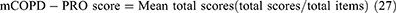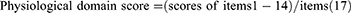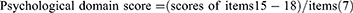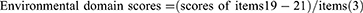Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 19
Association of Frailty with Patient-Report Outcomes and Major Clinical Determinants in Patients with Acute Exacerbation of Chronic Obstructive Pulmonary Disease
Authors Yang M , Liu Y , Zhao Y , Wang Z, He J, Wang Y, Anme T
Received 13 October 2023
Accepted for publication 7 April 2024
Published 12 April 2024 Volume 2024:19 Pages 907—919
DOI https://doi.org/10.2147/COPD.S444580
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Min Zhang
Mengjiao Yang,1,2,* Yang Liu,1,* Yangyang Zhao,3,* Ziwei Wang,4 Jie He,5 Yali Wang,2 Tokie Anme6
1Graduate School of Comprehensive Human Science, University of Tsukuba, Tsukuba, Japan; 2Department of Cardiovascular Surgery, Affiliated Hospital of North Sichuan Medical College, Nanchong City, Sichuan Province, People’s Republic of China; 3Department of Blood Transfusion, Affiliated Hospital of North Sichuan Medical College, Nanchong City, Sichuan Province, People’s Republic of China; 4Department of Respiratory and Critical Care Medicine, Affiliated Hospital of North Sichuan Medical College, Nanchong City, Sichuan Province, People’s Republic of China; 5Department of Nursing, Affiliated Hospital of North Sichuan Medical College, Nanchong City, Sichuan Province, People’s Republic of China; 6Faculty of Medicine, University of Tsukuba, Tsukuba, Ibaraki, Japan
*These authors contributed equally to this work
Correspondence: Tokie Anme, Faculty of Medicine, University of Tsukuba, 1-1-1 Tennodai, Tsukuba, Ibaraki, 3058577, Japan, Tel\Fax +81-29-853-3436, Email [email protected]
Purpose: This study aimed to explore the correlation of frailty status with disease characteristics and patient-reported outcomes (PROs) in patients with acute exacerbations of chronic obstructive pulmonary disease (AECOPD) and determine the sensitivity and specificity of modified COPD PRO scale (mCOPD-PRO) for detecting frailty.
Patients and Methods: This cross-sectional study surveyed 315 inpatients with AECOPD from a tertiary hospital in China from August 2022 to June 2023. Patient frailty and PROs were assessed using the validated FRAIL scale and mCOPD-PRO, respectively. Spearman’s ρ was used to assess the relevance of lung disease indicators commonly used in clinical practice, and ordinal logistic regression analyses were used to identify the variables associated with frailty status. The validity of mCOPD-PRO in discriminating frail or non-frail individuals was determined using the receiver operating characteristic curve.
Results: The participants (N=302, mean age 72.4± 9.1 years) were predominantly males (73.2%). Among them, 43 (14.3%) patients were not frail, whereas 123 (40.7%) and 136 (45.0%) patients were pre-frail and frail, respectively. The FRAIL scale was moderately correlated with the mCOPD-PRO scores (Spearman’s rank correlation coefficient [Rs]=0.52, P< 0.01) for all dimensions (Rs=0.43– 0.49, P< 0.01). Patients residing in rural areas (odds ratio [OR], 1.67; 95% confidence interval [95% CI], 1.01– 2.76) and with higher mCOPD-PRO scores (OR, 4.78; 95% CI, 2.75– 8.32) were more likely to be frail. Physically active patients (OR, 0.42; 95% CI, 0.21– 0.84) were less likely to be frail. In addition, mCOPD-PRO had good discriminate validity for detecting frailty (area under the curve=0.78), with a sensitivity and specificity of 84.6% and 60.8%, respectively. The optimal probability threshold for mCOPD-PRO was ≥ 1.52 points.
Conclusion: In patients with AECOPD, frailty is closely related to PROs and disease characteristics. Additionally, the mCOPD-PRO score can distinguish well between frail and non-frail patients. Our findings provide support for interventions targeting frail populations with AECOPD.
