Back to Journals » Cancer Management and Research » Volume 10
Association of IL-8 gene promoter -251 A/T and IL-18 gene promoter -137 G/C polymorphisms with head and neck cancer risk: a comprehensive meta-analysis
Authors Wang Z, Gao ZM, Huang HB, Sun LS, Sun AQ, Li K
Received 15 February 2018
Accepted for publication 13 April 2018
Published 10 August 2018 Volume 2018:10 Pages 2589—2604
DOI https://doi.org/10.2147/CMAR.S165631
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Antonella D'Anneo
Zheng Wang,1 Zi-Ming Gao,2 Hai-Bo Huang,2 Li-Sha Sun,2 An-Qi Sun,2 Kai Li2
1Department of Otorhinolaryngology, the First Affiliated Hospital of China Medical University, Shenyang 110001, China; 2 Department of Surgical Oncology, the First Affiliated Hospital of China Medical University, Shenyang 110001, China
Purpose: No consensus exists on the impact of polymorphisms in cytokines (such as interleukin IL-8 and IL-18) on cancer risk; moreover, there is very little evidence regarding head and neck cancer (HNC).
Methods: Thus, a meta-analysis including 22 studies with 4731 cases and 8736 controls was conducted to evaluate this association. The summary odds ratio (OR) and corresponding 95% confidence intervals (CIs) for C-X-C motif chemokine ligand 8 (CXCL8, which encodes IL-8) and IL-18 polymorphisms and HNC risk were estimated.
Results: The results showed a significantly increased risk of HNC susceptibility for IL18-137 G/C in five genetic models, but, interestingly, no significant association was found for the CXCL8-251 A/T polymorphism. When stratified by cancer type, an increased risk of nasopharyngeal cancer was found for both -137 G/C and -251A/T. When the studies were stratified by ethnicity and genotyping method, there were significant associations between Asian populations and polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) studies for -137 G/C, and African populations for -251 A/T in some genetic models. A positive association was also found between the population-based groups in some models for -137 G/C; conversely, significantly decreased risk was found among the -251 A/T hospital-based group. Meta-regression was also conducted. The publication year, control source, and cancer type contributed to CXCL8 -251 A/T heterogeneity; however, no factors were found that contributed to IL-18 -137 G/C heterogeneity. Marginal significance was found in the recessive model for IL-18 -137 G/C by Egger’s test, whereas no publication bias was detected for CXCL8 -251 A/T.
Conclusions: The results indicate that the IL-18 -137 G/C polymorphism is associated with HNC risk, especially nasopharyngeal cancer, in Asian populations and, when using PCR-RFLP, CXCL8 -251 A/T polymorphisms play a complex role in HNC development.
Keywords: interleukin-8, interleukin-18, polymorphism, head and neck cancer, meta-analysis
Introduction
Head and neck cancer (HNC), which encompasses malignant tumors of the larynx, pharynx, oral cavity, thyroid, and other related areas, is the 7th most common solid malignancy in the world, with approximately 686,000 new cases annually.1 Although the exact pathogenetic mechanisms of HNC are still undefined, evidence indicates that HNC carcinogenesis is a complicated, multistep, and multifactorial process, involving genetic factors, tobacco smoking, alcohol consumption, viral infection, ultraviolet radiation, lifestyle, and environmental factors.2–4 However, although many people are exposed to these extrinsic factors, only a small proportion develop HNC,5 and a family history of HNC in first degree relatives elevates the risk.6 This indicates the existence of genetic predisposition, and suggests that certain genetic factors, such as genes involved in inflammation, might be associated with the pathogenesis of HNC. Inflammation, which is associated with DNA damage, angiogenesis, proliferation, invasion, and metastasis, may play an important role in the oncogenesis and progression of HNC.7,8 Recent studies suggest an association between HNC and increased pro-inflammatory cytokines. Among these cytokines, interleukin (IL)-8 and IL-18 have attracted increasing attention.9–11
As a member of the chemokine family, IL-8 is encoded by the C-X-C motif chemokine ligand 8 (CXCL8) gene, which is located on chromosome 4q-13-21 in humans. CXCL8 consists of a proximal promoter region, four exons, and three introns.12 It is produced by a wide range of healthy cells (such as neutrophils, monocytes, endothelial cells, and fibroblasts), and several types of tumor cells.13,14 Present evidence suggests that IL-8 promotes angiogenesis, tumorigenesis, tissue invasion, and metastasis.15–17 Although CXCL8 contains several polymorphic sites, only three common polymorphisms have been reported in the coding sequence: +251 A/T, +396 G/T, and +781 C/T.18 Polymorphisms may play important roles in some cancers, including ovarian, breast, bladder, and others.19–22 An A/T single nucleotide polymorphism (SNP) is located at position -251 of CXCL8 in the transcription start site, which is associated with IL-8 expression.23–26 A large number of studies have investigated associations between the CXCL8 -251 A/T (rs4073) gene polymorphism and the risk of human cancers, with conflicting conclusions.27–32
IL-18 is also a pro-inflammatory cytokine. A member of the IL-1 super-family, it is known as an inducer of interferon-γ.33 IL-18 is produced by a number of cell types, such as activated blood and tissue monocytes/macrophages, Kupffer cells, T and B cells, osteoblasts, dendritic cells (DCs), microglia, and epithelial cells.34 IL-18 induces the activation of natural killer cells and the proliferation of activated T cells, affecting both innate and acquired immunity.35
IL-18 is also associated with tumorigenesis, and has been reported to contribute to both anticancer and procancer processes.36–39 The human IL-18 gene is located on chromosome 11q22.1–q22.3.40 Three SNPs in the IL-18 promoter regions have been identified that could alter IL-18 expression (-137, -607, and -656).41 However, the most commonly investigated and the most biochemically functional polymorphism is the -137 G>C (rs187238) polymorphism. Numerous studies have reported a role for the -137 G>C polymorphism in the susceptibility to various cancers, whereas the results of these studies mainly display that there is no association between IL-18 -137 G>C and cancer risk,42,43 but, interestingly, in our present meta-analysis, we got a completely different result.
