Back to Journals » Cancer Management and Research » Volume 14
Association of Hypertension and Breast Cancer: Antihypertensive Drugs as an Effective Adjunctive in Breast Cancer Therapy
Authors Fan Y, Khan NH , Farhan Ali Khan M, Ahammad MF, Zulfiqar T, Virk R, Jiang E
Received 23 November 2021
Accepted for publication 25 February 2022
Published 1 April 2022 Volume 2022:14 Pages 1323—1329
DOI https://doi.org/10.2147/CMAR.S350854
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Yong Teng
Yuanyuan Fan,1,* Nazeer Hussain Khan,1,* Muhammad Farhan Ali Khan,2 MD Faysal Ahammad,3 Tayyaba Zulfiqar,2 Razia Virk,4 Enshe Jiang5
1School of Life Sciences, Henan University, Kaifeng, Henan, 475004, People’s Republic of China; 2Department of Pharmacy, Quaid I Azam University, Islamabad, Pakistan; 3Key Laboratory of Natural Medicine and Immune Engineering, School of Medicine, Henan University, Kaifeng, People’s Republic of China; 4Department of Bio-Sciences, University Wah, Rawalpindi, Pakistan; 5Institute of Nursing and Health, Henan University, Kaifeng, 475004, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Enshe Jiang, Email [email protected]
Abstract: Breast cancer (BC) is the most common malignancy affecting women, and its incidence in younger women is rising worldwide. Early-onset of BC is a multi-step process involving various biological aggressive tumors such as triple negative and human epidermal growth factor 2 (HER2)-positive cancers. BC prevention is still arduous across the globe. A series of observational studies have established a conclusive non-genetic clinical link between hypertension (HTN) and the development of invasive BC. Those clinical associations have driven a pharmacological seek to use the anti-hypertension (AHTN) drugs as an effective adjunctive in BC therapy. The use of AHTN, especially beta-blockers and thiazides, has been recognized as a potent anti-tumor drug to mitigate BC progression, reduce the side effects of cancer treatment, and stop the reoccurrence of cancer in the survivors. Considering the dire need to disseminate the research on how AHTN drugs can be opted as the effective adjunctive therapy to cure the BC, the current review aimed to provide an update on novel understandings on association and mechanisms of AHTN-drugs against BC as an additional cancer therapy.
Keywords: breast cancer, beta blockers, hypertension, hypertensive drugs, thiazides
Introduction
Breast cancer (BC) is the most common malignancy affecting women, and its incidence in younger women is rising worldwide.1 Although significant advancements in breast cancer therapy have resulted in increased survival rates; however, owing to some clinical limitations, its prevention and prognosis yet remain a challenge all over the world.2,3 Breast cancer is the second most common cause of death in women.4 Early detection of breast cancer is one of the most effective ways to prevent it.5,6
According to recent reports, approximately 2.3 million new cases of BC are diagnosed each year, with a mortality rate of about 450,000 per year.1,7 Leading risk factors for BC include: age, genetic mutation (BRCA1 and BRCA2),8 lifestyle base-modified (non-genetic) risk factors,9,10 early menarche, nulliparity, first pregnancy after the age of 30 years, older age menopause, dense breast tissue,11 hormones replacement therapy,12 use of oral contraceptives,13 personal and family history of BC patent and other clinical complaints like hypertension (HTN).14
In cancer education, it is challenging but has fundamental importance to accurately evaluate the role of non-genetic clinical factors like HTN for estimating BC risk for individual women- the first essential step toward precision prevention.15,16 Considering the need to disseminate the literature on, how HTN is involved in BC development, and how the use of anti-hypertensive (AHTN) drugs can be opted as the effective adjunctive therapy to cure the BC, the current review aimed to provide an update on new understandings and the mechanisms of AHTN-drugs against BC progression.