Plain Language Summary: Patients with chronic obstructive pulmonary disease often have concomitant frailty that may lead to disease deterioration such as acute exacerbations, hospital readmissions, disability, and premature death. Patient-reported outcomes are often used in clinical practice to measure patients’ disease characteristics and overall status. Whether patients’ frailty state is associated with patient-reported outcomes and if so, which factors are associated with frailty remain unclear. This study, conducted in China, examined their relationship as well as identified factors associated with frailty states. 302 hospitalized patients with acute exacerbations of chronic obstructive pulmonary disease completed a questionnaire answering questions about disease severity, frailty state, anxiety, and depression. The findings suggest that people who live in rural areas, self-reported more severe overall conditions, and are physically inactive are more likely to be frail. Patient-reported outcomes can distinguish between frail and non-frail patients. Therefore, patient-reported outcomes can be used to assess the extent of frailty; early screening of AECOPD combined frailty population and implementation of interventions can help mitigate the adverse effects of frailty.
Keywords: frailty, COPD, COPD exacerbation, patient-report outcome, aging
Introduction
Frailty is a clinical syndrome associated with a multisystemic decline in physiological reserve and increased vulnerability to potentially contributing to chronic disease onset and altering the prognosis and treatment of some diseases.1–6 Frailty is a risk factor for chronic obstructive pulmonary disease (COPD) exacerbation and is associated with worsening outcomes, such as acute exacerbations of COPD (AECOPD), hospitalization, length of stay, hospital readmissions, and mortality.6–9 Previous studies have reported that up to 19% of patients with stable COPD6 and >50% of patients with AECOPD experience frailty,10 which is related to their shared pathophysiological mechanisms, such as chronic inflammation, impaired neuroendocrine regulation, and immune system dysfunction. With the increase in global aging and the lifespan of individuals with chronic diseases, assessing and improving the condition of frail individuals, especially those with chronic diseases, has become a priority in public health.3,11
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) recommends using patient-reported outcomes (PROs) to assess disease severity and prognosis in patients with COPD.12 PROs are any report provided directly by the patient regarding their health status,13 including symptoms, functional status, and health-related quality of life (HRQoL). AECOPD leads to a decline in patient’s overall health status and daily functioning, severely affecting their HRQoL.14,15 PROs not only reflect a patient’s symptom burden during AECOPD, such as dyspnea, cough, and sputum production but also provide valuable information regarding the impact of symptoms on the patient’s daily living activities and the effectiveness of treatment.16 Furthermore, PROs can predict disability and all-cause mortality;15,17 thus, they play a critical role in the disease.18
Although AECOPD has shown a strong association with PROs and frailty, the relationship between them has not been clarified. While some studies have reported that frailty is associated with PROs, albeit with weak or no correlation with the severity of disease characteristics (eg, lung function),19 others have reported that frailty may be an entity independent of PROs and physiological outcomes.20
Therefore, the current study aimed to 1) identify the association of frailty status (using the FRAIL scale)21 with PROs (using the modified PRO scale for COPD [mCOPD-PRO]22) and disease characteristics in patients hospitalized with AECOPD and 2) examine the discriminatory capacity of PROs in identifying frail individuals and the optimal cut-off value.
Methods
Design and Participants
The current study is a cross-sectional, observational study with data drawn from the respiratory critical care unit of a large tertiary care institution in Sichuan Province, China, between August 2022 and June 2023. We recruited 315 hospitalized AECOPD patients who met the following inclusion criteria: a diagnosis of COPD according to the GOLD guidelines,23 a diagnosis of AECOPD by a specialist respiratory team on admission, and a stay of more than 24 hours on the ward. The exclusion criteria were as follows: a diagnosis of pulmonary fibrosis, heart failure (New York Heart Association [NYHA] class III or IV), and inability to answer the self-report questions. After excluding 13 patients with missing data, 302 participants were finally included in the analysis.
Variables and Exposures
Frailty Assessment Using the FRAIL Scale
The FRAIL scale21 is a clinical frailty screening tool proposed by the International Task Force on Nutrition, Health and Aging in 2008. It is based on the phenotype model in the form of a simple self-report questionnaire with five components: fatigue, resistance, ambulation, illness, and loss of weight (Supplement 1).
