Back to Journals » Psychology Research and Behavior Management » Volume 16
Association of Long-Term HbA1c Variability with Anxiety and Depression in Patients with Type 2 Diabetes: A Cross-Sectional Retrospective Study
Authors Shi Q, Ding J, Su H, Du Y, Pan T, Zhong X
Received 14 October 2023
Accepted for publication 7 December 2023
Published 18 December 2023 Volume 2023:16 Pages 5053—5068
DOI https://doi.org/10.2147/PRBM.S441058
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Mei-Chun Cheung
Qian Shi,1,* Jingcheng Ding,1,* Hong Su,2 Yijun Du,1 Tianrong Pan,1 Xing Zhong1
1Department of Endocrinology, the Second Affiliated Hospital of Anhui Medical University, Hefei City, Anhui Province, 230601, People’s Republic of China; 2Department of Epidemiology and Health Statistics, Anhui Medical University, Hefei City, Anhui Province, 230601, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xing Zhong, No. 678 Furong Road, Hefei City, Anhui Province, 230601, People’s Republic of China, Tel +86 18905692292, Email [email protected]
Purpose: To explore the relationship between long-term glycemic variability and anxiety and depression in patients with type 2 diabetes.
Participants and Methods: A cohort comprising 214 individuals diagnosed with type 2 diabetes participated in this study. Comprehensive demographic and laboratory information was gathered for them. The evaluation of anxiety relied on the 7-item Generalized Anxiety Disorder Scale (GAD-7), while depression was assessed utilizing the 9-item Health Questionnaire (PHQ-9). Based on the presence or absence of anxiety and depression, participants were categorized into either the mood disorder or control groups. Subsequently, univariate and stepwise multiple binary logistic regression analyses were conducted to investigate the potential correlations between factors and the presence of anxiety and depression.
Results: The prevalence of anxiety disorders is 23%, and depression is 32%. The prevalence of smoking, diabetic autonomic neuropathy, stroke, and osteoporosis in the mood disorder group was significantly higher than that in the control group (P < 0.05), the glycated hemoglobin A1c variability score (HVS), mean hemoglobin A1c value, total cholesterol, urinary albumin/creatinine and systemic immune-inflammatory index (SII) were significantly higher in the control group (P < 0.05). The level of high-density lipoprotein in the mood disorder group was significantly lower than the control group (P < 0.05). In stepwise multiple binary logistic regression analyses, the main factors associated with anxiety were depression (P < 0.001, OR=117.581) and gender (P < 0.001, OR=9.466), and the main factors related to depression included anxiety (P < 0.001, OR=49.424), smoking (P=0.042, OR=2.728), HVS (P=0.004, OR=8.664), and SII (P=0.014, OR=1.002).
Conclusion: Persistent fluctuations in blood glucose levels have been linked to anxiety and depression. Consequently, maintaining an optimal level of glycemic control and minimizing fluctuations becomes imperative in the comprehensive management of diabetes.
Keywords: type 2 diabetes, anxiety, depression, long-term glycemic variability
Introduction
Diabetes, a pervasive chronic ailment, bears significant and far-reaching implications. The most recent statistics from the International Diabetes Federation (IDF) reveal that in 2021, the worldwide prevalence of diabetes among adults stood at a staggering 536.6 million individuals, constituting 10.5% of the global population. Projections for the year 2045 paint an even more concerning picture: 783.2 million (12.2%) people will have diabetes worldwide.1 Moreover, within China, the most recent estimations have witnessed a notable surge in the prevalence of diabetes mellitus, surging from 10.9% in 2013 to a marked 12.4% in 2018,2 significantly higher than the global average. Studies have shown that anxiety and depressive disorders in diabetic patients may be twice as high as in the general population.3 Diabetic individuals grappling with concurrent mood disorders exhibit diminished treatment adherence, suboptimal blood glucose management, elevated complication rates, lower quality of life, and elevated mortality risk compared to their counterparts within the ordinary diabetic population.4
Recently, there has been an interest in mental health and the influence of glucose variability on clinical outcomes in diabetes. This variability encompasses short-term fluctuations in blood glucose levels, including intra-day and inter-day variability, and long-term changes in blood glucose variability over extended periods.5 Experimental findings show that intermittent hyperglycemia catalyzes oxidative stress of microvascular and macrovascular complications compared to persistent hyperglycemia. Furthermore, hyperglycemia may be cheerful with the development of anxiety and depression.6,7 The fluctuations in blood glucose levels and psychological disorders exert unequivocal and detrimental effects on the prolonged outcomes of individuals with type 2 diabetes mellitus.8–10 Some studies have linked hyperglycemia and poor glycemic control to anxiety and depression,11–13 but other studies have found no correlation between glycemic control and mood problems.14,15 The correlation between blood glucose levels and anxiety and depression is inconsistent, and few are concerned about long-term blood sugar fluctuations.
This article investigates the prevalence of anxiety and depression among individuals diagnosed with type 2 diabetes mellitus and its relationship with prolonged blood glucose fluctuations. Timely identification and intervention in patients with type 2 diabetes who also exhibit mood disorders are expected to improve clinical outcomes.
