Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 15
Association of lncRNA LINC01173 Expression with Vitamin-D and Vitamin B12 Level Among Type 2 Diabetes Patients
Authors Aladel A , Verma AK, Dabeer S , Ahmad I , Alshahrani MY, AboHassan MS, Khan MI, Almutairi MG , Beg MMA
Received 14 April 2022
Accepted for publication 29 June 2022
Published 19 August 2022 Volume 2022:15 Pages 2535—2543
DOI https://doi.org/10.2147/DMSO.S369012
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ming-Hui Zou
Alanoud Aladel,1,* Amit K Verma,2,* Sadaf Dabeer,3 Irfan Ahmad,4 Mohammad Y Alshahrani,4 Mohammad S AboHassan,4 Mohammad Idreesh Khan,5,* Malak Ghazi Almutairi,6 Mirza Masroor Ali Beg7,8
1Department of Community Health Sciences, College of Applied Medical Sciences, King Saud University, Riyadh, Saudi Arabia; 2Department of Biotechnology, Jamia Millia Islamia, New Delhi, India; 3Division of Endocrinology Metabolism, and Lipids, School of Medicine, Emory University, Atlanta, GA, USA; 4Department of Clinical Laboratory Sciences, College of Applied Medical Sciences, King Khalid University, Abha, Saudi Arabia; 5Department of Clinical Nutrition, College of Applied Health Sciences in Arras, Qassim University, Buraidah, Saudi Arabia; 6Department of Nutrition, Almethnab General Hospital, Qassim Health Cluster, Ministry of Health, Al Mithnab, Saudi Arabia; 7Faculty of Medicine, Alatoo International University, Bishkek, Kyrgyzstan; 8Centre for Promotion of Medical Research, Bishkek, Kyrgyzstan
*These authors contributed equally to this work
Correspondence: Mirza Masroor Ali Beg, Email [email protected]
Background: Type 2 diabetes mellitus (T2DM) has risen to become the world’s most serious public health problem in recent years, and the role of long noncoding RNAs (lncRNAs) in the onset and progression of T2DM, as well as special attention to vitamins, has gotten a lot of attention recently.
Methods: The aim of the study was to analyze lncRNA LINC01173 expression along with assessment of vitamin-D and B12 among the T2DM cases. Quantitative RT-PCR was used to analyze the expression of lncRNA LINC01173. Vitamin-D and B12 were analyzed by chemiluminescence-based assay.
Results: The present study observed that the T2DM cases had 6.67-fold increased lncRNA LINC01173 expression compared to healthy controls. Expression of lncRNA LINC01173 was found to be associated with hypertension (p=0.03), wound healing (p=0.04), and blurred vision (p< 0.0001). It was observed that the T2DM cases with vitamin-D deficiency had a significant association with fasting glucose level (p=0.01) and HbA1C level (p=0.01) among the T2DM cases. The association of lncRNA LINC01173 with vitamin-D was analyzed and it was observed that the vitamin-D deficient cases had higher lncRNA LINC01173 expression compared to insufficient T2DM cases (p=0.01) and sufficient T2DM cases (p=0.0006). It was also observed that the T2DM cases with smoking had a 8.33-fold lncRNA LINC01173 expression while non-smokers had a 5.43-fold lncRNA LINC01173 expression (p< 0.0001).
Conclusion: The study concluded that the increased lncRNA LINC01173 expression was observed to be linked with alteration in vitamin-D level and smoking habit. Altered expression of lncRNA LINC01173 expression was linked with fasting glucose and HbA1C alteration. Collectively, lncRNA LINC01173 expression, vitamin-D alteration, as well as smoking habit may cause the disease severity and increase the pathogenesis of disease.