Each question on the self-report questionnaire could be scored 0 or 1 depending on the patient’s response. The total score could range from 0 to 5, with scores of 3–5, 1–2, and 0 categorized as frail, pre-frail and robust, respectively.
Patient-Reported Outcome Measurement
The mCOPD-PRO22 was used to assess the health status of patients with COPD, including 27 items in the Physiological (17), Psychological (7), and Environmental (3) domains (Supplement 2). The Cronbach’s alpha of the instrument is 0.954, and the correlation coefficients between the scores of the items and their domains range from 0.429–0.902.22 The Cronbach’s alpha for this study was 0.884. The mCOPD-PRO score and the scores for each domain were calculated using the following formulae:
The PRO score and scores for each dimension varied from 0–4. A higher score indicated poorer health status.
Descriptive Characteristics and Covariates
The demographic variables and disease information of the participants were collected. The demographic variables included age, sex, educational level, residential status, marital status, smoking history, and alcohol consumption. Disease information included body mass index (BMI), pulmonary function (GOLD stages I–IV), the COPD Assessment Test (CAT) score,24 the modified Medical Research Council (mMRC) score,25 and the nutritional risk screening (Nutritional risk screening, NRS2002) results.26 The use of NRS2002,26 a nutritional risk screening method for hospitalized patients, has been recommended by the European Society of Parenteral and Enteral Nutrition (ESPEN). It is based on a composite score of disease severity, impaired nutritional status, and age. The total score ranges from 1–7. A score of ≥3 indicates high risk, suggesting the requirement for nutritional support, whereas a score of 1–2 indicates low risk.
The CCI was used to assess the comorbidity burden.27 CCI can quantify comorbidities based on the number and severity of diseases that a patient has. It includes 19 diseases, with the weights of the items ranging from 1–6 and a total score ranging from 0–37. The Hospital Anxiety and Depression Scale (HADS)28 was used to assess anxiety and depression in patients. It consists of seven items each for anxiety (HADS-A) and depression (HADS-D), with a total score ranging from 0–21. A score of ≥11 indicated that the patient has symptoms of anxiety or depression.
Residential status was evaluated by asking the patient about their current living status. The question had two responses: “living alone” or “otherwise” (living with spouse or children or living in a nursing facility). Wealth status was evaluated by asking participants to describe how they felt financially about their current living situation. The question had four responses: “very difficult”, “fairly difficult”, “fairly comfortable”, and “very comfortable”. Participants with the responses of “very difficult” or “fairly difficult” were considered to have a poor wealth status, whereas those with the responses of “fairly comfortable” or “very comfortable” were considered to have a better-off wealth status. Physical activity status was evaluated by asking participants how often they exercised each week, with “no physical activity of any kind” being considered “sedentary”, “some type of exercise 2–3 times per week” being considered “moderate”, and “daily exercise or some type of exercise >3 times per week” being considered “active”. Lastly, the participants were asked whether they received domiciliary oxygen therapy. The question had two responses: Yes and No.
Statistical Analysis
Descriptive statistics were used to describe the demographic characteristics, disease characteristics, and frailty categories as measured by the FRAIL scale. The participants were described according to their frailty group. ANOVA was used for comparison of means and standard deviations comparison, the Kruskal–Wallis test was used for comparison of medians and interquartile ranges (IQRs), and the χ2 test or Fisher’s exact test was used for comparison of proportions.
Spearman correlation coefficient was used to assess the strength of the associations between frailty, PROs, and disease characterization variables. Ordinal logistic regression analyses were performed after a test of parallel lines to identify the variables associated with the patient’s frailty status. Hosmer-Lemeshow goodness of fit assessed the calibration of the model. Receiver operating curve (ROC) analysis was used to determine the optimal probability threshold by maximizing the Youden J index to identify the numerical values of PROs in frail vs non-frail patients. An optimal estimate of the area under the curve (AUC) for the 95% confidence interval (95% CI) was calculated subsequently.
R version 4.2.3 was used for data analyses, and GraphPad Prism 9.0.0 was used for graphing. A two-sided P-value of <0.05 was considered statistically significant.
