences of GDM, composite adverse pregnancy outcomes, preterm birth, and macrosomia increased in women with higher MST1 values. Serum MST1 in the first trimester was correlated with FPG, 1hr PG, 2hr PG, and HOMA-IR, while inversely correlated with HOMA-β in the second trimester. Furthermore, after adjusting for traditional risk factors, women with higher first-trimester MST1 values had greater odds of GDM, composite adverse pregnancy outcomes, preterm birth, and macrosomia (aOR 2.276, P=0.030; aOR 2.690, P=0.003; aOR 3.210, P=0.048; aOR 5.488, P=0.010).
Conclusion: Elevated levels of MST1 in the first trimester of pregnancies are associated with increased risks of GDM and adverse pregnancy outcomes.
Keywords: mammalian sterile line 20-like kinase 1, gestational diabetes mellitus, adverse pregnancy outcome, blood glucose
Background
Gestational diabetes mellitus (GDM) is a common complication during pregnancy characterized by glucose metabolic disorder. The prevalence of GDM varies across different regions, with rates ranging from 6.6% in Japan and Nepal to 45.3% in the United Arab Emirate.1,2 The prevalence of GDM is increasing globally. Several risk factors have been identified, including impaired glucose tolerance, family history of GDM or type 2 diabetes, advanced maternal age, obesity, and high parity.3 The exact etiology and pathophysiology of GDM are still not fully understood, but insulin resistance and impaired insulin secretion are believed to play a major role. Other factors such as inflammation, adipokines, oxidative stress, and placenta factors are also involved in the development of GDM.4
GDM occurring in early gestation can lead to adverse pregnancy outcomes, including premature delivery, pre-eclampsia, macrosomia, restricted fetal growth, and fetal distress5 It also increases the risk of metabolic syndrome, obesity, and type 2 diabetes for both the mother and the offspring6 High maternal glucose levels have been found to be significantly correlated with adverse pregnancy outcomes.7 Women diagnosed with GDM are at a 10-fold higher risk of developing type 2 diabetes after delivery compared to those with normal blood glucose levels.8 A recent study also showed that women with a history of GDM have a 63% increased risk of cardiovascular diseases. Even if blood glucose levels return to normal after delivery, the cardiovascular risk for these women remains elevated.9 GDM is also associated with higher risks of postpartum malignancy, ophthalmic, and renal diseases.10 Offspring of GDM women are more likely to develop obesity, metabolic, cardiovascular, and neuropsychiatric disorders.11 A French cohort study reported a 30% higher risk of cardiac malformations in the offspring of GDM patients.12 However, GDM is usually diagnosed in the late second trimester, highlighting the need to identify potential risk factors for glucose metabolic disorder in early pregnancy to enable early prevention of GDM and adverse pregnancy outcomes.
Mammalian sterile line 20-like kinase 1 (MST1) is a serine-threonine kinase that is widely expressed in mammals. It plays a crucial role in Hippo signaling pathway and is involved in various cellular processes, including proliferation, apoptosis, oxidative stress, and glycolipid metabolism regulation.13 Recent research has shown that MST1 can be activated by multiple diabetogenic conditions. Both in vitro and in vivo studies have demonstrated that overexpression of MST1 leads to a decrease in the survival of islet beta cells and a complete loss of glucose-stimulated insulin secretion.14 However, potent inhibitors of MST1 have been discovered to effectively prevent apoptosis in human islets and restore beta cell mass in animal models of diabetes.15
Based on these findings, it is possible that MST1 may also play a role in the development of glucose metabolic disorders during pregnancy. This study aims to investigate the involvement of MST1 in gestational glucose disorders and its potential impact on adverse pregnancy outcomes. By identifying and understanding the early signs of glucose disorders in pregnancy, this research could contribute to the early detection and prevention of such conditions, ultimately improving maternal and fetal health.