To our knowledge, no quantitative summary of the evidence regarding the association of CXCL8 and IL-18 polymorphisms with HNC risk has been reported, and existing studies regarding the association of CXCL8 or IL-18 with other types of cancers are contradictory. Therefore, we conducted a meta-analysis to quantitatively summarize the evidence and estimate relationships using subgroups analysis, meta regression, sensitivity analysis, and evaluation of publication bias.
Methods
Publication search and inclusion criteria
We searched the PubMed, Embase, Cochrane Library, and China National Knowledge Infrastructure databases for all potentially eligible articles up to November 31, 2017 on the association between CXCL8 and IL18 polymorphisms and HNC risk. The search terms used were “IL-8”, “CXCL8”, “IL-18”, “polymorphism”, “head and neck cancer”, “nasopharyngeal cancer”, “oral cancer”, “laryngeal cancer”, “esophageal cancer”, “thyroid cancer”, “tongue cancer”, and “mouth neoplasm”. We also screened the references of review articles and meta-analyses articles. Primary searches resulted in 176 abstracts (Figure 1). An additional six studies were added from meta-analyses and review articles, for a total of 182 studies. For inclusion, identified studies had to: (1) be case-controlled; (2) study the association between CXCL8 or IL-18 and HNC risk; (3) provide sufficient data to calculate a P-value and odds ratio (OR) with 95% confidence interval (CI); and (4) contain control subjects that conformed to the Hardy-Weinberg equilibrium (HWE).
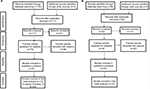  | Figure 1 Flowchart for identification of IL-8 (A) and IL-18 (B) studies. |
Data extraction
Data were extracted independently by two investigators from studies that met the inclusion criteria. Discrepancies were resolved by discussion between the authors to reach an agreement. The following information was recorded for each study: first author, year of publication, region, cancer type, genotyping methods, source of controls, the number of cases and controls for each genotype, and HWE score in the control groups (Table 1).
  | Table 1 Characteristics of literature included in the meta-analysis Note: Superscripted a, b, c and d are parts of one study by Campa et al.49 Abbreviations: HWE, Hardy Weinberg equilibrium; PCR-RFLP: polymerase chain reaction-restriction fragment length polymorphism; AS-PCR, alleles specific polymerase chain reaction; HNSCC, head and neck squamous cell carcinoma; PCR-HRM, polymerase chain reaction-high resolution melt; PCR-FRET, polymerase chain reaction-fluorescence resonance energy transfer. |
Statistical analysis
Statistical analyses were performed using Stata 14.0 software (Stata Corporation, College Station, TX, USA). To evaluate the deviation of the CXCL8 and IL-18 polymorphisms from HWE, a chi-square test was used to evaluate the control subjects, in which P<0.05 suggested a significant deviation from HWE. The OR corresponding to the 95% CI was used to evaluate the association between CXCL8 and IL-18 polymorphisms and HNC risk. The present study was performed using allelic, homozygote, heterozygote, dominant, and recessive models. Stratified analyses based on cancer types, ethnicity, genotyping methods, and the sources of controls and DNA samples were quantified with ORs and 95% CIs. If a cancer type was included in only one study, it was combined into the “other cancers” group. The statistical heterogeneity was evaluated using the Q-test and I2 statistics. When heterogeneity existed (P<0.10, I2>50%), the random effect model was used:44 otherwise, the Mantel-Haenszel method was applied to calculate ORs in a fixed-effect model.45 Stratified and meta-regression analyses were used to explore sources of heterogeneity. Moreover, sensitivity analysis was performed to assess the stability of the results by sequentially removing each study and evaluating the stability of the results. Publication bias was analyzed by Begg’s funnel plot and Egger’s test.46,47
Results
Study characteristics
After a comprehensive search, 182 relevant articles were initially retrieved. After screening the titles and abstracts, 160 articles were excluded, 13 papers were duplicated, 21 papers were reviews, 80 papers were not associated with SNPs in CXCL8 or IL-18, 27 papers were not about HNC, 16 papers lacked relative data, and three papers were not consistent with HWE (Figure 1). Finally, 22 articles but 25 case-control studies (15 for CXCL8 and 10 for IL-18) covering 4731 cases and 8736 controls met our inclusion criteria (Figure 1). In terms of genotyping methods, for CXCL8, five articles used polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP), two articles used TaqMan assays, and five articles used other methods; for IL18, four articles used PCR-RFLP, three articles used allele specific-PCR, and three articles used other methods. The genotype distributions of the studied SNPs in the control groups agreed with HWE (P>0.05). All Newcastle-Ottawa Scale (NOS) scores were greater than 5 stars, indicating good articles. The study characteristics are shown in Table 1.