Participants and Methods
Participants
This study consisted of patients with type 2 diabetes under treatment and follow-up care at the Second Affiliated Hospital of Anhui Medical University between February and May 2023. Based on screening psychological tools, these patients were categorized into the presence or absence of mood disorders, with the mood disorder group encompassing individuals with anxiety, depression, or a combination of both. The research adhered to the Declaration of Helsinki and received approval from the Ethics Committee of the Second Affiliated Hospital of Anhui Medical University (Ethics number YX2023-068). All subjects signed informed consent before starting the investigation.
Inclusion criteria comprised: (1) Patients diagnosed with type 2 diabetes mellitus;16 (2) Individuals aged 18 to 75 years; (3) Glycated hemoglobin (HbA1c) was measured at least three times in the year prior to enrollment; (4) Stable diabetes regimen for at least three months prior to enrollment. Exclusion criteria encompassed were: (1) Special forms of diabetes mellitus, such as maturity-onset diabetes of the young (MODY) and latent autoimmune diabetes in adults (LADA), gestational diabetes mellitus, and type 1 diabetes mellitus; (2) Patients experiencing acute diabetic complications, such as diabetic ketoacidosis and hyperosmotic hyperglycemia syndrome; (3) Concurrent severe medical conditions, such as significant cardiovascular and cerebrovascular diseases or cancer; (4) History of drug abuse, psychiatric conditions, or misuse of psychoactive substances; (5) Patients facing communication challenges or severe audio-visual impairments.
Data Collection
Comprehensive demographic and clinical data were documented: gender, age, body mass index (BMI), years of education, occupation, smoking and alcohol history, diabetes duration, glucose-lowering regimen, diabetic complications, and concurrent medical conditions. Laboratory parameters, including current fasting blood glucose (FPG), postprandial 2-hour blood glucose (PBG), fasting c-peptide, glycated hemoglobin (HbA1c), total cholesterol (TC), triglycerides (TG), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), alanine aminotransferase (ALT), alanine transaminase (AST), uric acid (UA), urinary albumin/creatinine ratio (ACR), and systemic immune-inflammatory index (SII), were collected from the study participants. The insulin resistance index (HOMA-IR) was calculated using HOMA2 v2.2.3 (available at homacalculator/https://www.dtu.ox.ac.uk/), and glomerular filtration rate (eGFR) was determined as well.
Anxiety and Depression Assessment
The 9-item Health Questionnaire (PHQ-9) consists of nine items, each measured using a 0 to 3, with total scores ranging from 1 to 27. A score of ≥5 indicates the presence of a depressive state. The PHQ-9 contains items based on the DSM-IV diagnostic criteria for major depressive disorder.17
The 7-item Generalized Anxiety Disorder Scale (GAD-7) consists of seven items, each measured using a score of 0 to 3. Its total score ranges from 0 to 21. The GAD-7 is a sound detection tool for GAD. A participant with a score of ≥ 5 indicates the presence of an anxiety state—the GAD-7 scale items based on DSM-IV criteria for generalized anxiety disorder.18
Long-Term Blood Glucose Fluctuations
In this study, the glycated hemoglobin A1c variability score (HVS) used as the evaluation index of long-term blood glucose fluctuation, namely, the number of HbA1c changes ≥0.5% from the previous time accounted for the percentage of the total number of HbA1c measurements. In clinical practice, individuals with an HVS of 60% or higher face a greater risk of multiple diabetes complications and death than their peers.19,20
Statistical Analysis
SPSS27. 0 (Statistical Product and Service Solutions, SPSS) was used for data management and analysis. Measurement data conforming to normal distribution were expressed as 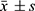 , and the T-test was used for intergroup comparisons. Measurement data with skewed distribution were described in median and interquartile range (P25, P75), and comparisons between groups were made using the U Mann–Whitney in nonparametric tests. Counting data was told by the number of instances, and the χ²-test was used for intergroup comparisons. Univariate logistic regression analyses were conducted to explore potential risk factors for anxiety and depression among patients diagnosed with type 2 diabetes mellitus. Variables with a significance level of p<0.05 were subsequently included as independent variables in stepwise multiple binary logistic regression analyses to ascertain their predictive significance regarding the occurrence of anxiety and depression in these patients. To assess the appropriateness of the stepwise multiple logistic regression model, the goodness of fit was evaluated employing the Hosmer-Lemeshow goodness-of-fit test, with a p-value exceeding 0.05 indicative of a well-fitted model. The model’s ability to discern whether a patient exhibited symptoms of anxiety or depression was gauged by calculating the area under the receiver operating characteristic (ROC) curve. An area under the curve (AUC) exceeding 0.7 was considered indicative of an acceptable model fit. Statistically significant differences were defined as p<0.05.