Materials and Methods
Study Design and Participants
This study was conducted at Taizhou People’s Hospital Preconception Cohort with the aim of investigating the risk factors associated with GDM and adverse pregnancy outcomes. The study enrolled a cohort of pregnancies from October 2019 to March 2021, comprising a total of 272 participants. These participants were recruited during their first antenatal care visit between 8 and 12 gestational weeks. They were followed longitudinally from their first antenatal care visit until the time of the oral glucose tolerance test (OGTT) at 24–28 gestational weeks, as well as through the postpartum period. Out of the initial 272 subjects, 27 were excluded due to a lack of OGTT and 14 were excluded due to a lack of pregnancy outcome data. Ultimately, 231 pregnancies were included in this study, of which 42 developed GDM (GDM group), while the remaining 189 participants had normal glucose tolerance (NGT group). A flow chart depicting the cohort study is presented in Figure 1.
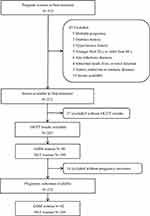 |
Figure 1 Flowchart of the study. |
Diagnosis and Intervention of GDM
Between 24 and 28 gestational weeks, all participants underwent a 75-g OGTT in the morning after fasting for at least 8 hr. The diagnosis of GDM was made according to the World Health Organization 2013 criteria.16 All patients diagnosed with GDM did not receive any hypoglycemic drugs and only received diet and exercise control.
Adverse Pregnancy Outcomes
Adverse pregnancy outcomes in this study were defined as pathological pregnancy and abnormal pregnancy. The composite adverse pregnancy outcomes included pre-eclampsia, preterm birth, macrosomia, and postpartum hemorrhage. Pre-eclampsia was defined as blood pressure ≥140mmHg and/or diastolic blood pressure ≥90mmHg after 20 weeks of gestation. Preterm birth was considered as delivery occurring between 24 and 37 weeks of gestation. Macrosomia is defined as a newborn weighing more than 4000g. A postpartum hemorrhage was defined as excessive bleeding exceeding 500mL within 24 hr after delivery. If a woman experienced multiple adverse pregnancy outcomes, it was considered as one composite adverse pregnancy outcome.
Data Collection Procedures
During the initial antenatal care visit to the hospital, which typically occur between 8 and 12 weeks of gestation, a standardized procedure was followed. This procedure involved administering a questionnaire to gather information on various factors such as height, pre-pregnancy weight, smoking and alcohol habits, parity, family history of diabetes, and impaired glucose tolerance with or without pregnancy. To determine the family history of diabetes, participants were asked if any of their first-degree relatives (parents, siblings, or children) had diabetes. Smoking habit was defined as self-reported smoking during pregnancy, while drinking habit was defined as the consumption of any alcoholic beverage 3 months before or during pregnancy. In addition to these questions, the participants’ preconception BMI (Body Mass Index) was calculated. This was done by dividing their pre-pregnancy weight in kilograms by the square of their height in meters. Overall, these standardized procedures were used to collect important information about the participants’ demographics, lifestyle factors, and medical history, which will be used for further analysis in the study.
Blood Sample Collection and Measurement of Serum MST1
Blood samples were collected in the morning after an overnight fast and analyzed in the hospital's central laboratory. Plasma glucose (PG) was assessed by the glucose oxidase method (Beckman Coulter Co., Ltd., USA). Insulin was detected by chemiluminescence assays (ARCHITECT Insulin Reagent Kit, Abbott Laboratories, USA). The homeostatic model was used to assess the insulin resistance HOMA-IR=FIns×FPG/22.5, and insulin beta cell function HOMA-β=20×Fins/(FPG-3.5). The remaining sera were stored at −80°C in the laboratory for long-term preservation. Serum MST1 levels were measured using an enzyme-linked immunosorbent assay (ELISA) kit (Shanghai Zhenke Biology Co., Ltd., China). The procedure involved adding specimens, standards, and HRP-labeled antibodies to a microtiter plate coated with MST1 capture antibodies. The plate was then incubated and thoroughly washed. The substrate TMB was added, which reacted with peroxidase to produce a blue color. The addition of acid converted the blue color to yellow, and the concentration of MST1 was determined by measuring the absorbance (OD) at a wavelength of 450 nm. The concentration of the sample was calculated based on this measurement. The ELISA kit used for MST1 measurement has an intra-assay CV(%) of less than 10% and an inter-assay CV(%) of less than 15%. The linear range of the assay is 0.625–20 ng/mL. Prior to the measurement, all reagents were stored at temperatures between 2°C and 8°C to maintain their stability. All ELISA experiments were performed twice, and the mean value was used for analysis.