Keywords: long noncoding RNA, vitamin-D, vitamin-B12, diabetes
Introduction
Type 2 diabetes mellitus is a long-term metabolic disorder marked by high blood glucose levels. According to the International Diabetes Federation (IDF), there were nearly 463 million diabetics worldwide in 2019, with the number expected to rise to 700 million by 2045.1 Type 1 diabetes mellitus, type 2 diabetes mellitus, and gestational diabetes mellitus are major categories of diabetes mellitus.2 Only type 2 diabetes mellitus (T2DM) accounts for approximately 90% of all diabetes mellitus cases worldwide.1 T2DM is caused by cell dysfunction or insulin resistance, and the risk of T2DM is influenced by both genetic and environmental factors.3 The regulatory network of pathophysiology of T2DM has continuously remained a focused area for researchers. In recent times, long noncoding RNAs (lncRNAs) have had widespread interest. A growing number of researchers have suggested that the lncRNAs play a vital role in progression and occurrence of T2DM.4,5 LncRNA also plays an important role in development of T2DM and its related complications.6,7
LncRNAs are nonprotein coding transcripts with more than 200 nucleotides.8 Previously, these lncRNAs were thought to be transcription noise; however, studies have revealed that lncRNA transcripts are involved in nearly all cell functions, including apoptosis,9 cell metabolism,10 differentiation,11,12 and proliferation.13,14 The abnormal expressions of lncRNA have been observed to play a crucial role in pancreatic β-cell regulation. β-cell exposure to frequently increased concentrations of glucose has a harmful influence on its function, which leads to faulty secretion of insulin and, finally, loss of cells by apoptosis in T2DM.3 Researchers found that the number of long noncoding RNAs (lncRNAs) was tightly regulated after performing whole genome transcriptome mapping. lncRNAs were activated in mature pancreatic cells but not in human embryonic pancreatic progenitor cells, implying that these lncRNAs are an important part of the – cell maturation and differentiation program.7 A report revealed that lncRNA alters the mRNA expression which contains risk for T2DM.6,15 In the last decade, studies have reported that lncRNAs exist in several fluids of the human body like plasma,16 urine,17 and serum.18 The probability of developing T2DM was substantially high in both males and females and it has been suggested that 25-OH D deficiency might contribute to T2DM development.19 Vitamin D deficiency is suggested to be one of the factors that influence many processes involved in T2DM.20 Vitamin B12 deficiency-associated peripheral neuropathy can also remain subclinical and interact with T2DM.21 It has also been said that T2DM patients who developed diabetic neuropathy had a lower level of vitamin B12.22
A previous study by Verma et al23 in 2022, to explore the association of FNDC-5 (Fibronectin type III domain-containing protein 5) and Selectin-E expression among obese and non-obese T2DM cases, observed significant involvement of FNDC-5 expression with serum vitamin-D and B12 level in an Indian population. Following the same methodology, we aimed to explore the role of lncRNAs LINC01173 expression and involvement of serum level of vitamin-D and B12 among the Saudi T2DM cases.
Materials and Methods
Sample Collection
This was a control based study which included 400 participants, 200 of whom were newly-diagnosed untreated T2DM and 200 were healthy controls. A 3 mL venous blood sample was withdrawn from all the Saudi-based study participants and collected in a plain tube, a glucose test was done from a 1 mL venous blood sample collected in a fluoride vial. For diagnosis of T2DM disease, all criteria were met, including fasting blood glucose (glucose level >126 mg/dL) and postprandial glucose (2-hour blood glucose >200 mg/dL) as well as glycated hemoglobin (HbA1C) being taken into consideration. The serum was collected from blood samples centrifuged at 1,500 rpm and stored at −80°C for further processing. T2DM cases with any other metabolic or organ-related disorder were excluded. T2DM cases from all age groups and both males and females were included.
This research study was approved by the research ethics committee, Ministry of Health, Saudi Arabia, and all participants gave their informed written consent before the study began. The present study was conducted at the College of Applied Medical Sciences, King Khalid University, Abha, Saudi Arabia and all the samples were taken from the associated general hospital.