, and the T-test was used for intergroup comparisons. Measurement data with skewed distribution were described in median and interquartile range (P25, P75), and comparisons between groups were made using the U Mann–Whitney in nonparametric tests. Counting data was told by the number of instances, and the χ²-test was used for intergroup comparisons. Univariate logistic regression analyses were conducted to explore potential risk factors for anxiety and depression among patients diagnosed with type 2 diabetes mellitus. Variables with a significance level of p<0.05 were subsequently included as independent variables in stepwise multiple binary logistic regression analyses to ascertain their predictive significance regarding the occurrence of anxiety and depression in these patients. To assess the appropriateness of the stepwise multiple logistic regression model, the goodness of fit was evaluated employing the Hosmer-Lemeshow goodness-of-fit test, with a p-value exceeding 0.05 indicative of a well-fitted model. The model’s ability to discern whether a patient exhibited symptoms of anxiety or depression was gauged by calculating the area under the receiver operating characteristic (ROC) curve. An area under the curve (AUC) exceeding 0.7 was considered indicative of an acceptable model fit. Statistically significant differences were defined as p<0.05.
Results
Demographic and Clinical Data of Patients from Different Groups
The total number of patients with mood disorders was 73, with a prevalence of 34.11%. There were 50 patients with anxiety, with a prevalence of 23%; 69 patients with depression, with a prevalence of 32%; and female type 2 diabetic patients were more prone to anxiety disorders. As shown in Table 1, the demographic comparison between the groups showed that the proportion of patients who smoked cigarettes and the prevalence of diabetic autonomic neuropathy, stroke, and osteoporosis were significantly higher in the group with mood disorders than in the control group (P < 0.05). There was no significant difference in the type of glucose-lowering medication used between the two groups (P > 0.05). Moreover, the glycated hemoglobin A1c variability score (HVS), average glycated hemoglobin value, total cholesterol (TC), urinary albumin/creatinine ratio (ACR), and systemic immune-inflammatory index (SII) in T2DM patients with mood disorders were significantly higher than those in the regular group (P < 0.05). The level of high-density lipoprotein cholesterol (HDL-C) was significantly lower than that in the standard group (P < 0.05).
 |
Table 1 Comparison of Demographics and Clinical Indicators Between Groups |
Univariate Analysis of Demographic and Clinical Indicators and Subject Anxiety
A logistic one-way regression analysis was conducted to explore the relationship between demographic and clinical indicators and the presence of anxiety (Table 2). Gender, years of education, depression, stroke, HVS, TC, and SII were associated with anxiety (P < 0.05). Conversely, no significant correlations were observed between anxiety and variables such as smoking, alcohol consumption, diabetes duration, or diabetes-related complications (P > 0.05).
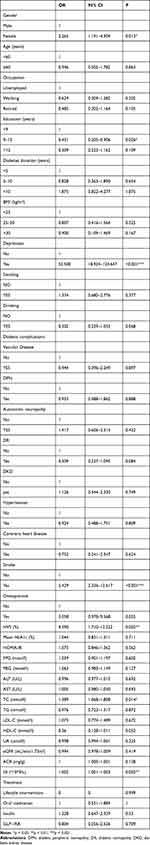 |
Table 2 Relationship Between Demographic and Clinical Indicators and Anxiety |
Univariate Analysis of Demographic and Clinical Indicators and Subject Depression
In Table 3, a comprehensive analysis of demographic and clinical indicators was conducted using logistic one-way regression analysis to investigate their relationship with depression. The results revealed significant correlations between depression, such as smoking, anxiety, type 2 diabetic autonomic neuropathy, and stroke (P < 0.05). Among the clinical parameters examined, glycated hemoglobin A1c variability score (HVS), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and systemic immune-inflammatory index (SII) exhibited significant associations with depression (P < 0.05). In contrast, alcohol consumption, gender, diabetes duration, other diabetes-related complications, and various laboratory indices did not display notable associations with depression (P > 0.05).
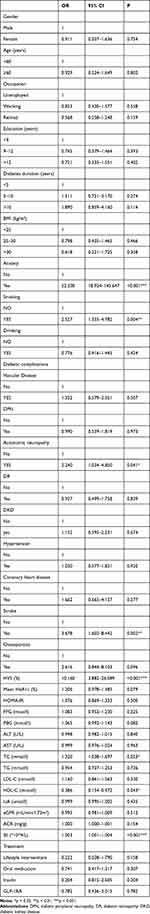 |
Table 3 Relationship Between Demographic and Clinical Indicators and Depression |
Multifactorial Stepwise Multiple Binary Logistic Regression Analysis of Anxiety
In the stepwise multiple binary logistic regression analysis, influential factors that were clinically relevant to anxiety, along with common confounders such as smoking and alcohol consumption, were taken into account. As outlined in Table 4, the factors significantly associated with anxiety encompassed depression (P < 0.001, OR = 117.581) and gender (P < 0.001, OR = 9.466). The logistic regression model exhibited a Hosmer-Lemeshow goodness-of-fit test result of 0.129, and the area under the ROC curve (AUC) was calculated at 0.916 (P < 0.001, 95% CI = 0.867–0.965), signifying the model distinguishing individuals experiencing anxiety.