Statistical Analysis
For variables that are normally distributed, means and standard deviations are calculated. For variables that are not normally distributed, medians and interquartile ranges are used. Percentages were calculated for categorical variables. The Kolmogorov–Smirnov method was used to test for normality. Student’s t-tests and Mann–Whitney test were used for continuous variables, while Chi-square tests for categorical variables between two groups. Correlation analysis was used to investigate the connection between MST1 and glucose metabolic variables. Logistic regression analysis was performed to examine the associations of MST1 with GDM and adverse pregnancy outcomes. SPSS 26.0 software (IBM SPSS Inc, Chicago, IL, USA) was used for all data analysis. Additionally, a restricted cubic spline (RCS) regression model with three knots was used to explore potential nonlinear relationships between continuous MST1 levels and the risks of GDM and adverse pregnancy outcomes. Receiver operating characteristic (ROC) curves were used to compare the predictability of MST1 for GDM. A significance level of P < 0.05 (two-tailed) was considered statistically significant.
Results
Characteristics of Women in GDM and NGT Groups
In total, 231 pregnancies were included in the study, and 42 (18.18%) of them developed GDM; thus, the remaining 189 participants were considered the NGT reference group (81.82%). The demographic characteristics of the study population in GDM and NGT groups are summarized in Table 1. Women with GDM had greater age than women with NGT. No significant differences in preconception BMI, family history of diabetes, smoking exposure, alcohol consumption, previous GDM, and parity were observed between the two groups. The levels of fasting plasma glucose (FPG), HOMA-IR, and MST1 started to increase in the GDM group between 8 and 12 gestational weeks. Similarly, the women with GDM also had higher levels of FPG, 1hr PG, 2hr PG, HOMA-IR, and MST1 between 24 and 28 gestational weeks. Interestingly, the HOMA-β index was equal between groups in the first trimester, but only presented to be lower in GDM group in the second trimester (Table 1).
 |
Table 1 Characteristics of Participants in GDM and NGT Group |
Furthermore, from the first trimester to the second trimester, HOMA-IR increased in both GDM and NGT groups. However, HOMA-β increased and FPG decreased only in NGT group, while MST1 increased only in GDM group (Supplementary Table 1).
Incidence of GDM and Adverse Pregnancy Outcomes in Low and High MST1 Groups
The participants were divided into two groups based on their median level of MST1 (6.77 pg/mL) in the first trimester: the low MST1 group (below 6.77 pg/mL) and the high MST1 group (equal to or above 6.77 pg/mL). Compared to the low MST1 group, women in the high MST1 group had higher incidence of GDM (12.17% vs 24.13%, P=0.018), composite adverse pregnancy outcomes (15.65% vs 29.31%, P=0.003), preterm birth (3.48% vs 12.07%, P=0.029), and macrosomia (2.61% vs 12.07%, P=0.012) (Table 2).
 |
Table 2 Incidence of GDM and Adverse Pregnancy Outcomes Between Groups Categorized by MST1 |
Correlations of MST1 in First Trimester with Glucose Metabolic Factors in Second Trimester
MST1 in the first trimester was correlated with FPG, HOMA-IR, 1hr PG, and 2hr PG levels in the second trimester, with the co-efficient r values 0.393, 0.217, 0.130, and 0.143, respectively (P<0.05). On the contrary, MST1 in the first trimester was inversely correlated with HOMA-β in the second trimester (r = −0.233; P=0.000).