Ethics Statement
This study was conducted in accordance with the Declaration of Helsinki, and approved by the Medical Ethics Committee of Affiliated Hospital of North Sichuan Medical College (approval No. 2022ER444-1, No. 2023ER324-1) and the Institutional Review Board of the Institute of the University of Tsukuba (approval No.1841). All participants provided written informed consent.
Results
Among the 302 patients hospitalized for AECOPD during the study period, 221 (73.2%) were male patients, with a mean (standard deviation [SD]) age of 72.4 (9.1) years and a BMI of 21.3 (3.9) kg/m2. The patients in the study population were categorized as frail (136; 45.0%), pre-frail (123; 40.7%), and non-frail (43; 14.3%). Table 1 presents the baseline characteristics of the three groups. A decrease in the mean BMI (SD) of each group (robust was 22.1 [3.7] kg/m2, pre-frail was 21.9 [3.9] kg/m2, and frail was 20.5 [3.8] kg/m2), an increase in the proportion of patients residing in rural areas (robust was 32.6%, pre-frail was 50.4%, and frail was 56.6%), a decrease in the proportion of those who were physically active (robust was 62.8%, pre-frail was 33.3%, and frail was 13.9%), and a decrease in the median PRO scores and scores for each dimension (median PROs [IQR] scores: 1.04 (0.65–1.33), 1.48 (1.19–1.85), and 2.11 (1.67–2.56) for the robust, pre-frail, and frail groups, respectively) were observed as the frailty status progressed. Compared with the non-frail participants, participants with frailty (pre-frail, frail) had a longer duration of illness, poorer lung function (GOLD stages II–IV), more severe dyspnea (mMRC scores) and symptoms (CAT scores), increased rates of anxiety and depression, higher nutritional risk, and greater requirement for domiciliary oxygen therapy.
 |
Table 1 Baseline Characteristics and Distribution of Frailty by Frail Scale Among Hospitalized Individuals with AECOPD (N=302) |
Higher levels of frailty were associated with higher (worse) PRO scores (Table 2). The Kruskal–Wallis test revealed that the differences in the health status indices between the frailty quartiles were evident in the PRO score and the three subscales (all P<0.001). This pattern was observed in the PRO score (Figure 1). Tables 3 and 4 present the correlation matrices between disease characteristics and frailty and between frailty and PRO scores, respectively. The results indicate that the majority of disease characteristics were significantly and positively correlated with frailty and PRO scores, with the exception of CCI. Frailty was moderately correlated with the PRO score (Rs=0.520); in contrast, it was correlated with all dimensions of PROs (all P<0.001) with a medium effect size (Rs=0.433–0.492).
 |
Table 2 Mean PROs Score by Frailty Status |
 |
Table 3 Spearman Correlation Matrices for the Association of Frailty with PROs and the Disease Characteristics in Patients with AECOPD |
 |
Table 4 Correlation Between Frailty and PROs Score |
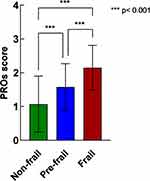 |
Figure 1 Mean PROs score according to the extent of frailty. Abbreviation: PROs, patient-reported outcomes. |
Ordinal logistic regression analysis was performed after passing the parallel lines test (P=0.060) to identify the variables that detected the frailty status. The following variables that were significant in univariate analysis were included in the regression models (using the enter method, Hosmer- Lemeshow χ2=10.19, P=0.252): age (years), BMI (kg/m2), residential status, physical activity, mCOPD-PRO score, course, GOLD stage, CAT score, mMRC score, anxiety (HADS-A≥11), and the requirement of domiciliary oxygen therapy. The results revealed that patients residing in rural areas (odds ratio [OR], 1.67; 95% CI, 1.01–2.76) and patients with higher mCOPD-PRO scores (OR, 4.78; 95% CI, 2.75–8.32) were more likely to be frail. In contrast, physically active patients (OR, 0.42; 95% CI, 0.21–0.84) were less likely to be frail (Table 5).