Quantitative synthesis
As shown in Tables 2 and 3, when all eligible studies were pooled together, a significant association between the 137 G/C polymorphism and HNC was observed under all gene models: (C vs G: OR=1.31, 95% CI=1.09–1.57, Pheterogeneity=0.002; CC vs GG: OR=1.69, 95% CI=1.20–2.38, Pheterogeneity=0.175; GC vs GG: OR=1.27, 95% CI=1.01–1.59, Pheterogeneity=0.004; GC+CC vs GG: OR=1.32, 95% CI=1.06–1.65, Pheterogeneity=0.002;CC vs GG+GC: OR=1.53, 95%CI=1.13–2.06, Pheterogeneity=0.321), whereas no significant association was found between the 251 A/T polymorphism and HNC risk.
Furthermore, to evaluate the effect of specific factors on the results, we performed subgroup analysis, concentrating on cancer type, ethnicity, genotyping method, and DNA sample (Tables 2 and 3 and Figure 2). Stratified analysis revealed that the overall association was partly attributed to nasopharyngeal cancer for -251 A/T (AT vs TT: OR=1.39, 95%CI=1.01–1.92, Pheterogeneity=0.242) and -137 G/C (C vs G: OR=1.48, 95% CI=1.20–1.83, Pheterogeneity=0.222; CC vs GG: OR=2.01, 95% CI=1.30–3.10, Pheterogeneity=0.598; GC vs GG: OR=1.53, 95% CI=1.22–1.93, Pheterogeneity=0.365; GC+CC vs GG: OR=1.60, 95% CI=1.26–2.02, Pheterogeneity=0.306; CC vs GG+GC: OR=1.72, 95% CI=1.12–2.63, Pheterogeneity=0.664). In the subgroup analysis based on ethnicity, the results suggested a significantly increased risk of HNC in an African population with -251 A/T (A vs T: OR=1.63, 95% CI=1.19–2.22, Pheterogeneity=NA; AA vs TT: OR=2.46, 95% CI=1.31–4.64, Pheterogeneity=NA; AA+AT vs TT: OR=1.81, 95% CI=1.15–2.84, Pheterogeneity=NA; AA vs AT+TT: OR=1.91, 95% CI=1.08–3.39, Pheterogeneity=NA) and in an Asian population for -137 G/C (C vs G: OR=1.40, 95% CI=1.13–1.74, Pheterogeneity=0.012; CC vs GG: OR=2.03, 95% CI=1.38–2.99, Pheterogeneity=0.332; GC+CC vs GG: OR=1.41, 95% CI=1.06–1.86, Pheterogeneity=0.004; CC vs GG+GC: OR=1.83, 95% CI=1.28–2.61, Pheterogeneity= 0.469); however, no significant association was found in Asian or European populations for -251 A/T or in European or African populations for -137 G/C. In subgroup analysis by genotyping methods, a significantly elevated risk was found in the PCR-RFLP group for -137 G>C (C vs G: OR=1.38, 95% CI=1.04–1.83, Pheterogeneity=0.050; CC vs GG: OR=1.84, 95% CI=1.19–2.83, Pheterogeneity=0.503; GC+CC vs GG: OR=1.44, 95% CI=1.03–2.03, Pheterogeneity=0.053; CC vs GG+GC: OR=1.61, 95% CI=1.06–2.46, Pheterogeneity=0.663), but no significant association was found for -251 A/T with any genotyping method. Stratification by the control source showed significant associations between the population-based group (C vs G: OR=1.27, 95% CI=1.02–1.59, Pheterogeneity=0.016; GC+CC vs GG: OR=1.30, 95% CI=1.01–1.67, Pheterogeneity=0.035) and the hospital-based group (CC vs GG: OR=2.14, 95% CI=1.11–4.12, Pheterogeneity=0.137, CC vs GG+GC: OR=1.92, 95% CI=1.04–3.52, Pheterogeneity=0.181) and -137 G>C, whereas significantly decreased risk was found in the hospital-based group for -251 A/T (AA vs AT+TT: OR=0.54, 95% CI=0.32–0.89, Pheterogeneity=0.312).
Evaluation of heterogeneity
There was heterogeneity among the studies in all overall comparisons and subgroup analyses for CXCL8 -251 A/T and some overall comparisons and subgroup analyses (C vs G, GC vs GG, GC/CC vs GG) for IL-18 -137 G/C. To explore the sources of heterogeneity, we performed meta-regression using publication year, cancer type, ethnicity, source of controls, sample size (≤500 and >500 subjects) and genotyping method as covariables. The results suggested that the publication year (AA vs AT/TT: P=0.020; AA vs TT: P=0.041), source of control (AA vs AT/TT: P=0.024), and cancer type (AA/ATvs TT: P=0.047; AT vsTT: P=0.036) may contribute to the heterogeneity for CXCL8 -251 A/T; however, for IL18 -137 G/C, no factors contributing to the heterogeneity were found. Moreover, for CXCL8 -251 A/T, our meta-regression analyses revealed that the publication year could explain 88.38% (AA vs AT/TT), 54.62% (AA vs TT) of the τ2, the source of controls could explain 73.22% (AA vs AT/TT) of the τ2, and the cancer type could explain almost 100% (AA/AT vs TT; AT vs TT) of the τ2.