Association Between MST1 in the First Trimester and the Risk of GDM
After adjusting for traditional risk factors such as age, preconception BMI, family history of diabetes, smoking and alcohol habits, previous GDM, and parity, logistic regression analysis showed that higher MST1 levels in the first trimester were associated with an increased risk of GDM (adjusted odds ratio [aOR] 1.341 [95% CI 1.075–1.672]; P=0.009). Pregnant women with MST1 levels ≥6.77 pg/mL had approximately 2.3-fold higher odds of GDM compared to those with MST1 levels <6.77 pg/mL (aOR 2.276 [95% CI 1.084–4.870]; P=0.030) (Table 3).
 |
Table 3 Associations of MST1 with GDM |
We also investigated the nonlinear relationship among MST1 with GDM. The RCS regression model revealed that higher MST1 levels in the first trimester were associated with an increased risk of GDM in a nonlinear fashion (Figure 2a). Furthermore, combining MST1 with traditional risk factors improved the predictive ability for GDM compared to using MST1 or traditional factors alone (area under the receiver operating characteristic curve [AUC]: 0.710, 0.635, and 0.677, respectively) (Supplementary Figure 1).
 |
Figure 2 (a) RCS regression analysis of MST1 and the risk of GDM. (b) RCS regression analysis of MST1 and the risk of adverse pregnancy outcomes. |
Association Between MST1 in the First Trimester and the Risks of Adverse Pregnancy Outcomes
Similarly, after adjusting for traditional risk factors and GDM, pregnancies with higher MST1 levels in the first trimester had approximately 2.7-fold higher odds of composite adverse pregnancy outcomes (aOR 2.690 [95% CI 1.386–5.221]; P=0.003). The subgroup analysis also showed that participants with higher MST1 levels had higher odds of preterm birth and macrosomia (aOR=3.210 [95% CI 1.073–10.404], P=0.048; aOR=5.488 [95% CI 1.498–20.103], P=0.010) (Table 4). Furthermore, the RCS regression model revealed that higher MST1 levels in the first trimester were associated with an increased risk of composite adverse pregnancy outcomes in a nonlinear fashion (Figure 2b).
 |
Table 4 Associations of MST1 with Adverse Pregnancy Outcomes |
Discussion
GDM is a condition characterized by carbohydrate intolerance that is first recognized during pregnancy. It affects approximately 10–15% of all pregnant women and shares similarities with type 2 diabetes, primarily involving insulin resistance and dysfunction of islet beta cells. As pregnancy progresses, insulin sensitivity decreases, leading to a 2–2.5 times increase in insulin secretion to maintain normal blood glucose levels. However, prolonged excessive insulin secretion gradually impairs the function of islet beta cells. When compensatory insulin secretion is insufficient to overcome insulin resistance, blood glucose levels rise, resulting in GDM.17 Once diagnosed with GDM, both the mother and fetus are exposed to various risks, including polyhydramnios, pre-eclampsia, macrosomia, preterm birth, and respiratory distress due to dysglycemia.18 Current recommendations suggest an OGTT between 24 and 28 weeks of gestation to identify GDM. However, if we can detect GDM earlier, we can better alleviate the adverse effects of high glucose on both the mother and the offspring. Therefore, it is crucial to identify and prevent GDM in the early stages of pregnancy to potentially improve pregnancy outcomes, reduce maternal and infant complications, and lower the risks of metabolic disorders and cardiovascular diseases in the postpartum period.19,20
MST1, a ubiquitously expressed serine/threonine kinase, as well as a key component of the Hippo signaling pathway, is extensively expressed in various human tissues and plays a crucial role in essential biological processes, including cell apoptosis, proliferation, morphogenesis, differentiation, adhesion, and migration. The mammalian Hippo pathway has emerged as a complex signal network through a series of kinase cascades. MST1 interacts with the regulatory protein Salvador (Sav1) and phosphorylates and activates large tumour suppressors 1 and 2 (LATS1/2). Then, activated LATS1/2 phosphorylates the downstream effector Yes-associated protein (YAP), leading to its cytoplasmic sequestration and ubiquitin-dependent degradation by binding 14-3-3 proteins. Thereby, YAP transcriptional activity is prevented, resulting in resistance to proliferation and increase in apoptosis in multiple tissues. In addition, MST1 promotes cell death through regulation of multiple downstream targets such as histone H2B, FOXO family members, as well as stress kinase c-Jun-N-terminal kinase (JNK) and caspase-3 activation.21,22 MST1 was initially identified as a target for tumor suppression. Previous research discovered that