Total RNA Extraction
Total RNA extraction was done from whole blood from all the study participants, using a RNA extraction kit (GeneAid, Taiwan) and further stored at −70°C in 2 mL nuclease free eppendorf tubes. The concentration and quality of total RNA was assessed by taking OD on a A260/280 ratio by nano-spectrophotometry method.
Complementary DNA Synthesis and QRT-PCR for lncRNAs Expression
Of the total extracted RNA from study participants, 100 ng were used to make cDNA using a kit (Verso, Thermo Scientific, USA) according to the kit’s instructions. Expression of lncRNAs LINC01173 was done by quantitative real-time PCR using SYBR green dye using specific primer sequences (forward primer: 5’-CTAGGCTCAGACCTGGCAAC-3’ and reverse primer: 5’-GGCCTTGGGGAAACGAAGAT-3’). The program followed: qRT-PCR for lncRNAs LINC01173, and β-actin was performed for 40 cycles, initial denaturation was at 94°C for 50 seconds, annealing temperature for, LINC01173, and β-actin was at 60°C for 50 seconds, extension at 72°C for 50 seconds, and a 20 µL reaction volume was used. To confirm the target amplification, an additional step at 72°C for 10 minutes was performed to finish the reaction and a melting curve examination was performed between 35°C and 90°C and every reaction was done in duplicate. The lncRNAs LINC01173 expression level was calculated using the relative quantification by 2−(ΔΔCT) method.
Vitamin D and B12 Level Assessment
Serum sample of T2DM cases were thawed and used in a chemiluminescence-based assay to determine serum vitamin D levels (Vitros XT7600 integrated system). Serum 25(OH) D levels ≤20 ng/mL was considered as Vitamin-D deficiency, 25(OH) D level <30 ng/mL considered as insufficiency and sufficiency as levels of 30 ng/mL or above.24 Vitamin B12 deficiency was defined as a serum concentration of less than 148 pmol/L, with 148 pmol/L being considered normal.25
Statistical Analysis
All the statistical testing was done using Graph Pad Prism software version 6.05. The Mann–Whitney U-test was used to determine whether there were any significant differences between the groups. The relative cycle threshold (Ct) method was used to analyze the QRT-PCR results and lncRNAs LINC01173, expression levels were calculated by the relative quantification method using the 2–(ΔΔCt). Up-regulation or down-regulation of expression was defined as a result of more than or less than one. All values were normalized relative to the control values, which were depicted as a value of 1. P-values less than 0.05 were considered to be significant.
Results
Demographic Characteristics
The demographic characteristics of T2DM cases and healthy controls are depicted in Table 1. In brief, a total of 400 study subjects were recruited, among whom 200 were newly-diagnosed T2DM cases and 200 were healthy controls. Among the T2DM cases, 54.5% were males and 45.5% were females, while, among the healthy controls, 57.5% were males and 42.5% were females. T2DM cases ≤40 years were 23.5% and >40 years were 76.5%, while, among the healthy controls, ≤40 years were 24.5% while >40 years were 75.5%; more details are described in Table 1.
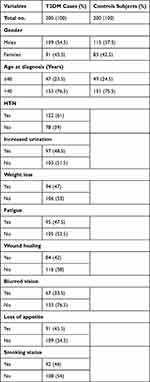 |
Table 1 Demographic and Clinical Characteristics of Enrolled Cases with Type 2 Diabetes Mellitus and Healthy Controls |
Association of lncRNA LINC01173 Expression Among the T2DM Cases with Clinic-Pathological Features
It was observed that lncRNAs LINC01173 showed an overall 6.67-fold relative expression among the T2DM cases compared to healthy controls (Table 2). T2DM cases in the age group ≤40 years showed a 5.60-fold lncRNAs LINC01173 expression while those >40 years showed a 7.12-fold lncRNAs LINC01173 (p=0.04). T2DM cases with hypertension history had a 7.11-fold lncRNAs LINC01173 expression while non-hypertensive cases had a 5.23-fold lncRNAs LINC01173 expression (p=0.03). T2DM cases with wound healing capacity had a 6.40-fold lncRNAs LINC01173 expression while, in contrast, had 7.27-fold lncRNAs LINC01173 expression (p=0.04). It was also observed that T2DM cases with blurred vision had a 7.10-fold lncRNAs LINC01173 in contrast with no blurred vision T2DM cases, who had a 4.27-fold lncRNAs LINC01173 expression (p<0.0001).