 |
Table 4 Multifactorial Stepwise Multiple Logistic Regression Analysis of Anxiety |
Multifactorial Stepwise Multiple Binary Logistic Regression Analysis of Depression
In the stepwise multiple binary logistic regression analysis, we considered factors that held clinical significance concerning depression, in addition to accounting for standard confounding variables such as smoking and alcohol consumption, as identified in the single-factor analysis. In Table 5, depression displayed associations with anxiety (P < 0.001, OR=49.424), smoking (P=0.042, OR=2.728), glycated hemoglobin A1c variability score (HVS) (P=0.004, OR=8.664), and systemic immune-inflammatory index (SII) (P=0.014, OR=1.002). The logistic regression model’s assessment yielded a Hosmer-Lemeshow goodness-of-fit test result of 0.681, and the area under the ROC curve (AUC) was computed as 0.912 (P < 0.001, 95% CI=0.869–0.954). These findings underscore the model’s efficacy in discerning the presence of depression among the study participants.
 |
Table 5 Multifactorial Stepwise Multiple Logistic Regression Analysis of Depression |
Discussion
This article investigated the prevalence of anxiety and depression in patients with type 2 diabetes mellitus and examined its relationship to demographic and clinical indicators, particularly long-term blood glucose fluctuations.
Research findings indicate that the prevalence of anxiety disorders within the general population typically falls within the range of 7.3% to 25%.21–23 The INTERPRET-DD study, encompassing data from 3170 diabetes patients across 18 countries, conducted an estimation that placed the prevalence of anxiety, encompassing all forms of anxiety disorders, at 15%,24 and meta-analysis showed that the estimated prevalence of depression in patients with diabetes ranged from 18% to 46%.25 Moreover, 40% of diabetic patients suffer from increased anxiety.26 Our study encompassed a cohort of 214 diabetic patients, among whom 50 individuals (23%) exhibited comorbid anxiety, while 69 individuals (32%) displayed comorbid depression. These prevalence rates closely align with those reported in previous studies. Prior research has indicated a relatively broad spectrum of prevalence rates for comorbid anxiety and depression. For instance, Anderson et al reported prevalence rates of comorbid depression in diabetic patients ranging from 11% to 30%, depending on whether standardized diagnostic interviews or self-report questionnaires were utilized.27
Mood disorders and diabetes demonstrate a dynamic interaction, with an interdependent relationship prevailing between anxiety and depression.23,28,29 Type 2 diabetes mellitus (T2DM) can elevate the likelihood of experiencing depressive symptoms to a certain degree, while conversely, depression can heighten the risk of developing T2DM.30 Individuals exhibiting anxiety symptoms may face an elevated risk of developing type 2 diabetes. Conversely, those diagnosed with diabetes encounter a 41% higher risk of developing anxiety disorders than adults without diabetes.31 Our study showed that patients presenting with anxiety are more likely to be depressed when compared to individuals without such comorbidity and vice versa. This observation further underscores the bidirectional relationship between anxiety and depression in individuals with type 2 diabetes. Our findings align closely with those reported in a domestic study comprising 893 diabetic patients.32
Glycated hemoglobin falls short of capturing the nuances of glycemic fluctuations. In contrast, glycated hemoglobin A1c variability measures long-term blood glucose variability, presenting a potentially superior indicator of diabetes-related risk. In our study, both the HVS and average HbA1c levels were notably elevated in patients diagnosed with type 2 diabetes mellitus with mood disorders. In the correlation analysis, only HbA1c variability was positively associated with depression. Furthermore, a cross-sectional study involving diabetic patients aged 20 to 75 demonstrated that poor self-perceived glycemic control was positively linked with anxiety but exhibited no significant correlation with depression scores.33 A study of 179 T2DM patients also showed that anxiety was associated with poor glycemic control, while depression was not associated with it.34
Our stepwise multiple binary logistic regression unveiled that the glycated hemoglobin A1c variability score (HVS) was a significant and noteworthy risk factor for the co-occurrence of depression in type 2 diabetes mellitus. These are similar to the study of Alejandro et al, who observed a positive and statistically significant association between depression, anxiety, and HbA1c coefficient of variation (HbA1c-CV) among patients with type 1 diabetes.35 A study on elderly patients with type 2 diabetes also found that those with higher HbA1c variability were more likely to develop depressive symptoms.36 Moreover, short-term blood glucose fluctuations are also positively associated with anxiety and depression.13 Therefore, people with diabetes with high blood sugar fluctuations may be more prone to anxiety and depression disorders.
Extensive fluctuation range of blood glucose may affect oxidative stress, chronic low-grade inflammation, and endothelial dysfunction.37 Commonly, there are shared underlying mechanisms contributing to the development of depression and anxiety about factors such as lipid metabolism, advanced glycosylation products, neuroinflammation, and neurotransmitter imbalances.38 Our study findings indicated that the systemic immune-inflammatory index (SII) stands as a risk factor for individuals with type 2 diabetes combined with anxiety and depression. The systemic immune-inflammatory index (SII) is an objective gauge of the equilibrium between systemic inflammation and the immune response status within the host. This index considers the roles of neutrophils, platelets, and lymphocytes, each contributing to distinct immune and inflammatory pathways.39 In a follow-up study of patients after COVID-19 infection, baseline SII was positively correlated with depression and anxiety scores at follow-up.40 Another prospective research on type 2 diabetes showed that SII levels were associated with an increased risk of depression in diabetic patients.41 Our study findings align with prior research. This relationship is grounded in the response of activated inflammatory cells to blood sugar fluctuations, resulting in heightened secretion of inflammatory factors, including interleukin-6 (IL-6), interleukin-18 (IL-18), and tumor necrosis factor α (TNF-α).42,43 In contrast, tumor necrosis factor-α (TNF-α) and interleukin-6 (IL −6), interleukin-1β (IL-1β), and C-reactive protein (CRP) were increased in the blood, and cerebrospinal fluid of depressed patients.44,45 Chronic neuroinflammation leads to immune dysregulation of the central nervous system and depressive episodes.46–48 Thus, the mechanisms leading to the development of mood disorders in type 2 diabetes may be related to chronic low-grade inflammation.