Sensitivity analysis
To evaluate the sensitivity of the meta-analysis, we omitted one study at a time and checked for significant differences. There were no significant differences observed upon removal of any of the studies, indicating that the results are statistically reliable (Figure 3).
  | Figure 3 Sensitivity analysis of the overall ORs. The results were calculated through omitting each eligible study. (A) IL-8 -251 A/T in A versus T model; (B) IL-18 -137 G/C in C versus G model. Note: Superscripted a, b, c and d are parts of one study by Campa et al.49 Abbreviations: OR, odds ratio; CI, confidence interval. |
Publication bias
A Begg funnel plot was generated, and Egger’s test was performed to evaluate the publication bias of the studies included in our analysis. Figure 3 displays funnel plots examining the CXCL8 -251 A/T and IL-18 -137 G/C polymorphisms and cancer risk. There was marginal significance in the recessive model (CC vs GC+GG: P=0.048) for IL-18 -137 G/C in the Egger’s test, while no publication bias was detected in any genetic comparison for CXCL8 -251 A/T (Figure 4).
Discussion
To our knowledge, this is the first meta-analysis to assess the association of the CXCL8 -251 A/T and IL-18 -137 G/C polymorphisms with HNC risk. In our present study, a significantly elevated risk was observed for IL-18 -137 G/C, but no strong association between the CXCL8 -251 A/T polymorphism and HNC risk was found in the overall analysis.
Further subgroup analyses revealed that the association between IL-18 -137 G/C and HNC risk was more predominant among nasopharyngeal cancer groups, Asian populations, studies using PCR-RFLP for genotyping, and population- and hospital-based studies. To elaborate more specifically, C alleles carriers and CC genotypes were significantly associated with an increased risk in nasopharyngeal cancer groups, Asian populations, and samples analyzed by PCR-RFLP. Moreover, an association between C allele carriers and increased risk was found in the population-based group, whereas, in the hospital-based group, an increased risk for HNC was found with the CC genotype.
Conversely, stratified analysis of CXCL8 -251 A/T provided evidence that A allele carriers and AA genotypes were associated with a significantly increased risk in African populations. Moreover, AT genotypes were associated with a significantly increased risk in the nasopharyngeal cancer group compared with the TT genotype, whereas the AA genotype was associated with a significantly decreased risk in hospital-based studies.
To some extent, our results are distinctly different from those of previous studies. Several studies in recent years have investigated these polymorphisms, with conflicting results. For CXCL8 -251 A/T, our pooled results are in accordance with some previous studies,24,25 but contrast with studies reporting a positive association between the CXCL8 -251 A/T polymorphism and cancer risk.19,48 On the other hand, for IL-18 -137 G/C, our pooled results were completely opposite to those reported by Yang et al43 and Mi et al.42
Although the results of overall analysis are sometimes the same, similar or different trends may be found in subgroup analyses. For example, for CXCL8-251 A/T, stratification by ethnicity revealed a significant association for African populations with carriers of the A allele and homozygous AA genotypes, whereas, among non-African populations, there were no strong associations in any of the genetic models. Our results are in accordance with Gao et al,28 who also observed significantly elevated risks in African populations. However, diverse results were observed in other previous studies, Wang et al22 found that carriers of the -251 A allele among African and Asian populations were at a higher risk for cancer. Wang et al27 found that there were significant risks among Asians for both A allele carriers and AA individuals; however, no significant associations were found in non-Asian populations. On the contrary, when the IL-18 -137 G/C, data was stratified by ethnicity, C alleles carriers and CC genotypes in Asian populations were significantly associated with an increased risk of HNC. This result is consistent with the reports of Yang et al43 and Mi et al,42 who found that C alleles and CC genotypes elevated the risk of cancers. These results indicate that CXCL8 -251 A/T and IL-18 -137 G/C polymorphisms may be crucial in HNC patients of specific ethnicities. The reasons for these discrepancies are not known. We hypothesize that they may be attributable to gene–environmental interactions. Variation in the allele frequency of particular polymorphisms might differ among ethnicities as a result of disparate environmental effects, and it can be inferred from the different allele distribution, in accordance with natural selection principles, that the rare allele carriers in the African populations for CXCL8 or Asian populations for IL-18 might be eliminated compared to in other ethnicities. This might explain why a significant association was observed in certain ethnicities, but not others. It should be noted that, for decades, nasopharyngeal cancer was more common in African and Asian populations compared with other ethnicities, consistent with the associations between nasopharyngeal cancer and the two polymorphisms studied here in these populations.49 The differences could also be due to small sample size or potential reporting bias in our study. For example, the African CXCL8 -251 A/T cohort contained approximately 329 subjects, and there is only one study in the African group.50 Therefore, it was underpowered to find causal positive or negative associations.