Hypertension and Breast Cancer
In today’s life, systemic hypertension (HTN) is an emerging critical public health issue. It is a well-known cause of various life-threatening complications such as cerebrovascular accidents, coronary artery disease, cognitive heart failure, peripheral arterial diseases, renal failure, and associated with the well-known the onset of carcinogenesis.17–19 According to reports, it has been estimated that the global burden of adults with HTN will reach 1.56 billion in 2025 with a major proportion from low and middle-income countries (LMIC).20,21 Published research reveals that both the burden and impact of HTN are more among the elderly population and vary in both sexes.22 However, data on the female reproductive timing and HTN reveals that females are more prone to HTN.23
Clinically, hypertension is characterized by an elevated blood pressure level.24 It is the most common chronic illness among the elderly, affecting 61% of women.25 Through the years of research, several observational and case-control studies have established the link between the postmenopausal hypertension in women and breast cancer.26–29
Researchers have examined that the prevalence of hypertension and female breast cancer is on the rise with age and consider postmenopausal estrogen withdrawal as one of the possible reasons for this escalation.30,31 It has also been proposed that as BC and HTN share common pathophysiology pathways mediated by fatty tissue, it is the factor that may lead to chronic inflammation and BC onset.32 Another possible justification of this association lies in the role of HTN in inhibiting the inflammation and increasing the apoptosis, which may lead to the development of BC in breast tissues33? Studies have reported that women who used antihypertensive medications showed an increased improvement in the BC treatment compared to those without prescriptions of antihypertensive drugs.31,34
Although several well-established strong and absolute connections between BC and HTN have been identified; however, the links between the use of anti-hypertension drugs and BC treatment are under preclinical and clinical trials. Ambivalent variables that extend to specific features of these drugs and level of HTN like severity, type (systolic or diastolic), duration, and age need further large-scale clinical data attestation.
Antihypertensive Drugs: An Effective Adjunctive Anticancer Therapy
To treat the complex illness of BC, most common therapies include radiotherapy, chemotherapy and surgery; however, pharmacological approaches are repurposing new drugs to develop a more efficient treatment with better results.35 The use of AHTN drugs against the treatments of BC is one of those strategies of drugs development, repositioning and repurposing the possible pharmacological alternatives for cancer treatment.14 Currently available AHTN drug products are considered a reservoir of agents with the potential to make important contributions in the oncology field.36 The most often used drugs to treat hypertension include angiotensin-converting enzyme inhibitors (ACEIs), angiotensin receptor blockers (ARBs), b-blockers (BBs), calcium channel blockers (CCBs), and diuretics37 (Figure 1). Certain antihypertensive drugs, like BBs and thiazides, have been speculated to impact cancer cell proliferation through several pathways, providing justification for their putative links to breast cancer. A number of epidemiologic studies have also been carried out to see if they have any impact on breast cancer incidence and outcomes.38–40
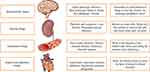 |
Figure 1 Anti-hypertensive drugs used to relieve the hypertensive patients by their action to respective body organs and physiological process. |
Β-Blockers (BBs) and BC
BB drug therapy reduces secondary cancer formation and improves cancer-specific survival in breast cancer. Compared to non-hypertensive breast cancer patients or those treated with other antihypertensive drugs, patients who started and maintained antihypertensive beta-blocker therapy prior to their breast cancer diagnosis had fewer distant metastases.41 BBs may also be beneficial as part of breast cancer therapy’s supportive care. A study of 174 breast cancer patients found that those who used BBs experienced 32% fewer cancer-related intrusive thoughts than those who did not take BBs.42,43 Surprisingly, long-term usage of BBs was found to have a protective impact against breast cancer risk.44 Furthermore, no connection with any antihypertensive medication was seen in the prognostic study.44,45 Breast cancer–specific mortality (19–22) was 48% to 81% lower in women who used BBs, and breast cancer recurrence/distant metastases were 48% to 57% lower in women who used BBs. When compared to users of other classes of antihypertensive treatments, they did not have an elevated risk of cancer.46
Mechanistically justification of this therapy lies in the way of blocking the action of endogenous catecholamines on the β-adrenergic receptor part of the autonomic nervous system, which is known to participate in blood pressure control.42,46
Researchers found that B-blockers have the potential to act on receptors associated with mechanisms that trigger tumorigenesis, angiogenesis, and tumor metastasis and exert the anti-tumor effects.47 Another possible way of b-blockers such as propranolol and β-AR to relieve the BC cells is their ability to interfere with angiogenesis and modulate the expression and activation of angiogenic signalling pathways, including angiopoietin/TIE2, VEGF, and hypoxia inducible factor48–52 (Figure 2).