Yang and others found that female patients were susceptible to anxiety, depression, and cognitive impairment.13 A 4-year prospective study in Malaysia also similarly showed a higher prevalence of depression among female patients than males.49 Our study uncovered that female patients diagnosed with type 2 diabetes exhibited a heightened susceptibility to anxiety disorders, although no gender disparity was observed among patients with depression. This observation may be attributed to the dominance of middle-aged and elderly individuals within our study cohort, who, in addition to facing social and familial pressures, are influenced by hormonal fluctuations characteristic of the perimenopausal period.50 Univariate analysis unveiled that patients with type 2 diabetes possessing a moderate level of education manifested a greater likelihood of experiencing anxiety symptoms. A Greek study involving 170 adults with type 2 diabetes yielded congruent findings, indicating that individuals with primary school education were more prone to exhibiting symptoms of depression and anxiety when contrasted with their counterparts with higher education levels.51 Nonetheless, when integrated into the stepwise multiple logistic regression model, education level did not emerge as an independent predictor of anxiety among individuals with type 2 diabetes. Conversely, the influence of smoking on depression remains a relatively less explored area in the literature. Our study showed that smoking patients were more likely to have depressive symptoms, a trend that resonates with the findings of Rubin et al. Their research suggested that individuals with depression often smoke to alleviate mood disturbances, while long-term smoking increases the incidence of depression.52
Regarding cardiovascular disease and chronic complications of diabetes, this study showed that stroke was positively associated with anxiety and depression in patients with type 2 diabetes. A multicenter prospective study in the UK showed an increased risk of stroke in depressed patients,53 while Walter and others showed that survivors of stroke had an increased risk of depression,54 and patients with anxiety, which was similar to the results of this study.55 A meta-analysis has illuminated that individuals with diabetic complications, encompassing conditions such as diabetic retinopathy, nephropathy, neuropathy, microvascular complications, and sexual dysfunction, exhibit an elevated likelihood of experiencing mood disorders.56 Nonetheless, this study exclusively established a heightened likelihood of depression among patients with type 2 diabetes who also had autonomic neuropathy, failing to identify significant correlations between depression and other chronic diabetic complications. This outcome could be attributed to our study cohort’s notably elevated prevalence of chronic diabetes-related complications. Furthermore, our study outcomes underscore the significance of abnormal lipid metabolism as a risk factor for anxiety and depression in individuals. Elevated blood lipid levels are closely intertwined with factors such as obesity and insulin resistance, which, in turn, can provoke anxiety and depression-like behaviors.57 Abnormal blood lipid metabolism has been confirmed to be associated with various psychiatric disorders.58–61 Consequently, in managing diabetes, it is necessary to strengthen the management of blood lipids.
The limitations of this study encompass: (a) As a single-center study, the prevalence of anxiety and depression cannot be generalized to the overall diabetic population in China; (b) The analysis of mood disorders in patients with type 2 diabetes mellitus in this paper predominantly relies on basic demographic and laboratory indicators, with personality traits, quality of life, and religious beliefs remaining unexamined for their influence on the patient’s mood disorders; (c) This study adopts a cross-sectional retrospective approach, underscoring the necessity for future prospective studies to ascertain whether long-term glucose fluctuations can predict the onset of anxiety and depression in patients with type 2 diabetes; (d) Finally, The univariate analysis screening (UAS) may mistakenly exclude essential covariates in the multiple logistic regression. Stepwise multifactor regression was employed to perform regression, beginning with significant predictors in the model. However, this approach may exclude other significant predictors from the final model, losing valuable information and potential predictive power.
Conclusion
Anxiety and depression represent prevalent complications in individuals with type 2 diabetes.3,30 These mood disorders are intricately linked to long-term blood glucose fluctuations, alongside additional risk factors such as dyslipidemia, smoking, and gender. Mechanistically, the pathogenesis of anxiety and depression may be entwined with chronic low-grade systemic inflammation. Consequently, the imperative for effective diabetes management encompasses maintaining reasonable glycemic control while minimizing glucose fluctuations.