Cancer type was also used as a stratifying factor, and we found significantly elevated risk for nasopharyngeal cancer in the heterozygote model (AT vs TT) for CXCL8-251 A/T and in all genetic models for IL18 -137 G/C; however, we did not find any associations with any other cancer types in any genetic models. This is consistent with the results of Gao et al28 and Wang et al.22 These findings indicate that the A allele of CXCL8 -251 A/T and the C allele of IL-18 -137 G/C are risk factors for developing nasopharyngeal cancer. The mechanism for these findings is not well understood.
However, a number of studies have investigated the significant biochemical functions of CXCL8 or IL-18, and the effects of particular polymorphisms. The A/T and G/C SNPs studied here are, respectively, associated with the production of CXCL8, IL-8 transcriptional activity, and the expression of IL-8 and IL-18,23–26,41 which are associated with cancer characteristics, such as increased proliferation, invasion, and migration.16,51,52 Hence, the A allele, C allele, and CC genotype may contribute to tumorigenesis and metastasis.
Selection bias in control subject recruitment was a significant source of concern. When stratified by control source, we found significantly decreased risk among hospital-based studies for CXCL8 -251 A/T (AA vs AT+TT), whereas, for IL-18 -137 G>C, we observed significantly increased risk for C allele carriers (C vs G, GC+CC vs GG) among the population-based group and for the CC genotype (CC vs GG, CC vs GG+GC) among the hospital-based group. Considering that hospital-based controls lack the representativeness of population-based controls, and the amount of studies used was small, we must be cautious with our conclusions. When stratified by genotyping methods, we did not find any statistical association in any genetic models for CXCL8 -251 A/T; however, a strong association was found in all genetic models except the heterozygote model (GC vs GG) for IL-18 -137 G/C. This is the first report of this association in the literature.
One of the major concerns in a meta-analysis is heterogeneity among the included studies, because imprecise results may be obtained as a result of non-homogeneous data. In our study, the Q-test and I2 statistics were used to test the significance of heterogeneity. Significant heterogeneity was found in all pooled and subgroup analyses for CXCL8 -251 A/T and some for IL-18 -137 G/C, and meta-regression was performed for the corresponding genetic models according to cancer type, ethnicity, source of controls, sample size, genotyping method, and publication year to explore its source. We found that publication year, source of controls, and cancer type were the main sources of heterogeneity for CXCL8 -251 A/T; however, these did not impact the overall summary effect. Meanwhile we did not find any factors contributing to heterogeneity in the three corresponding genetic models (C vs G, GC vs GG and GC +CC vs GG) for IL-18 -137 G/C. This is a common phenomenon in meta-regression analyses, and is permitted.
Importantly, sensitivity analyses indicated that the estimated summary effect was robust, and did not change when individual studies were excluded.
There were several limitations to this meta-analysis. First, in the stratified analyses, we could not perform subgroup analysis by age, smoking status, alcohol consumption, treatment, and other risk factors because of insufficient data in the included studies. A more precise analysis could be conducted if these data were comprehensive. Second, tumorigenesis depends on both genetic and environmental factors, but gene–gene and gene–environment interactions were not taken into account in our meta-analysis, again because of a lack of data. Third, heterogeneity is a potential problem which may preclude the acquisition of accurate results in a meta-analysis. Significant heterogeneity was observed in some genetic models, which may have resulted from the publication year, source of controls, and cancer type. Fourth, although most controls were selected from healthy populations, a few studies selected controls from inpatients with cancer-free histories or no family history of cancer and other diseases. In addition, we used Begg’s funnel plot and Egger’s test to assess the publication bias, and found a marginal significance in the recessive model for IL-18 -137 G/C by Egger’s test, possibly because only published studies were included in the meta-analysis. Last, most of the subjects included were of Asian and European ancestry, with only one study for each of CXCL8 -251A/T and IL-18 -137 G/C regarding African ethnicity; therefore, potential selective bias could exist which might contribute to insufficient statistical power.
In conclusion, the present meta-analysis suggests that the IL-18 -137 G/C polymorphism contributes to the susceptibility of HNC, especially among nasopharyngeal cancer groups, Asian populations, studies using PCR-RFLP for genotyping, and population- and hospital-based studies in some genetic comparisons. The CXCL8 -251 A/T polymorphism acted as an important genetic factor for HNC development, especially among African populations and nasopharyngeal cancer groups, and, interestingly, a decreased risk was found in the hospital-based group. However, due to the limitations mentioned above, investigations, using unbiased methods, well-matched controls, and with larger sample size and examining the effect of gene–gene and gene–environment interactions, as well as more types of interleukins, should be conducted in the future.
Acknowledgments
Funding: This study was supported by grants from the Natural Science Foundation of Liaoning Province (No. 2015020500). Ethical approval: This article does not contain any studies with human participants or animals performed by any of the authors.
Disclosure
The authors report no conflicts of interest in this work.