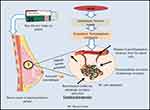 |
Figure 2 Purpose mechanism of action of beta- blocker to kill the progressive breast cancer cells by blocking the adrenergic receptors activation. |
Thiazides and BC
Besides the B-blocker, diuretics are considered as the potential molecular target to cure BC. Breast cancer is associated with a variety of antihypertensive medications including all types of diuretics: thiazide; loop and potassium-sparing diuretics. Long-term use of diuretics might protect against breast cancer.53 Longer life expectancy is linked to a higher risk of having a second primary cancer (SPC), which is defined as malignant tumors.54
Diuretics work by reducing salt reabsorption at various locations in the nephron, resulting in increased sodium and water losses in the urine. The second class of diuretics, known as aquatics, works by inhibiting vasopressin receptors in the connecting tubule and collecting duct, preventing water reabsorption.55–57 Blood pressure lowering drugs were linked to reduced risk of breast cancer. These findings support a link between treated hypertension, diuretic use, and the risk of breast cancer in women aged 50–75 years.56
What Consequences Does Hypertension Have on Women?
In comparison to hypertensive men, hypertensive women develop arterial stiffness, heart failure with preserved ejection fraction, atrial fibrillation, and dementia at a later age.58 A BP target of 140/90 mm Hg is recommended by most major treatment guidelines. Systolic blood pressure (SBP) rises after menopause, which is assumed to be due to the loss of endogenous estrogen’s vasodilator effects, increased arterial stiffness and salt sensitivity, decreased endothelial nitric oxide generation, and increased angiotensin II receptor expression. Importantly, isolated SBP elevation in both sexes is a sensitive predictor of future cardiovascular diseases.49,59 Obesity, which affects up to 40% of postmenopausal women, and greater rates of depression and anxiety are other characteristics that predispose to the development of hypertension and disproportionately affect postmenopausal women. Finally, increased physical activity and balanced nutrition intake can counteract the effects of cancer on arterial stiffness and blood pressure.60
Conclusion
In a nutshell, HTN and female BC becoming more common as people get older. Treating HTN is beneficial as a breast cancer treatment. In hypertensive patients, ARBs, ACEi, CCBs and BBs are commonly employed. Research has established the clinical findings on the BB involvement in mitigating the risk of BC progression and recurrence, however, there is a scarcity of data on ACE inhibitors and CCBs against BC risk. The goal of this mini narrative review is to highlight the interplay of HTN and BC treatment and to exert the treatment recommendations for female hypertensive patients. Moreover, this review raised the concern and need for in-depth exploration to identify the potential links of AHTN drug use and BC outcomes as a future perspective.
Future Perspective
The majority of regularly given antihypertensive drugs are safe for older breast cancer survivors in terms of outcomes. The positive connections between the use of diuretics and b-blockers and the likelihood of poor breast cancer outcomes identified in this study need to be clarified and confirmed. Given the growing number of antihypertensive medications on the market, identifying potential links between their use and adverse breast cancer outcomes could aid clinicians and women with breast cancer in weighing the benefits and risks of various treatment options when it comes to managing hypertension.
Abbreviations
HTN, hypertension; AHTN, Anti-hypertension; BC, Breast Cancer; ACEIs, angiotensin-converting enzyme inhibitors; ARBs, angiotensin II receptor blockers; BBs, b-blockers (BBs); CCBs, calcium channel blockers; AT1R, angiotensin receptor subtype 1; RAS, renin-angiotensin; GPCRs, G-protein coupled receptors.
Acknowledgments
The authors gratefully acknowledge the assistance and motivation energy of Professor Xing- Ying Ji to accomplish this manuscript. Hussain pays countless thanks to his Piare Lala for his support and presence in the PhD journey.
Funding
This work was supported by Henan Provincial Science and Technology Research Project [No. 212102310147] and the National Natural Science Foundation of China (No. 81900375).
Disclosure
The authors report no other potential conflicts of interest for this work.
References
1. DeSantis CE, Ma J, Gaudet MM, et al. Breast cancer statistics, 2019. CA Cancer J Clin. 2019;69(6):438–451. doi:10.3322/caac.21583
2. Moo T-A, Sanford R, Dang C, Morrow M. Overview of breast cancer therapy. PET Clin. 2018;13(3):339–354. doi:10.1016/j.cpet.2018.02.006
3. Fang X, Cao J, Shen A. Advances in anti-breast cancer drugs and the application of nano-drug delivery systems in breast cancer therapy. J Drug Deliv Sci Technol. 2020;57:101662. doi:10.1016/j.jddst.2020.101662
4. Azamjah N, Soltan-Zadeh Y, Zayeri F. Global trend of breast cancer mortality rate: a 25-year study. APJCP. 2019;20(7):2015. doi:10.31557/APJCP.2019.20.7.2015
5. Naeem M, Hayat M, Qamar SA, et al. Risk factors, genetic mutations and prevention of breast cancer. Int J Biosci. 2019;14(4):492–496.