in patients with colorectal cancer, the serum MST1 concentration detected using ELISA was a potential biomarker for early detection, prognosis, and prediction of distant metastasis of this disease.23 Besides cancer, MST1 is also involved in the regulation of cardiovascular disease. MST1 up-regulation promoted the apoptosis of vascular smooth muscle cells (VSMC) and the occurrence of aortic dissection, while down-regulation of it decreased VSMC apoptosis and aortic dissection formation.24 In a retrospective cohort study involved 160 patients with acute type A aortic dissection (ATAAD), the greater circulating MST1 detected using ELISA was correlated with the 30-day mortality in these patients who underwent emergency surgery.25
Recent studies have shown that MST1 also plays a crucial role in insulin secretion and beta cell dysfunction, making it a potential target for the treatment of glucose metabolism disorders.26 Ardestani et al found that MST1 was activated in the islets of individuals with type 2 diabetes, obese diabetic mice, and hyperglycemic mice fed with a high-fat, high-sucrose diet. Immunostaining for pMST1 was significantly increased in pancreatic islets of poorly controlled type 2 diabetes patients and db/db mice, while there was minimal expression in cells from individuals without diabetes or control mice.27 Overexpression of MST1 alone was sufficient to induce apoptosis in both human and mouse beta cells. This overexpression also led to a marked reduction in PDX1, a key regulator of beta cell survival, resulting in a complete loss of glucose-stimulated insulin secretion.14 Additionally, MST1 overexpression abolished the enhancement of glucose-induced insulin secretion by GLP-1, while the GLP-1 analogue liraglutide was able to restore PDX1 expression by inactivating MST1.28 On the contrary, MST1 deficiency attenuated the reduction in PDX1 caused by high glucose treatment, suggesting that MST1 is necessary for the decrease in PDX1 levels in a diabetic environment.15 Silencing the MST1 gene improved beta cell function under diabetogenic conditions. In MST1-deficient mice, the survival of beta cells and their ability to secrete insulin in response to glucose stimulation were significantly improved, particularly in high glucose environments. Furthermore, potent inhibitors of MST1 showed efficacy in preventing apoptosis in human islets and restoring beta cell mass in animal models of diabetes.14,29 We reported that the therapy of neratinib (a potent triple kinase inhibitor of HER2/EGFR and MST1) effectively restored normoglycemia in a patient with breast cancer and type 2 diabetes and suggested neratinib as a potent therapeutic regimen for cancer-diabetes comorbidity.30 Given the role of MST1 in beta cell function, it is likely to play a crucial role in the pathological process of glucose metabolism disorders during pregnancy. However, there have been no reports on the association of MST1 with diabetes in human. This prospective cohort study aims to investigate the association between MST1 levels and the risks of GDM and adverse pregnancy outcomes in pregnancies. The results showed that elevated serum MST1 levels were associated with increased risks of GDM and adverse pregnancy outcomes, suggesting that MST1 may be involved in the development and consequences of glucose metabolism disorders during pregnancy.
The development of GDM is believed to be caused by dysfunction of beta cells due to increased insulin resistance and hyperinsulinemia, as well as placental hormones, neurohormonal dysfunction, chronic inflammation, adipokines, and oxidative stress.31 Our results showed that insulin resistance naturally increased both in women with or without GDM during pregnancy. However, women without GDM were able to maintain normal blood glucose levels throughout pregnancy due to adequate compensation of insulin secretion by islet beta cells. However, women with GDM had higher insulin resistance but inadequate compensation due to insulin secretion. These results were similar with a previous study.32 Meanwhile, levels of MST1 also increased from the first trimester to the second trimester in women with GDM. To further investigate the potential mechanism of MST1 and the development of GDM, we examined its correlations with glucose metabolic factors. We found that levels of MST1 in the first trimester of pregnancy were positively correlated with insulin resistance and blood glucose levels, while inversely correlated with beta cell function in the second trimester. Based on previous research on MST1 and glucose metabolism, it is speculated that MST1 may increase insulin resistance, decrease beta cell function, and lead to glucose intolerance during pregnancy.