 |
Table 5 Ordinal Logistic Regression Analysis of Frailty in Patients with AECOPD |
Figure 2 presents the ROC curves for the PRO scores distinguishing between frail and non-frail individuals. The AUC for the ability of PROs to detect frailty on the FRAIL scores was 0.780 (0.728, 0.832), and the optimal probability threshold for PROs was ≥1.52 points, at which point the sensitivity and specificity were 84.6% and 60.8%, respectively (Figure 2A). Furthermore, PROs were able to differentiate between frailty and non-frailty in all dimensions (0.747–0.771). The AUC for the physiological domain was 0.755 (0.700, 0.809), and the cut-off value was 1.70, with a sensitivity and specificity of 86.8% and 59.0%, respectively (Figure 2B). The AUC for the psychological domain was 0.746 (0.690, 0.802), and the cut-off value was 1.36, with a sensitivity and specificity were 64.0% and 78.2%, respectively (Figure 2C). The AUC for the environmental domain was 0.764 (0.710, 0.818), and the cut-off value was 1.84, with a sensitivity and specificity of 74.3% and 68.1%, respectively (Figure 2D).
Discussion
This cross-sectional study of patients hospitalized with AECOPD revealed that PROs corresponded to and were significantly correlated with the physiological and clinical variables that correspond to important differences in frailty on the FRAIL scale. Ordinal logistic regression analyses revealed that the residential status, physical activity status, and mCOPD-PRO score were associated with frailty. Furthermore, the mCOPD-PRO score had a good ability to discriminate frail from non-frail patients.
Frailty is associated with the exacerbation of COPD.29 The pathogenesis of COPD involves a complex interaction between environmental exposures and genetic susceptibility.30 COPD causes extrapulmonary manifestations, such as cardiovascular disease and skeletal muscle disorders (eg, muscular dystrophy and muscle weakness),29,31 that are similar to those of frailty. Accelerated lung aging is one of the mechanisms intrinsic to this process.32 The frailty status based on the FRAIL scale indicated that frailty was prevalent in these patients (85.7%), with 40.7% pre-frail and 45.0% frail. This result is consistent with the results of the study by Chin et al,33 who used the Clinical Frailty Scale (CFS) to detect frailty in 86% of such patients included in their study, which is higher than the patients with COPD. In addition, the results of two previous studies7,10 on patients hospitalized with AECOPD revealed that the incidence of COPD exacerbations in frail individuals was 42% higher than that in non-frail individuals (incidence rate ratio [IRR] 1.42, 95% CI 0.94–2.17). However, as this study used a different assessment tool, caution should be exercised in interpreting this result, and more evidence is needed to validate the use of the FRAIL scale in this population.
The PRO tool is increasingly used in clinical practice as a valuable measure to assess the impact of medical interventions on the HRQoL of patients with COPD.34,35 As patients and physicians do not always share the same view regarding the importance of the disease,36 understanding PROs can help assess the patient’s overall health status. Engström et al37 reported that tools for the comprehensive assessment of the impact of COPD can detect the impact of a specific disease and the overall burden of the disease on daily functioning and emotional well-being. The CAT and mMRC are commonly used as symptom-reporting tools and for predicting the severity of debilitation in patients with COPD.38–40 The St. George’s Respiratory Questionnaire (SGRQ)41 is commonly used to assess the patient’s general health status. Kusunose et al20 identified the SGRQ score as the only predictor of frailty. However, the SGRQ assessment is more complex and does not apply to routine screening of patients in clinical practice. The mCOPD-PRO was used to assess symptoms and provide a comprehensive measure of disease-specific health in the present study. The results of the present study revealed that the mCOPD-PRO was closely associated with the CAT score, mMRC score, and frailty status (Rs=0.365–0.688).
Patients with COPD who are physically inactive are more likely to be frail. This finding may be attributed to the fact that individuals are often affected by the disease with varying degrees of dyspnea, and those with severe symptoms are more inclined to have a sedentary lifestyle. Moreover, these patients were more likely to have deficits in muscle strength and mass.42–45 As the level of dyspnea increases, the patient’s physical activity decreases, which increases the likelihood of muscle atrophy and loss of muscle strength, further contributing to a decrease in the patient’s activity tolerance.42 Exercise training is an important component of pulmonary rehabilitation for patients with COPD.45 As evidenced by multiple studies,46–48 exercise training is effective in reversing the systemic consequences of COPD, particularly skeletal muscle dysfunction,46 improving exercise tolerance, reducing symptoms of dyspnea, and increasing the HRQoL of patients with COPD.47–49 Residing in rural areas is associated with frailty as access to smoking cessation programs, early diagnosis, and treatment is lesser in these areas,50 which may contribute to higher morbidity and mortality rates.51,52 Healthcare providers and community partners that serve rural residents can help increase the access and participation of adults with COPD in healthcare interventions, including pulmonary rehabilitation, self-management, and patient education resources.