6. Khan NH, Duan S-F, Wu -D-D, et al. Better reporting and awareness campaigns needed for breast cancer in Pakistani women. Cancer Manag Res. 2021;13:2125. doi:10.2147/CMAR.S270671
7. Azubuike SO, Muirhead C, Hayes L, et al. Rising global burden of breast cancer: the case of sub-Saharan Africa (with emphasis on Nigeria) and implications for regional development: a review. World J Surg Oncol. 2018;16(1):1–13. doi:10.1186/s12957-018-1345-2
8. Venkitaraman AR. How do mutations affecting the breast cancer genes BRCA1 and BRCA2 cause cancer susceptibility? DNA Repair (Amst). 2019;81:102668. doi:10.1016/j.dnarep.2019.102668
9. Parada H
10. Khan NH, Ullah F, Khan TA, et al. Personal-care cosmetic practices in Pakistan: current perspectives and management. Clin Cosmet Investig Dermatol. 2021;14:9. doi:10.2147/CCID.S270667
11. Johansson AL, Andersson TM-L, Hsieh -C-C, et al. Tumor characteristics and prognosis in women with pregnancy‐associated breast cancer. Int J Cancer Res. 2018;142(7):1343–1354. doi:10.1002/ijc.31174
12. Kotsopoulos J, Gronwald J, Karlan BY, et al. Hormone replacement therapy after oophorectomy and breast cancer risk among BRCA1 mutation carriers. JAMA Oncol. 2018;4(8):1059–1065. doi:10.1001/jamaoncol.2018.0211
13. Bonfiglio R, Di Pietro M. The impact of oral contraceptive use on breast cancer risk: state of the art and future perspectives in the era of 4P medicine. Semin Cancer Biol. 2021;72:11–18.
14. Zheng G, Sundquist J, Sundquist K, et al. Beta-blockers use and risk of breast cancer in women with hypertension. Cancer Epidemiol Biomark Prev. 2021;30(5):965–973. doi:10.1158/1055-9965.EPI-20-1599
15. French DP, Southworth J, Howell A, et al. Psychological impact of providing women with personalised 10-year breast cancer risk estimates. Br J Cancer. 2018;118(12):1648–1657. doi:10.1038/s41416-018-0069-y
16. Lee A, Mavaddat N, Wilcox AN, et al. BOADICEA: a comprehensive breast cancer risk prediction model incorporating genetic and nongenetic risk factors. Genet Med. 2019;21(8):1708–1718. doi:10.1038/s41436-018-0406-9
17. Kidoguchi S, Sugano N, Tokudome G, et al. New concept of onco-hypertension and future perspectives. Hypertension. 2021;77(1):16–27. doi:10.1161/HYPERTENSIONAHA.120.16044
18. Xu C. The Elabela in hypertension, cardiovascular disease, renal disease, and preeclampsia: an update. J Hypertens. 2021;39(1):12–22. doi:10.1097/HJH.0000000000002591
19. Kovarik JJ, Morisawa N, Wild J, et al. Adaptive physiological water conservation explains hypertension and muscle catabolism in experimental chronic renal failure. Acta Physiologica. 2021;232(1):e13629. doi:10.1111/apha.13629
20. Zhou B, Bentham J, Di Cesare M, et al. Worldwide trends in blood pressure from 1975 to 2015: a pooled analysis of 1479 population-based measurement studies with 19· 1 million participants. Lancet. 2017;389(10064):37–55.
21. Zhou B, Perel P, Mensah GA, Ezzati M. Global epidemiology, health burden and effective interventions for elevated blood pressure and hypertension. Nat Rev Cardiol;2021. 1–18. doi:10.1038/s41569-020-00473-5
22. Leppänen T, Kulkas A, Töyräs J, et al. Polysomnographic characteristics of severe obstructive sleep apnea vary significantly between hypertensive and normotensive patients of both genders. Sleep Breath. 2021;25(1):105–116. doi:10.1007/s11325-020-02047-8
23. Tirpude GS, De S. Knowledge regarding risk factors of hypertension among non-healthcare workers working in hospital. Age. 2021;21(30):75.