References
Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–386. | ||
Sabir M, Baig RM, Mahjabeen I, Kayani MA. Novel germline CDK4 mutations in patients with head and neck cancer. Hered Cancer Clin Pract. 2012;10(1):11. | ||
Smith EM, Rubenstein LM, Haugen TH, Pawlita M, Turek LP. Complex etiology underlies risk and survival in head and neck cancer human papillomavirus, tobacco, and alcohol: a case for multifactor disease. J Oncol. 2012;2012:571862. | ||
Akhtar S, Sheikh AA, Qureshi HU. Chewing areca nut, betel quid, oral snuff, cigarette smoking and the risk of oesophageal squamous-cell carcinoma in South Asians: a multicentre case-control study. Eur J Cancer. 2012;48(5):655–661. | ||
Sturgis EM, Wei Q. Genetic susceptibility-molecular epidemiology of head and neck cancer. Curr Opin Oncol. 2002;14(3):310–317. | ||
Negri E, Boffetta P, Berthiller J, et al. Family history of cancer: pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. Int J Cancer. 2009;124(2):394–401. | ||
Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002; 420(6917):860–867. | ||
Yapijakis C, Serefoglou Z, Vylliotis A, et al. Association of polymorphisms in Tumor Necrosis Factor Alpha and Beta genes with increased risk for oral cancer. Anticancer Res. 2009;29(6):2379–2386. | ||
Liao B, Zhong BL, Li Z, Tian XY, Li Y, Li B. Macrophage migration inhibitory factor contributes angiogenesis by up-regulating IL-8 and correlates with poor prognosis of patients with primary nasopharyngeal carcinoma. J Surg Oncol. 2010;102(7):844–851. | ||
Visciano C, Liotti F, Prevete N, et al. Mast cells induce epithelial-to-mesenchymal transition and stem cell features in human thyroid cancer cells through an IL-8-Akt-Slug pathway. Oncogene. 2015;34(40):5175–5186. | ||
Jablonska E, Puzewska W, Grabowska Z, Jablonski J, Talarek L. VEGF, IL-18 and NO production by neutrophils and their serum levels in patients with oral cavity cancer. Cytokine. 2005;30(3):93–99. | ||
Mukaida N, Shiroo M, Matsushima K. Genomic structure of the human monocyte-derived neutrophil chemotactic factor IL-8. J Immunol. 1989;143(4):1366–1371. | ||
Rollins BJ. Chemokines. Blood. 1997;90(3):909–928. | ||
Baggiolini M, Clark-Lewis I. Interleukin-8, a chemotactic and inflammatory cytokine. FEBS Lett. 1992;307(1):97–101. | ||
Ju D, Sun D, Xiu L, Meng X, Zhang C, Wei P. Interleukin-8 is associated with adhesion, migration and invasion in human gastric cancer SCG-7901 cells. Med Oncol. 2012;29(1):91–99. | ||
Ding S, Tang Z, Jiang Y, et al. IL-8 is involved in estrogen-related receptor α-regulated proliferation and migration of colorectal cancer cells. Dig Dis Sci. 2017;62:3438–3446. | ||
Konno H, Ohta M, Baba M, Suzuki S, Nakamura S. The role of circulating IL-8 and VEGF protein in the progression of gastric cancer. Cancer Sci. 2003;94(8):735–740. | ||
Rafrafi A, Chahed B, Kaabachi S, et al. Association of IL-8 gene polymorphisms with non small cell lung cancer in Tunisia: A case control study. Hum Immunol. 2013;74(10):1368–1374. | ||
Koensgen D, Bruennert D, Ungureanu S, et al. Polymorphism of the IL-8 gene and the risk of ovarian cancer. Cytokine. 2015;71(2):334–338. | ||
Huang Q, Wang C, Qiu LJ, Shao F, Yu JH. IL-8-251A>T polymorphism is associated with breast cancer risk: a meta-analysis. J Cancer Res Clin Oncol. 2011;137(7):1147–1150. | ||
Ahirwar DK, Mandhani A, Mittal RD. IL-8 -251 T > A polymorphism is associated with bladder cancer susceptibility and outcome after BCG immunotherapy in a northern Indian cohort. Arch Med Res. 2010;41(2):97–103. | ||
Wang N, Zhou R, Wang C, et al. -251 T/A polymorphism of the interleukin-8 gene and cancer risk: A HuGE review and meta-analysis based on 42 case-control studies. Mol Biol Rep. 2012;39(3):2831–2841. | ||
Canedo P, Castanheira-Vale AJ, Lunet N, et al. The interleukin-8-251*T/*A polymorphism is not associated with risk for gastric carcinoma development in a Portuguese population. Eur J Cancer Prev. 2008;17(1):28–32. | ||
Ohyauchi M, Imatani A, Yonechi M, et al. The polymorphism interleukin 8 -251 A/T influences the susceptibility of Helicobacter pylori related gastric diseases in the Japanese population. Gut. 2005;54(3):330–335. | ||
Sun L, Mao D, Cai Y, et al. Association between higher expression of interleukin-8 (IL-8) and haplotype -353A/-251A/+678T of IL-8 gene with preeclampsia: a case-control study. Medicine. 2016;95(52):e5537. | ||