Acknowledgments
The authors gave thanks to all participants in the study and the members of the survey teams. This study was supported by grants from Natural Science Research Project of Universities in Anhui Province (2023AH053182), the Clinical Research Incubation Program of the Second Affiliated Hospital of Anhui Medical University (2021LCZD14), Anhui Medical University Scientific Research Fund Project (2020xkj200, 2022xkj177), Endocrinology-Epidemiology and Biostatistic Co-Construction Project of Anhui Medical University (2023). Qian Shi and Jingcheng Ding are the co-first authors of this study. Xing Zhong is the correspondence for this study.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sun H, Saeedi P, Karuranga S, et al. IDF Diabetes Atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119. doi:10.1016/j.diabres.2021.109119
2. Wang L, Peng W, Zhao Z, et al. Prevalence and treatment of diabetes in China, 2013–2018. JAMA. 2021;326(24):2498–2506. doi:10.1001/jama.2021.22208
3. Woon LSC, Sidi HB, Ravindran A, et al. Depression, anxiety, and associated factors in patients with diabetes: evidence from the anxiety, depression, and personality traits in diabetes mellitus (ADAPT-DM) study. BMC Psychiatry. 2020;20(1):227. doi:10.1186/s12888-020-02615-y
4. Egede LE, Ellis C. Diabetes and depression: global perspectives. Diabetes Res Clin Pract. 2010;87(3):302–312. doi:10.1016/j.diabres.2010.01.024
5. Rodbard D. Glucose variability: a review of clinical applications and research developments. Diabetes Technol Ther. 2018;20(S2):S25–S215. doi:10.1089/dia.2018.0092
6. de Ornelas Maia AC, de Azevedo Braga A, Brouwers A, Nardi AE, e Silva AC. Prevalence of psychiatric disorders in patients with diabetes types 1 and 2. Compr Psychiatry. 2012;53(8):1169–1173. doi:10.1016/j.comppsych.2012.03.011
7. Buchberger B, Huppertz H, Krabbe L, Lux B, Mattivi JT, Siafarikas A. Symptoms of depression and anxiety in youth with type 1 diabetes: a systematic review and meta-analysis. Psychoneuroendocrinology. 2016;70:70–84. doi:10.1016/j.psyneuen.2016.04.019
8. Egede LE, Dismuke CE. Serious psychological distress and diabetes: a review of the literature. Curr Psychiatry Rep. 2012;14(1):15–22. doi:10.1007/s11920-011-0240-0
9. Kilpatrick ES, Rigby AS, Atkin SL. A1C variability and the risk of microvascular complications in type 1 diabetes: data from the diabetes control and complications trial. Diabetes Care. 2008;31(11):2198–2202. doi:10.2337/dc08-0864
10. Šoupal J, Škrha J, Fajmon M, et al. Glycemic variability is higher in type 1 diabetes patients with microvascular complications irrespective of glycemic control. Diabetes Technol Ther. 2014;16(4):198–203. doi:10.1089/dia.2013.0205
11. Hamer M, Batty GD, Kivimaki M. Haemoglobin A1c, fasting glucose and future risk of elevated depressive symptoms over 2 years of follow-up in the English longitudinal study of ageing. Psychol Med. 2011;41(9):1889–1896. doi:10.1017/S0033291711000079
12. Lustman PJ, Anderson RJ, Freedland KE, de Groot M, Carney RM, Clouse RE. Depression and poor glycemic control: a meta-analytic review of the literature. Diabetes Care. 2000;23(7):934–942. doi:10.2337/diacare.23.7.934
13. Yang W, Liu M, Tian Y, et al. The increased prevalence of depression and anxiety in T2DM patients associated with blood glucose fluctuation and sleep quality. BMC Endocr Disord. 2022;22(1):232. doi:10.1186/s12902-022-01147-8
14. Mansori K, Shiravand N, Shadmani FK, et al. Association between depression with glycemic control and its complications in type 2 diabetes. Diabetes Metab Syndr. 2019;13(2):1555–1560. doi:10.1016/j.dsx.2019.02.010
15. Bazelmans E, Netea-Maier RT, Vercoulen JH, Tack CJ. Surprisingly few psychological problems and diabetes-related distress in patients with poor glycaemic control. Neth J Med. 2016;74(1):16–21.
16. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2011;34(Suppl 1):S62–69. doi:10.2337/dc11-S062
17. Nouwen A, Deschênes SS, Balkhiyarova Z, Albertorio-Díaz JR, Prokopenko I, Schmitz N. Measurement invariance testing of the patient health questionnaire-9 (PHQ-9) across people with and without diabetes mellitus from the NHANES, EMHS and UK Biobank datasets. J Affect Disord. 2021;292:311–318. doi:10.1016/j.jad.2021.05.031
18. Toussaint A, Hüsing P, Gumz A, et al. Sensitivity to change and minimal clinically important difference of the 7-item Generalized Anxiety Disorder Questionnaire (GAD-7). J Affect Disord. 2020;265:395–401. doi:10.1016/j.jad.2020.01.032
19. Forbes A, Murrells T, Mulnier H, Sinclair AJ. Mean HbA1c, HbA1c variability, and mortality in people with diabetes aged 70 years and older: a retrospective cohort study. Lancet Diabetes Endocrinol. 2018;6(6):476–486. doi:10.1016/S2213-8587(18)30048-2
20. Li S, Nemeth I, Donnelly L, Hapca S, Zhou K, Pearson ER. Visit-to-Visit HbA1c variability is associated with cardiovascular disease and microvascular complications in patients with newly diagnosed type 2 diabetes. Diabetes Care. 2020;43(2):426–432. doi:10.2337/dc19-0823
21. Stein DJ, Scott KM, de Jonge P, Kessler RC. Epidemiology of anxiety disorders: from surveys to nosology and back. Dialogues Clin Neurosci. 2017;19(2):127–136. doi:10.31887/DCNS.2017.19.2/dstein
22. Smith KJ, Béland M, Clyde M, et al. Association of diabetes with anxiety: a systematic review and meta-analysis. J Psychosom Res. 2013;74(2):89–99. doi:10.1016/j.jpsychores.2012.11.013
23. Light RW, Merrill EJ, Despars JA, Gordon GH, Mutalipassi LR. Prevalence of depression and anxiety in patients with COPD. Relationship to functional capacity. Chest. 1985;87(1):35–38. doi:10.1378/chest.87.1.35
24. Chaturvedi SK, Manche Gowda S, Ahmed HU, et al. More anxious than depressed: prevalence and correlates in a 15-nation study of anxiety disorders in people with type 2 diabetes mellitus. Gen Psychiatr. 2019;32(4):e100076. doi:10.1136/gpsych-2019-100076
25. Renn BN, Feliciano L, Segal DL. The bidirectional relationship of depression and diabetes: a systematic review. Clin Psychol Rev. 2011;31(8):1239–1246. doi:10.1016/j.cpr.2011.08.001
26. Grigsby AB, Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. Prevalence of anxiety in adults with diabetes: a systematic review. J Psychosom Res. 2002;53(6):1053–1060. doi:10.1016/s0022-3999(02)00417-8
27. Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care. 2001;24(6):1069–1078. doi:10.2337/diacare.24.6.1069
28. Choi KW, Kim YK, Jeon HJ. Comorbid anxiety and depression: clinical and conceptual consideration and transdiagnostic treatment. Adv Exp Med Biol. 2020;1191:219–235. doi:10.1007/978-981-32-9705-0_14
29. Tiller JWG. Depression and anxiety. Med J Aust. 2013;199(S6):S28–31. doi:10.5694/mja12.10628
30. Ogurtsova K, da Rocha Fernandes JD, Huang Y, et al. IDF Diabetes Atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract. 2017;128:40–50. doi:10.1016/j.diabres.2017.03.024
31. Mersha AG, Tollosa DN, Bagade T, Eftekhari P. A bidirectional relationship between diabetes mellitus and anxiety: a systematic review and meta-analysis. J Psychosom Res. 2022;162:110991. doi:10.1016/j.jpsychores.2022.110991
32. Sun N, Lou P, Shang Y, et al. Prevalence and determinants of depressive and anxiety symptoms in adults with type 2 diabetes in China: a cross-sectional study. BMJ Open. 2016;6(8):e012540. doi:10.1136/bmjopen-2016-012540
33. Collins MM, Corcoran P, Perry IJ. Anxiety and depression symptoms in patients with diabetes. Diabet Med. 2009;26(2):153–161. doi:10.1111/j.1464-5491.2008.02648.x
34. Gonzalez Heredia T, González-Ramírez LP, Hernández-Corona DM, Maciel-Hernández EA. Anxious depression in patients with Type 2 Diabetes Mellitus and its relationship with medication adherence and glycemic control. Glob Public Health. 2021;16(3):460–468. doi:10.1080/17441692.2020.1810735
35. Déniz-García A, Díaz-Artiles A, Saavedra P, Alvarado-Martel D, Wägner AM, Boronat M. Impact of anxiety, depression and disease-related distress on long-term glycaemic variability among subjects with Type 1 diabetes mellitus. BMC Endocr Disord. 2022;22(1):122. doi:10.1186/s12902-022-01013-7
36. Ravona-Springer R, Heymann A, Schmeidler J, et al. Hemoglobin A1c variability predicts symptoms of depression in elderly individuals with type 2 diabetes. Diabetes Care. 2017;40(9):1187–1193. doi:10.2337/dc16-2754
37. Klimontov VV, Saik OV, Korbut AI. Glucose Variability: how Does It Work? Int J Mol Sci. 2021;22(15):7783. doi:10.3390/ijms22157783
38. Kim YK, Kim OY, Song J. Alleviation of depression by glucagon-like peptide 1 through the regulation of neuroinflammation, neurotransmitters, neurogenesis, and synaptic function. Front Pharmacol. 2020;11:1270. doi:10.3389/fphar.2020.01270