 |
Table 2 Long Non-Coding LINC01173 Expression in Type 2 Diabetes Mellitus Cases |
Association of Vitamin-D and B12 Level with Glucose Parameter Among T2DM Cases
Based on the status of Vitamin-D level among the T2DM cases (Deficient, Insufficient, Sufficient), glucose parameters were compared (Table 3). T2DM cases with vitamin-D deficiency showed a 284.5 mg/dL fasting glucose level while insufficient and sufficient T2DM cases had 250.2 mg/dL and 247.2 mg/dL fasting glucose levels, respectively (p=0.01). No such association with post prandial glucose level was observed with vitamin level. It was observed that the T2DM cases with vitamin deficiency had a 7.85% HbA1C level while insufficient and sufficient T2DM cases had 7.21% and 7.31% HbA1C level, respectively (p=0.01). No such association of glucose parameters was observed with B12 level.
 |
Table 3 Comparison of Glucose Parameter with Status of Vitamin-D and B12 Among the T2DM Cases |
Association of Long Non-Coding LINC01173 with Vitamin-D and B12 Among T2DM Cases
The association of lncRNA LINC01173 with vitamin-D was analyzed based on the level of vitamin-D (vitamin-D deficient, vitamin-D insufficient, and vitamin-D sufficient) (Figure 1A). It was observed that the vitamin-D deficient cases had higher lncRNA LINC01173 expression compared to insufficient T2DM cases (p=0.01) and sufficient T2DM cases (p=0.0006). Vitamin-D deficient T2DM cases showed a 10.78 (SD=7.41) fold higher lncRNA LINC01173 expression while vitamin-D insufficient had a 6.25 (SD=3.53) fold expression lncRNA LINC01173 and insufficient T2DM cases had a 5.96 (SD=3.33) fold lncRNA LINC01173 expression. Based on the B12 level two groups were made (deficient and normal level) and lncRNA LINC01173 expression was analyzed (Figure 1B), no such significant difference was observed in expression of lncRNA LINC01173 expression with respect to B12 deficient (6.67±3.75) and normal level (6.5±4.12).
Smoking and Their Association with lncRNA LINC01173 Expression
Based on the smoking habit of T2DM cases, lncRNA LINC01173 expression was analyzed. It was observed that the T2DM cases with smoking had a 8.33-fold (SD=3.96) lncRNA LINC01173 expression while non-smokers had a 5.43-fold (SD=3.58) lncRNA LINC01173 expression (p<0.0001) (Figure 2).