Zhang X, Zhang B, Zhang M, et al. Interleukin-8 gene polymorphism is associated with acute coronary syndrome in the Chinese Han population. Cytokine. 2011;56(2):188–191. | ||
Wang XB, Li YS, Li J, Han Y, Liu ZD. Interleukin-8 -251A/T gene polymorphism and lung cancer susceptibility: a meta-analysis. J Cell Mol Med. 2015;19(6):1218–1222. | ||
Gao LB, Pan XM, Jia J, et al. IL-8 -251A/T polymorphism is associated with decreased cancer risk among population-based studies: evidence from a meta-analysis. Eur J Cancer. 2010;46(8):1333–1343. | ||
Liu L, Zhuang W, Wang C, Chen Z, Wu XT, Zhou Y. Interleukin-8 -251 A/T gene polymorphism and gastric cancer susceptibility: a meta-analysis of epidemiological studies. Cytokine. 2010;50(3):328–334. | ||
Wang ZM, Wang CN, Zhao ZG, et al. Association between -251A>T polymorphism in the interleukin-8 gene and oral cancer risk: a meta-analysis. Gene. 2013;522(2):168–176. | ||
Cheng D, Hao Y, Zhou W, Ma Y. Positive association between Interleukin-8 -251A > T polymorphism and susceptibility to gastric carcinogenesis: a meta-analysis. Cancer Cell Int. 2013;13(1):100. | ||
Xue H, Liu J, Lin B, Wang Z, Sun J, Huang G. A meta-analysis of interleukin-8 -251 promoter polymorphism associated with gastric cancer risk. PLoS One. 2012;7(1):e28083. | ||
Okamura H, Tsutsi H, Komatsu T, et al. Cloning of a new cytokine that induces IFN-gamma production by T cells. Nature. 1995;378(6552):88–91. | ||
Tschoeke SK, Oberholzer A, Moldawer LL. Interleukin-18: a novel prognostic cytokine in bacteria-induced sepsis. Crit Care Med. 2006;34(4):1225–1233. | ||
Kito T, Kuroda E, Yokota A, Yamashita U. Cytotoxicity in glioma cells due to interleukin-12 and interleukin-18-stimulated macrophages mediated by interferon-gamma-regulated nitric oxide. J Neurosurg. 2003;98(2):385–392. | ||
Udagawa N, Horwood NJ, Elliott J, et al. Interleukin-18 (interferon-gamma-inducing factor) is produced by osteoblasts and acts via granulocyte/macrophage colony-stimulating factor and not via interferon-gamma to inhibit osteoclast formation. J Exp Med. 1997;185(6):1005–1012. | ||
Chen L, Yuan W, Chen Z, et al. Vasoactive intestinal peptide represses activation of tumor-associated macrophages in gastric cancer via regulation of TNFα, IL-6, IL-12 and iNOS. Int J Oncol. 2015;47(4):1361–1370. | ||
Kim KE, Song H, Kim TS, et al. Interleukin-18 is a critical factor for vascular endothelial growth factor-enhanced migration in human gastric cancer cell lines. Oncogene. 2007;26(10):1468–1476. | ||
Riedel F, Adam S, Feick P, Haas S, Gotte K, Hormann K. Expression of IL-18 in patients with head and neck squamous cell carcinoma. Int J Mol Med. 2004;13(2):267–272. | ||
Nolan KF, Greaves DR, Waldmann H. The human interleukin 18 gene IL18 maps to 11q22.2-q22.3, closely linked to the DRD2 gene locus and distinct from mapped IDDM loci. Genomics. 1998;51(1):161–163. | ||
Giedraitis V, He B, Huang WX, Hillert J. Cloning and mutation analysis of the human IL-18 promoter: a possible role of polymorphisms in expression regulation. J Neuroimmunol. 2001;112(1–2):146–152. | ||
Mi YY, Yu QQ, Yu ML, et al. Review and pooled analysis of studies on -607(C/A) and -137(G/C) polymorphisms in IL-18 and cancer risk. Med Oncol. 2011;28(4):1107–1115. | ||
Yang X, Qiu MT, Hu JW, et al. Association of interleukin-18 gene promoter -607 C>A and -137G>C polymorphisms with cancer risk: a meta-analysis of 26 studies. PLoS One. 2013;8(9):e73671. | ||
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. | ||
Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22(4):719–748. | ||
Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101. | ||
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. | ||
Shimizu Y, Kondo S, Shirai A, Furukawa M, Yoshizaki T. A single nucleotide polymorphism in the matrix metalloproteinase-1 and interleukin-8 gene promoter predicts poor prognosis in tongue cancer. Auris Nasus Larynx. 2008;35(3):381–389. | ||
Campa D, Hashibe M, Zaridze D, et al. Association of common polymorphisms in inflammatory genes with risk of developing cancers of the upper aerodigestive tract. Cancer causes & control: CCC. 2007;18(4):449–455. | ||
Ben Nasr H, Chahed K, Mestiri S, Bouaouina N, Snoussi K, Chouchane L. Association of IL-8 (-251)T/A polymorphism with susceptibility to and aggressiveness of nasopharyngeal carcinoma. Hum Immunol. 2007;68(9):761–769. | ||
Kietthubthew S, Wickliffe J, Sriplung H, Ishida T, Chonmaitree T, Au WW. Association of polymorphisms in proinflammatory cytokine genes with the development of oral cancer in Southern Thailand. Int J Hyg Environ Health. 2010;213(2):146–152. | ||