24. Kovacs G, Zeder K, Rosenstock P, et al. Clinical impact of the new definition of precapillary pulmonary hypertension. Chest. 2021;159(5):1995–1997. doi:10.1016/j.chest.2020.11.070
25. Ali DH, Kiliç B, Hart HE, et al. Therapeutic inertia in the management of hypertension in primary care. J Hypertens. 2021;39(6):1238–1245. doi:10.1097/HJH.0000000000002783
26. Soltani S, Benisi-Kohansal S, Azadbakht L, et al. Association between adherence to “dietary approaches to stop hypertension” eating plan and breast Cancer. Nutr Cancer. 2021;73(3):433–441. doi:10.1080/01635581.2020.1756354
27. Wang W, He Q, Zhang H, et al. A narrative review on the interaction between genes and the treatment of hypertension and breast cancer. Ann Transl Med. 2021;9:45.
28. Yue W, Tran HT, Wang J-P, et al. The hypertension related Gene G-protein coupled receptor kinase 4 contributes to breast cancer proliferation. Breast Cancer. 2021;15:11782234211015753. doi:10.1177/11782234211015753
29. Zhang Z, Cui F, Cao C, et al. Single-cell RNA analysis reveals the potential risk of organ-specific cell types vulnerable to SARS-CoV-2 infections. Comput Biol Med. 2022;140:105092. doi:10.1016/j.compbiomed.2021.105092
30. Potmešil P, Szotkowská R. Drug-induced liver injury after switching from tamoxifen to anastrozole in a patient with a history of breast cancer being treated for hypertension and diabetes. Ther Adv Chronic Dis. 2020;11:2040622320964152. doi:10.1177/2040622320964152
31. Zhao Y, Wang Q, Zhao X, et al. Effect of antihypertensive drugs on breast cancer risk in female hypertensive patients: evidence from observational studies. Clin Exp Hypertens. 2018;40(1):22–27. doi:10.1080/10641963.2017.1288736
32. Laforest S, Ennour-Idrissi K, Ouellette G, et al. Associations between markers of mammary adipose tissue dysfunction and breast cancer prognostic factors. Int J Obes. 2021;45(1):195–205. doi:10.1038/s41366-020-00676-3
33. Han H, Sung YJ, Kim JY, et al. Hypertension and breast cancer risk: a systematic review and meta-analysis. Sci Rep. 2017;7(1):1–9. doi:10.1038/s41598-016-0028-x
34. Xie Y, Wang M, Xu P, et al. Association between antihypertensive medication use and breast cancer: a systematic review and meta-analysis. Front Pharmacol. 2021;12:1169. doi:10.3389/fphar.2021.609901
35. Kirtonia A, Gala K, Fernandes SG, et al. Repurposing of drugs: an attractive pharmacological strategy for cancer therapeutics. Semin Cancer Biol. 2021;68:258–278.
36. Carlos-Escalante JA, de Jesús-sánchez M, Rivas-Castro A, et al. The use of antihypertensive drugs as coadjuvant therapy in cancer. Front Oncol. 2021;11:1595. doi:10.3389/fonc.2021.660943
37. Laurent S. Antihypertensive drugs. Pharmacol Res. 2017;124:116–125. doi:10.1016/j.phrs.2017.07.026
38. González‐Pérez A, Ronquist G, García Rodríguez LA. Breast cancer incidence and use of antihypertensive medication in women. Pharmacoepidemiol Drug Saf. 2004;13(8):581–585. doi:10.1002/pds.910
39. Powe DG, Voss MJ, Zänker KS, et al. Beta-blocker drug therapy reduces secondary cancer formation in breast cancer and improves cancer specific survival. Oncotarget. 2010;1(7):628. doi:10.18632/oncotarget.197
40. Devore EE, Kim S, Ramin CA, et al. Antihypertensive medication use and incident breast cancer in women. Breast Cancer Res Treat. 2015;150(1):219–229. doi:10.1007/s10549-015-3311-9
41. Botteri E, Munzone E, Rotmensz N, et al. Therapeutic effect of β-blockers in triple-negative breast cancer postmenopausal women. Breast Cancer Res Treat. 2013;140(3):567–575. doi:10.1007/s10549-013-2654-3
42. Phadke S, Clamon G. Beta blockade as adjunctive breast cancer therapy: a review. Crit Rev Oncol Hematol. 2019;138:173–177. doi:10.1016/j.critrevonc.2019.04.006
43. Wan Y, Li X. A novel drug quality control technology in cold chain logistics based on port transportation. J Coast Res. 2020;103(SI):696–700. doi:10.2112/SI103-142.1
44. Leung HW, Hung -L-L, Chan ALF, et al. Long-term use of antihypertensive agents and risk of breast cancer: a population-based case–control study. Cardiol ther. 2015;4(1):65–76. doi:10.1007/s40119-015-0035-1
45. Stanek B, Frey B, Hülsmann M, et al. Prognostic evaluation of neurohumoral plasma levels before and during beta-blocker therapy in advanced left ventricular dysfunction. J Am Coll Cardiol. 2001;38(2):436–442. doi:10.1016/S0735-1097(01)01383-3
46. Chen L, Chubak J, Boudreau DM, et al. Use of antihypertensive medications and risk of adverse breast cancer outcomes in a SEER–medicare population. Cancer Epidemiology Biomarkers & Prevention. 2017;26(11):1603–1610. doi:10.1158/1055-9965.EPI-17-0346
47. Parada-Huerta E, Alvarez-Dominguez T, Uribe-Escamilla R, et al. Metastasis risk reduction related with beta-blocker treatment in Mexican women with breast cancer. Asian Pac J Cancer Prev. 2016;17(6):2953–2957.