Additionally, we evaluated the association between MST1 and adverse pregnancy outcomes. Our study found that high levels of MST1 in the first trimester were associated with the risks of composite adverse pregnancy outcomes, particularly preterm birth and macrosomia. A previous study showed that compared with healthy pregnancies, MST1 of placenta in pregnancies with severe preeclampsia was high-expressed, while YAP1 and TAZ, the downstream factors of Hippo signaling pathway, were down-expressed. The staining intensity of MST1 was also stronger in placenta for the severe preeclampsia group.33 Sharma et al found that MST1 protein was detected in the cytoplasm during various stages of bovine embryos, phosphorylated YAP and TAZ were detected in the nucleus during the blastocyst stages, suggesting that MST1 could regulate embryonic development through Hippo signaling pathway.34 The knockout of MST1 in mouse embryonic stem cells could result in a disruption of differentiation into mesendoderm lineage, indicating that MST1 plays a role in early placental development, regulating vascular patterning, primitive hematopoiesis, cell proliferation, and survival.35,36 Therefore, we speculate that MST1 may be involved in the regulation of embryo and placental development, leading to adverse pregnancy outcomes.
However, there are several limitations to consider in this study. Firstly, the sample size was relatively small, and limited to a Chinese population. Therefore, our findings may not be generalizable to a larger and more diverse population. We recommend conducting a large-scale cohort study to validate the results. Secondly, only maternal blood samples were collected in this study, which may not provide a comprehensive understanding of the relationship between MST1 and gestational diabetes. It would be beneficial to collect biological samples from cord blood and placenta to gain a more complete picture of the impact of MST1 on newborns. Thirdly, the study did not investigate the signaling mechanism of MST1 in relation to glucose uptake and utilization during gestation. Understanding this mechanism could provide valuable insights into the underlying processes involved in the development of gestational diabetes.
In conclusion, this cohort study suggests that elevated serum levels of MST1 in early pregnancy may be associated with increased risks of GDM and adverse pregnancy outcomes, such as preterm birth and macrosomia. These findings suggest that MST1 may serve as an early predictor and prognostic indicator for GDM and adverse pregnancy outcomes.
Abbreviations
MST1, mammalian sterile line 20-like kinase 1; GDM, Gestational diabetes mellitus; FPG, fasting plasma glucose; 1hrPG, 1-hr postprandial plasma glucose; 2hrPG, 2-hr postprandial plasma glucose; HOMA-IR, homeostatic model assessment of insulin resistance; HOMA-β, homeostasis model assessment of β cell function.
Informed Consent
All of the participants provided informed consent.
Author Contributions
Bai-Bing Gao and Qing-qing Zhang are co-first authors. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by the Six Talent Peaks Project in Jiangsu Province (WSN-336); the Scientific Research Project of Health Commission in Jiangsu Province (LGY2020067); and the Scientific Research Project, Taizhou School of Clinical Medicine, Nanjing Medical University (TZKY20220110).
Disclosure
The authors declare no conflict of interest.