 |
Figure 2 lncRNA LINC01173 expression and its association with smoking and nonsmoking habit of T2DM cases. |
Discussion
The role of long noncoding RNAs (lncRNAs) in the occurrence and progression of T2DM has gotten a lot of attention in recent years. Despite their lack of ability to code for proteins, there is growing evidence that lncRNAs play an important role in gene regulation.26 In the pathogenesis of T2DM and T2DM-related complications, lncRNAs also play an important role.27 It has been demonstrated that lncRNAs have clinical utility and can be used as biomarkers for disease diagnosis.28 There are only a few studies done to explore the role of LINC01173 expression in T2DM, therefore the present study analyzed the expression of lncRNA LINC01173 among the T2DM cases and observed to be high compared to control. It was observed that the T2DM cases with hypertension, no wound healing and who had blurred vision had higher lncRNA LINC01173 comparatively. A study by Hu et al26 reported that the expression of lncRNA LINC01173 expression was high among the obese T2DM cases. We explored the association of lncRNA LINC01173 expression with Vitamin-D and B12 level and it was observed that the T2DM cases with Vitamin-D deficiency had higher expression of lncRNA LINC01173 compared to insufficient and sufficient T2DM cases while no significant association was observed with B12 level among the T2DM cases. Vitamin D has been found to play a role in glucose homeostasis via a number of mechanisms related to the insulin signaling pathway and takes part in nourishing of normal level of Ca2+ and reactive oxygen species (ROS) both in pancreatic beta cells. It is well known that T2DM is linked to increased oxidative stress and vitamin D deficiency.29 Vitamin D supplementation was also found to improve glycemic control and reduce insulin resistance in people at high risk of developing diabetes.30 In diabetic patients, vitamin D may help to reduce the negative effects of oxidative stress. Diabetes is linked to an increased level of oxidative damage, including DNA damage, as a result of chronic hyperglycemia.31 Vitamin D is also involved in a number of cellular processes that maintain glucose and lipid metabolism homeostasis, and growing evidence suggests that vitamin D deficiency is linked to the development of diabetes, insulin resistance, insulin signaling, and inflammation.32 In the present study it was observed that the T2DM cases with vitamin-D deficiency had high fasting glucose and HbA1C compared to insufficient and sufficient. The impact of vitamin D on the risk of developing type 2 diabetes has been studied in a Nurses Health Study which suggested a reduced vitamin-D level in T2DM.33 Hang Zhao et al34 suggested that Vitamin D had a clinically positive impact on glucose level, particularly on hemoglobin HbA1c reduction, and hyperglycemia induced-oxidative stress. Vitamin D has also been suspected as a diabetes risk modifier based on basic and animal studies and vitamin D insufficiency has long been suspected as a risk factor for diabetes.35 It was also observed that the T2DM cases with GAD autoantibody showed higher lncRNA LINC01173 expression while lower expression was observed comparatively. T2DM cases with smoking and habit also showed higher lncRNA LINC01173 expression compared to nonsmoker T2DM cases.
Conclusion
The present study concludes that the risk of T2DM may be associated with higher expression of lncRNA LINC01173 and alteration in vitamin-D level, and smoking habit was linked with altered lncRNA LINC01173 expression. Alteration in vitamin-D was also linked with fasting glucose and HbA1C alteration. Collectively, increased expression of lncRNA LINC01173, vitamin-D alteration, and smoking habit may be the cause of disease severity and increase the pathogenesis of disease.
Data Sharing Statement
We confirm that the data used during the research will not be shared with anybody/broadcasted in any public domain. The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics and Informed Consent
This research study was ethically approved by the research ethics committee, Ministry of Health, K.S.A., and all participants gave their written informed consent before the study began, and all the ethical principles regarding human experimentation were followed and the study was conducted in accordance with the Declaration of Helsinki.
Acknowledgments
This work is supported by Scientific Research Deanship at King Khalid University, Abha, Saudi Arabia for their financial support through the Small Research Group Project under grant number (RGP.01-115-43). Alanoud Aladel would also like to extend her appreciation to the deanship of scientific research at King Saud University, Riyadh, Saudi Arabia for funding this work through the research chair’s programme.
Disclosure
The authors declare that they have no conflicts of interest.
References
1. IDF Diabetes Atlas, 9th edn. Brussels, and Belgium, “IDF Diabetes Atlas, 9th edn”; 2019. Available from: https://www.diabetesatlas.org.
2. Ogurtsova K, da Rocha Fernandes JD, Huang Y, et al. IDF Diabetes Atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract. 2017;128:40–50. doi:10.1016/j.diabres.2017.03.024
3. Ruan Y, Lin N, Ma Q, et al. Circulating LncRNAs analysis in cases with type 2 diabetes reveals novel genes influencing glucose metabolism and Islet β-cell function. CPB. 2018;46(1):335–350.