48. Snyder EM, Sprissler R, Olson TP. The importance of use of genetics to guide hypertension therapy: using β-blockade as an example. Adv Mol Pathol. 2021;4:117–125. doi:10.1016/j.yamp.2021.06.005
49. Cooper H, Mishriky R, Antoun Reyad A. Efficacy and safety of cariprazine in acute management of psychiatric disorders: a meta-analysis of randomized controlled trials. Psychiatr Danub. 2020;32(1):36–45. doi:10.24869/psyd.2020.36
50. Lai W-F, Huang E, Lui K-H. Alginate‐based complex fibers with the Janus morphology for controlled release of co‐delivered drugs. Asian J Pharm Sci. 2021;16(1):77–85. doi:10.1016/j.ajps.2020.05.003
51. Khan MFA, Salman M, Khan NH, et al. Evaluation of errors in prescription writing: a cross-sectional study at community pharmacies and tertiary care hospitals of Lahore, Pakistan. Bangladesh J Medical Sci. 2019;18(2):260–266. doi:10.3329/bjms.v18i2.40695
52. Long Q, Zheng H, Liu X, et al. Perioperative intervention by β-Blockade and NF-κB suppression reduces the recurrence risk of endometriosis in mice due to incomplete excision. Reprod Sci. 2019;26(5):697–708. doi:10.1177/1933719119828066
53. Alhanafy AM, Labeeb A, Khalil A. The role of diuretics in treatment of aromatase inhibitors induced musculoskeletal symptoms in women with non metastatic breast cancer. APJCP. 2018;19(12):3525. doi:10.31557/APJCP.2018.19.12.3525
54. Bailey S, Ezratty C, Mhango G, Lin J. Clinical and sociodemographic risk factors associated with the development of second primary cancers among postmenopausal breast cancer survivors; 2021.
55. Santala EE, Murto MO, Artama M, et al. Angiotensin receptor blockers associated with improved breast cancer survival—a nationwide cohort study from Finland. Cancer Epidemiol Biomark Prev. 2020;29(11):2376–2382. doi:10.1158/1055-9965.EPI-20-0711
56. Largent J, McEligot AJ, Ziogas A, et al. Hypertension, diuretics and breast cancer risk. J Hum Hypertens. 2006;20(10):727–732. doi:10.1038/sj.jhh.1002075
57. Cho MA, Jeong SY, Sohn I, et al. Impact of angiotensin receptor blockers, beta blockers, calcium channel blockers and thiazide diuretics on survival of ovarian cancer patients. Cancer Res Treat. 2020;52(2):645. doi:10.4143/crt.2019.509
58. Lee SY, Kim MT, Jee SH, et al. Does long-term lactation protect premenopausal women against hypertension risk? A Korean women’s cohort study. Prev Med. 2005;41(2):433–438. doi:10.1016/j.ypmed.2004.11.025
59. Wenger NK, Ferdinand KC, Bairey Merz CN, et al. Women, hypertension, and the systolic blood pressure intervention trial. Am J Med. 2016;129(10):1030–1036. doi:10.1016/j.amjmed.2016.06.022
60. Hall JE, Mouton AJ, da Silva AA, et al. Obesity, kidney dysfunction, and inflammation: interactions in hypertension. Cardiovasc Res. 2021;117(8):1859–1876. doi:10.1093/cvr/cvaa336
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