References
1. Modzelewski R, Stefanowicz-Rutkowska MM, Matuszewski W, Bandurska-Stankiewicz EM. Gestational diabetes mellitus-recent literature review. J Clin Med. 2022;11(19):19. doi:10.3390/jcm11195736
2. Dalfra MG, Burlina S, Del Vescovo GG, Lapolla A. Genetics and epigenetics: new insight on gestational diabetes mellitus. Front Endocrinol. 2020;11:602477. doi:10.3389/fendo.2020.602477
3. Zhang Y, Xiao CM, Zhang Y, et al. Factors associated with gestational diabetes mellitus: a meta-analysis. J Diabetes Res. 2021;2021:6692695. doi:10.1155/2021/6692695
4. Plows JF, Stanley JL, Baker PN, Reynolds CM, Vickers MH. The pathophysiology of gestational diabetes mellitus. Int J Mol Sci. 2018;19(11):3342. doi:10.3390/ijms19113342
5. Sweeting AN, Ross GP, Hyett J, et al. Gestational diabetes mellitus in early pregnancy: evidence for poor pregnancy outcomes despite treatment. Diabetes Care. 2016;39(1):75–81. doi:10.2337/dc15-0433
6. Vounzoulaki E, Khunti K, Abner SC, Tan BK, Davies MJ, Gillies CL. Progression to type 2 diabetes in women with a known history of gestational diabetes: systematic review and meta-analysis. BMJ. 2020;369:m1361. doi:10.1136/bmj.m1361
7. Holt RI. The hyperglycemia and adverse pregnancy outcomes trial: answers but still more questions about the management of gestational diabetes. Diabet Med. 2008;25(9):1013–1014. doi:10.1111/j.1464-5491.2008.02559.x
8. Liu B, Cai J, Xu Y, et al. Early diagnosed gestational diabetes mellitus is associated with adverse pregnancy outcomes: a prospective cohort study. J Clin Endocrinol Metab. 2020;105(12):e4264–e4274. doi:10.1210/clinem/dgaa633
9. Gunderson EP, Sun B, Catov JM, et al. Gestational diabetes history and glucose tolerance after pregnancy associated with coronary artery calcium in women during midlife: the CARDIA study. Circulation. 2021;143(10):974–987. doi:10.1161/CIRCULATIONAHA.120.047320
10. Bianco ME, Josefson JL. Hyperglycemia during pregnancy and long-term offspring outcomes. Curr Diab Rep. 2019;19(12):143. doi:10.1007/s11892-019-1267-6
11. Ye W, Luo C, Huang J, Li C, Liu Z, Liu F. Gestational diabetes mellitus and adverse pregnancy outcomes: systematic review and meta-analysis. BMJ. 2022;377:e067946. doi:10.1136/bmj-2021-067946
12. Fajans SS, Bell GI, Polonsky KS. Molecular mechanisms and clinical pathophysiology of maturity-onset diabetes of the young. N Engl J Med. 2001;345(13):971–980. doi:10.1056/NEJMra002168
13. Pombo CM, Iglesias C, Sartages M, Zalvide JB. MST kinases and metabolism. Endocrinology. 2019;160(5):1111–1118. doi:10.1210/en.2018-00898
14. Ardestani A, Paroni F, Azizi Z, et al. MST1 is a key regulator of beta cell apoptosis and dysfunction in diabetes. Nat Med. 2014;20(4):385–397. doi:10.1038/nm.3482
15. Ardestani A, Maedler K. MST1: a promising therapeutic target to restore functional beta cell mass in diabetes. Diabetologia. 2016;59(9):1843–1849. doi:10.1007/s00125-016-3892-9
16. López Stewart G. Diagnostic criteria and classification of hyperglycaemia first detected in pregnancy: a World Health Organization Guideline. Diabet Res Clin Pract. 2014;103(3):341–363. doi:10.1016/j.diabres.2013.10.012
17. Law KP, Zhang H. The pathogenesis and pathophysiology of gestational diabetes mellitus: deductions from a three-part longitudinal metabolomics study in China. Clin Chim Acta. 2017;468:60–70. doi:10.1016/j.cca.2017.02.008
18. Cefalu WT, Berg EG, Petersen MP, Darsow T. American Diabetes Association’s Standards of Care: a paradigm shift in the dissemination of information. Diabetes Care. 2018;41(3):387–388. doi:10.2337/dci17-0064