Zhang LY, Du CQ, Niu WW, et al. Association of an interleukin-8 promoter polymorphism with esophageal squamous cell carcinoma in the Taihang mountain region. Chinese Journal of Clinical Oncology. 2008;35(10):591–595. | ||
Liu CM, Yeh CJ, Yu CC, et al. Impact of interleukin-8 gene polymorphisms and environmental factors on oral cancer susceptibility in Taiwan. Oral Dis. 2012;18(3):307–314. | ||
Wei YS, Lan Y, Tang RG, et al. Single nucleotide polymorphism and haplotype association of the interleukin-8 gene with nasopharyngeal carcinoma. Clinical Immunol. 2007;125(3):309–317. | ||
Tai SH, Wei YS, Wang P, et al. [Genetic polymorphisms of interferon-gamma and interleukin-8 in patients with nasopharyngeal carcinoma]. Sichuan Da Xue Xue Bao Yi Xue Ban. 2007;38(5):862–865. | ||
Kilic I, Guldiken S, Sipahi T, et al. Investigation of VEGF and IL-8 gene polymorphisms in patients with differentiated thyroid cancer. Clin Lab. 2016;62(12):2319–2325. | ||
Savage SA, Abnet CC, Mark SD, et al. Variants of the IL8 and IL8RB genes and risk for gastric cardia adenocarcinoma and esophageal squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2004;13(12):2251–2257. | ||
Abdolahi F, Dabbaghmanesh MH, Haghshenas MR, Ghaderi A, Erfani N. A gene-disease association study of IL18 in thyroid cancer: genotype and haplotype analyses. Endocrine. 2015;50(3):698–707. | ||
Babar M, Ryan AW, Anderson LA, et al. Genes of the interleukin-18 pathway are associated with susceptibility to Barrett’s esophagus and esophageal adenocarcinoma. Am J Gastroenterol. 2012;107(9):1331–1341. | ||
Farhat K, Hassen E, Bouzgarrou N, Gabbouj S, Bouaouina N, Chouchane L. Functional IL-18 promoter gene polymorphisms in Tunisian nasopharyngeal carcinoma patients. Cytokine. 2008;43(2):132–137. | ||
Asefi V, Mojtahedi Z, Khademi B, Naeimi S, Ghaderi A. Head and neck squamous cell carcinoma is not associated with interleukin-18 promoter gene polymorphisms: A case-control study. J Laryngolo Otol. 2009;123(4):444–448. | ||
Tsai HT, Hsin CH, Hsieh YH, et al. Impact of interleukin-18 polymorphisms -607A/C and -137G/C on oral cancer occurrence and clinical progression. PLoS ONE. 2013;8(12). | ||
Pratesi C, Bortolin MT, Bidoli E, et al. Interleukin-10 and interleukin-18 promoter polymorphisms in an Italian cohort of patients with undifferentiated carcinoma of nasopharyngeal type. Cancer Immunol Immunother. 2006;55(1):23–30. | ||
Nong LG, Luo B, Zhang L, Nong HB. Interleukin-18 gene promoter polymorphism and the risk of nasopharyngeal carcinoma in a Chinese population. DNA Cell Biol. 2009;28(10):507–513. | ||
Wei YS, Lan Y, Liu YG, Tang H, Tang RG, Wang JC. Interleukin-18 gene promoter polymorphisms and the risk of esophageal squamous cell carcinoma. Acta Oncol. 2007;46(8):1090–1096. | ||
Chung JH, Lee YC, Eun YG, et al. Single Nucleotide Polymorphism of Interleukin-18 and Interleukin-18 Receptor and the Risk of Papillary Thyroid Cancer. Exp Clin Endocrinol Diabetes. 2015;123(10):598–603. | ||
Liu C, Ma SJ, Zhang WB, Gu HY, Ding GW, Chen SC. Association of interleukin-8 polymorphism and susceptibility to esophageal cancer. Chin J Exp Surg. 2011;28(8):1261–1263. | ||
Hu YP, Liu B, Su T, Cheng J, Zhao W, Yang HD. IL-8-251 single nucleotide polymorphism in the recurrence of squamouse cell carcinoma of tongue. J Pract Stomatol. 2012(03):328–332. | ||
Pan GG, Luo B, Teng YJ, Liang LN. Research on interleukin-18 gene promoter polymorphisms and genetic susceptibility of nasopharyngeal carcinoma. Laboratory Medicine. 2013(06):457–461. | ||
Zhang Y, Zeng X, Lu H, Li Y, Ji H. Association between Interleukin-8-251A/T polymorphism and gastric cancer susceptibility: a meta-analysis based on 5286 cases and 8000 controls. Int J Clin Exp Med. 2015;8(12):22393–22402. | ||
Gai XJ, Wei YM, Li BS. Progress of relative factors about the prognosis of nasopharyngeal carcinoma. Chinese Journal of Cancer Prevention and Treatment. 2016;23(20):1398–1402. | ||
Kim KE, Song H, Hahm C, et al. Expression of ADAM33 is a novel regulatory mechanism in IL-18-secreted process in gastric cancer. J Immunol. 2009;182(6):3548–3555. | ||
Kim J, Kim C, Kim TS, et al. IL-18 enhances thrombospondin-1 production in human gastric cancer via JNK pathway. Biochem Biophys Res Commun. 2006;344(4):1284–1289. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