39. Huang H, Liu Q, Zhu L, et al. Prognostic value of preoperative systemic immune-inflammation index in patients with cervical cancer. Sci Rep. 2019;9(1):3284. doi:10.1038/s41598-019-39150-0
40. Mazza MG, De Lorenzo R, Conte C, et al. Anxiety and depression in COVID-19 survivors: role of inflammatory and clinical predictors. Brain Behav Immun. 2020;89:594–600. doi:10.1016/j.bbi.2020.07.037
41. Wang J, Zhou D, Dai Z, Li X. Association between systemic immune-inflammation index and diabetic depression. Clin Interv Aging. 2021;16:97–105. doi:10.2147/CIA.S285000
42. Zhang C, Bi Y, Jin G, Gan H, Yu L. High and fluctuating glucose levels increase the expression and secretion of interleukin‑18 in mouse peritoneal macrophages. Mol Med Rep. 2015;12(2):2715–2720. doi:10.3892/mmr.2015.3753
43. Li-Bo Y, Wen-Bo Q, Xiao-Hong L, You-Lun F, Tie Z. Intermittent high glucose promotes expression of proinflammatory cytokines in monocytes. Inflamm Res. 2011;60(4):367–370. doi:10.1007/s00011-010-0279-0
44. Smith KJ, Au B, Ollis L, Schmitz N. The association between C-reactive protein, Interleukin-6 and depression among older adults in the community: a systematic review and meta-analysis. Exp Gerontol. 2018;102:109–132. doi:10.1016/j.exger.2017.12.005
45. Hestad KA, Engedal K, Whist JE, et al. Patients with depression display cytokine levels in serum and cerebrospinal fluid similar to patients with diffuse neurological symptoms without a defined diagnosis. Neuropsychiatr Dis Treat. 2016;12:817–822. doi:10.2147/NDT.S101925
46. Carvalho LA, Torre JP, Papadopoulos AS, et al. Lack of clinical therapeutic benefit of antidepressants is associated overall activation of the inflammatory system. J Affect Disord. 2013;148(1):136–140. doi:10.1016/j.jad.2012.10.036
47. Leonard BE. Inflammation and depression: a causal or coincidental link to the pathophysiology? Acta Neuropsychiatr. 2018;30(1):1–16. doi:10.1017/neu.2016.69
48. Felger JC, Treadway MT. Inflammation effects on motivation and motor activity: role of dopamine. Neuropsychopharmacology. 2017;42(1):216–241. doi:10.1038/npp.2016.143
49. Trento M, Trevisan M, Raballo M, et al. Depression, anxiety, cognitive impairment and their association with clinical and demographic variables in people with type 2 diabetes: a 4-year prospective study. J Endocrinol Invest. 2014;37(1):79–85. doi:10.1007/s40618-013-0028-7
50. Stute P, Lozza-Fiacco S. Strategies to cope with stress and anxiety during the menopausal transition. Maturitas. 2022;166:1–13. doi:10.1016/j.maturitas.2022.07.015
51. Sympa P, Vlachou E, Kazakos K, Govina O, Stamatiou G, Lavdaniti M. Depression and self-efficacy in patients with type 2 diabetes in northern Greece. Endocr Metab Immune Disord Drug Targets. 2018;18(4):371–378. doi:10.2174/1871530317666171120154002
52. Boden JM, Fergusson DM, Horwood LJ. Cigarette smoking and depression: tests of causal linkages using a longitudinal birth cohort. Br J Psychiatry. 2010;196(6):440–446. doi:10.1192/bjp.bp.109.065912
53. Harshfield EL, Pennells L, Schwartz JE, et al. Association between depressive symptoms and incident cardiovascular diseases. JAMA. 2020;324(23):2396–2405. doi:10.1001/jama.2020.23068
54. Swardfager W, MacIntosh BJ. Depression, type 2 diabetes, and poststroke cognitive impairment. Neurorehabil Neural Repair. 2017;31(1):48–55. doi:10.1177/1545968316656054
55. Scott KM. Depression, anxiety and incident cardiometabolic diseases. Curr Opin Psychiatry. 2014;27(4):289–293. doi:10.1097/YCO.0000000000000067
56. Clouse RE, Lustman PJ, Freedland KE, Griffith LS, McGill JB, Carney RM. Depression and coronary heart disease in women with diabetes. Psychosom Med. 2003;65(3):376–383. doi:10.1097/01.psy.0000041624.96580.1f
57. Lam YY, Tsai SF, Chen PC, Kuo YM, Chen YW. Pioglitazone rescues high-fat diet-induced depression-like phenotypes and hippocampal astrocytic deficits in mice. Biomed Pharmacother. 2021;140:111734. doi:10.1016/j.biopha.2021.111734
58. Loas G, Dalleau E, Lecointe H, Yon V. Relationships between anhedonia, alexithymia, impulsivity, suicidal ideation, recent suicide attempt, C-reactive protein and serum lipid levels among 122 inpatients with mood or anxious disorders. Psychiatry Res. 2016;246:296–302. doi:10.1016/j.psychres.2016.09.056
59. Cavicchioli FL, Maes M, Roomruangwong C, et al. Associations between severity of anxiety and clinical and biological features of major affective disorders. Psychiatry Res. 2018;260:17–23. doi:10.1016/j.psychres.2017.11.024
60. Wang J, Jiang C, Chen L, et al. A cross-sectional study to investigate the correlation between depression comorbid with anxiety and serum lipid levels. Compr Psychiatry. 2016;69:163–168. doi:10.1016/j.comppsych.2016.05.005
61. De Berardis D, Serroni N, Campanella D, et al. Alexithymia, suicide ideation, C-reactive protein, and serum lipid levels among outpatients with generalized anxiety disorder. Arch Suicide Res. 2017;21(1):100–112. doi:10.1080/13811118.2015.1004485
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