4. Ponting CP, Oliver PL, Reik W. Evolution and functions of long noncoding RNAs. Cell. 2009;136(4):629–641. doi:10.1016/j.cell.2009.02.006
5. Chen Z. Progress and prospects of long noncoding RNAs in lipid homeostasis. Mol Metab. 2016;5(3):164–170. doi:10.1016/j.molmet.2015.12.003
6. Akerman I, Tu Z, Beucher A, et al. Human pancreatic β cell lncRNAs control cell-specific regulatory networks. Cell Metab. 2017;25(2):400–411. doi:10.1016/j.cmet.2016.11.016
7. Il Jang W, Kim M-S, Kang SH, et al. Association between metformin use and mortality in cases with type 2 diabetes mellitus and localized resectable pancreatic cancer: a nationwide population-based study in Korea. Oncotarget. 2017;8(6):9587–9596. doi:10.18632/oncotarget.14525
8. He X, Ou C, Xiao Y, Han Q, Li H, Zhou S. LncRNAs: key players and novel insights into diabetes mellitus. Oncotarget. 2017;8(41):71325–71341. doi:10.18632/oncotarget.19921
9. Lu W, Huang SY, Su L, Zhao BX, Miao JY. Long noncoding RNA LOC100129973 suppresses apoptosis by targeting miR-4707-5p and miR-4767 in vascular endothelial cells. Sci Rep. 2016;6(1):21620. doi:10.1038/srep21620
10. Liu X, Xiao Z-D, Han L, et al. LncRNA NBR2 engages a metabolic checkpoint by regulating AMPK under energy stress. Nat Cell Biol. 2016;18(4):431–442. doi:10.1038/ncb3328
11. Alvarez-Dominguez JR, Bai Z, Xu D, et al. De Novo reconstruction of adipose tissue transcriptomes reveals long non-coding RNA regulators of brown adipocyte development. Cell Metab. 2015;21(5):764–776. doi:10.1016/j.cmet.2015.04.003
12. Qi M, Zhou Q, Zeng W, et al. Analysis of long non-coding RNA expression of lymphatic endothelial cells in response to type 2 diabetes. CPB. 2017;41(2):466–474.
13. Luo G, Liu D, Huang C, et al. LncRNA GAS5 Inhibits Cellular Proliferation by Targeting P27Kip1. Mol Cancer Res. 2017;15(7):789–799. doi:10.1158/1541-7786.MCR-16-0331
14. Jin F, Wang N, Zhu Y, You L, Wang L, De W, Tang W. Downregulation of long noncoding RNA Gas5 affects cell cycle and insulin secretion in mouse pancreatic β cells. CPB. 2017;43(5):2062–2073.
15. Senée V, Chelala C, Duchatelet S, et al. Mutations in GLIS3 are responsible for a rare syndrome with neonatal diabetes mellitus and congenital hypothyroidism. Nat Genet. 2006;38(6):682–687. doi:10.1038/ng1802
16. Tan L, Yang Y, Shao Y, Zhang H, Guo J. Plasma lncRNA‑GACAT2 is a valuable marker for the screening of gastric cancer. Oncol Lett. 2016;12(6):4845–4849. doi:10.3892/ol.2016.5297
17. Terracciano D, Ferro M, Terreri S, et al. Urinary long noncoding RNAs in nonmuscle-invasive bladder cancer: new architects in cancer prognostic biomarkers. Transl Res. 2017;184:108–117. doi:10.1016/j.trsl.2017.03.005
18. Shen X, Zhang Y, Wu X, et al. Upregulated lncRNA-PCAT1 is closely related to clinical diagnosis of multiple myeloma as a predictive biomarker in serum. Cancer Biomark. 2017;18(3):257–263. doi:10.3233/CBM-160158
19. Albannawi G, Alsaif S, Alsaif G, et al. Vitamin D deficiency among type 2 diabetes patients in Saudi Arabia: a systematic review. Int J Diabetes Dev Ctries. 2020;3(12)197–203.