19. Lowe WL, Scholtens DM, Kuang A, et al.; Group, H. F.-u. S. C. R. Hyperglycemia and Adverse Pregnancy Outcome Follow-up Study (HAPO FUS): maternal gestational diabetes mellitus and childhood glucose metabolism. Diabetes Care. 2019;42(3):372–380. doi:10.2337/dc18-1646
20. Johns EC, Denison FC, Norman JE, Reynolds RM. Gestational diabetes mellitus: mechanisms, treatment, and complications. Trends Endocrinol Metab. 2018;29(11):743–754. doi:10.1016/j.tem.2018.09.004
21. Bi W, Xiao L, Jia Y, et al. c-Jun N-terminal kinase enhances MST1-mediated pro-apoptotic signaling through phosphorylation at serine 82. J Biol Chem. 2010;285(9):6259–6264. doi:10.1074/jbc.M109.038570
22. Cheung W, Ajiro K, Samejima K, et al. Apoptotic phosphorylation of histone H2B is mediated by mammalian sterile twenty kinase. Cell. 2003;113(4):507–517. doi:10.1016/s0092-8674(03)00355-6
23. Yu J, Zhai X, Li X, et al. Identification of MST1 as a potential early detection biomarker for colorectal cancer through a proteomic approach. Sci Rep. 2017;7(1):14265. doi:10.1038/s41598-017-14539-x
24. Shi Y, Liu B, Wang C, Yang C. MST1 down-regulation in decreasing apoptosis of aortic dissection smooth muscle cell apoptosis. Eur Rev Med Pharmacol Sci 2018;22(7):2044–2051. doi:10.26355/eurrev_201804_14734
25. Tian C, Qiu M, Lv H, Yue F, Zhou F. Quantitative proteomic analysis of serum reveals MST1 as a potential candidate biomarker in spontaneously diabetic cynomolgus monkeys. ACS omega. 2022;7(50):46702–46716. doi:10.1021/acsomega.2c05663
26. Galan JA, Avruch J. MST1/MST2 protein kinases: regulation and physiologic roles. Biochemistry. 2016;55(39):5507–5519. doi:10.1021/acs.biochem.6b00763
27. Ardestani A, Maedler K. MST1 deletion protects beta-cells in a mouse model of diabetes. Nutr Diabetes. 2022;12(1):7. doi:10.1038/s41387-022-00186-3
28. Gao T, Zhou D, Yang C, et al. Hippo signaling regulates differentiation and maintenance in the exocrine pancreas. Gastroenterology. 2013;144(7):1543–1553, 1553 e1. doi:10.1053/j.gastro.2013.02.037
29. Wu Y, Qi Z, Wang B, et al. Discovery of IHMT-MST1-58 as a novel, potent, and selective MST1 inhibitor for the treatment of type 1/2 diabetes. J Med Chem. 2022;65(17):11818–11839. doi:10.1021/acs.jmedchem.2c00926
30. Angelis V, Johnston S, Ardestani A, Maedler K. Case report: neratinib therapy improves glycemic control in a patient with type 2 diabetes and breast cancer. Front Endocrinol. 2022;13:830097. doi:10.3389/fendo.2022.830097
31. Kuhl C. Insulin secretion and insulin resistance in pregnancy and GDM. Implications for diagnosis and management. Diabetes. 1991;40(Suppl 2):18–24. doi:10.2337/diab.40.2.S18
32. Landon MB, Gabbe SG. Gestational diabetes mellitus. Obstet Gynecol. 2011;118(6):1379–1393. doi:10.1097/AOG.0b013e31823974e2
33. Liu R, Wei C, Ma Q, Wang W. Hippo-YAP1 signaling pathway and severe preeclampsia (sPE) in the Chinese population. Pregnancy Hypertens. 2020;19:1–10. doi:10.1016/j.preghy.2019.11.002
34. Sharma J, Madan P. Characterisation of the Hippo signalling pathway during bovine preimplantation embryo development. Reprod Fertil Dev. 2020;32(4):392–401. doi:10.1071/RD18320
35. Oh S, Lee D, Kim T, et al. Crucial role for Mst1 and Mst2 kinases in early embryonic development of the mouse. Mol Cell Biol. 2009;29(23):6309–6320. doi:10.1128/MCB.00551-09
36. Sun X, Ren Z, Cun Y, et al. Hippo-YAP signaling controls lineage differentiation of mouse embryonic stem cells through modulating the formation of super-enhancers. Nucleic Acids Res. 2020;48(13):7182–7196. doi:10.1093/nar/gkaa482
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