20. Al Dawish MA, Robert AA, Braham R, et al. Diabetes Mellitus in Saudi Arabia: a review of the recent literature. Curr Diabetes Rev. 2016;12(4):359–368. doi:10.2174/1573399811666150724095130
21. Briani C, Dalla Torre C, Citton V, et al. Cobalamin deficiency: clinical picture and radiological findings. Nutrients. 2013;5(11):4521–4539. doi:10.3390/nu5114521
22. Wang D, Zhai JX, Liu DW. Serum folate, vitamin B12 levels and diabetic peripheral neuropathy in type 2 diabetes: a meta-analysis. Mol Cell Endocrinol. 2017;443:72–79. doi:10.1016/j.mce.2017.01.006
23. Verma AK, Aladel A, Dabeer S, et al. Clinical Importance of FNDC-5 and selectin-E mRNA expression among type 2 diabetics with and without obesity, diabetes, metabolic syndrome and obesity. Targets Ther. 2022;15:1011.
24. Lips P. Vitamin D deficiency and secondary hyperparathyroidism in the elderly: consequences for bone loss and fractures and therapeutic implications. Endocr Rev. 2001;22(4):477–501. doi:10.1210/edrv.22.4.0437
25. Finkelstein JL, Layden AJ, Stover PJ. Vitamin B-12 and Perinatal Health. Adv Nutr. 2015;6(5):552–563. doi:10.3945/an.115.008201
26. Hu W, Ding Y, Wang S, Xu L, Yu H. The construction and analysis of the aberrant lncRNA-miRNA-mRNA network in adipose tissue from type 2 diabetes individuals with obesity. J Diabetes Res. 2020;2020:3980742. doi:10.1155/2020/3980742
27. Abdulle LE, Hao JL, Pant OP, et al. MALAT1 as a diagnostic and therapeutic target in diabetes-related complications: a promising long-noncoding RNA. Int J Med Sci. 2019;16(4):548–555. doi:10.7150/ijms.30097
28. Amir H, Khan MA, Feroz S, et al. CARLo-7-A plausible biomarker for bladder cancer. Int J Exp Pathol. 2019;100(1):25–31. doi:10.1111/iep.12305
29. Lee WC, Mokhtar SS, Munisamy S, Yahaya S, Rasool AHG. Vitamin D status and oxidative stress in diabetes mellitus. Cell Mol Biol. 2018;64(7):60–69. doi:10.14715/cmb/2018.64.7.11
30. Mirhosseini N, Vatanparast H, Mazidi M, Kimball SM. Vitamin D supplementation, glycemic control, and insulin resistance in prediabetics: a meta-analysis. J Endocr Soc. 2018;2(7):687–709. doi:10.1210/js.2017-00472
31. Elmarakby AA, Sullivan JC. Relationship between oxidative stress and inflammatory cytokines in diabetic nephropathy. Cardiovasc Ther. 2012;30(1):49–59. doi:10.1111/j.1755-5922.2010.00218.x
32. Szymczak-Pajor I, Drzewoski J, Śliwińska A. The molecular mechanisms by which vitamin D prevents insulin resistance and associated disorders. Int J Mol Sci. 2020;21(18):6644. doi:10.3390/ijms21186644
33. Mitri J, Dawson-Hughes B, Hu FB, Pittas AG. Effects of vitamin D and calcium supplementation on pancreatic β cell function, insulin sensitivity, and glycemia in adults at high risk of diabetes: the Calcium and Vitamin D for Diabetes Mellitus (CaDDM) randomized controlled trial. Am J Clin Nutr. 2011;94(2):486–494. doi:10.3945/ajcn.111.011684
34. Zakhary CM, Rushdi H, Hamdan JA, et al. Protective role of vitamin D therapy in diabetes mellitus type II. Cureus. 2021;13(8):e17317. doi:10.7759/cureus.17317
35. Mathieu C, Badenhoop K. Vitamin D and type 1 diabetes mellitus: state of the art. Trends Endocrinol Metab. 2005;16(6):261–266. doi:10.1016/j.tem.2005.06.004
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