Notably, no significant differences in the CCI index and nutritional risk were observed between the frail and non-frail groups. This result was unexpected as previous studies have shown that frailty and multimorbidity coexist to limit health and survival in older adults.53,54 When combined with multiple diseases, the interaction of various chronic diseases leads to endothelial dysfunction/injury, a common feature of these diseases, which in turn exacerbates cerebral ischemia and hypoxia, thereby reducing cognitive function.55 Moreover, this condition accelerates the decline of organ function and leads to the dysregulation of a wide range of physiological regulatory systems that underlie frailty.56 The young age and low comorbidity burden of the study population may have influenced the result of this study. In addition, malnutrition and unintentional weight loss are common in patients with COPD57 and are associated with decreased HRQoL58 and increased healthcare utilization.59 Patients at high nutritional risk were found to have poorer health outcomes; however, an association with the extent of frailty was not observed despite the significant difference in the BMI of patients with different frailty statuses. This may be attributed to the normal range of BMI and lower nutrition risk status of the participants in the present study. The findings of the present study suggest that frailty is highly prevalent in patients with AECOPD and is associated with a range of lung disease characteristics. Moreover, the mCOPD-PRO is a reliable patient-reported outcome tool that can be used in clinical practice to assess frailty status.
The strengths of this study include the use of the FRAIL scale in patients with AECOPD and the identification of associations between multiple lung disease characteristics and PROs. In addition, a comprehensive, patient-reported assessment tool for disease-specific health conditions was used in this study, and its reliability was also determined. Nevertheless, this study also has some limitations. First, causality for frailty could not be assessed as this was a cross-sectional study. Second, the lack of statistical significance of disease burden and nutritional risk (which were not found to be associated with frailty) may be due to the younger age of the study population in the present study. This may have introduced bias and affected the association of frailty with these factors, a possibility that cannot be overlooked. Third, the measures of frailty status and PROs in this study involved subjective evaluations, and the gap between what was reported and what occurred may be biased. Future studies could introduce objective evaluations, such as readmission rates and mortality rates, to explore the consistency of these outcomes. Further detailed investigations are expected to elucidate the relationship between frailty and COPD. Further evidence on the role of frailty prevention in improving the health status of patients can be gathered by investigating these limitations.
Conclusion
In conclusion, this cross-sectional result suggests that a high proportion of patients with AECOPD are frail or pre-frail. This study investigated the factors associated with frailty status and the findings suggest that there is a need to understand patients’ backgrounds, disease severity, and lifestyles, as people who live in rural areas, have poorer self-reported outcomes, and are physically inactive are more likely to be frail. Early screening of AECOPD combined frailty population and implementation of interventions is beneficial in mitigating the adverse effects of frailty on them. The findings may provide support for more effective interventions for the vulnerable population.
Abbreviations
COPD, chronic obstructive pulmonary disease; GOLD, Global Initiative for Chronic Obstructive Lung Disease; PROs, patient-reported outcomes; mCOPD-PRO, modified patient-reported outcome scale for chronic obstructive pulmonary disease; AECOPD, acute exacerbation of chronic pulmonary disease; NYHA, New York Heart Association; mMRC, modified Medical Research Council; CCI, Charlson Comorbidity Index; BMI, body mass index; CAT, COPD assessment test; NRS2022, Nutritional risk screening; ESPEN, European Society of Parenteral and Enteral Nutrition; HADS, Hospital Anxiety and Depression Scale; IQR, interquartile ranges; ROC, receiver operating curve; AUC, area under the curve; 95% CI, 95% confidence interval; SD, standard deviation; OR, odds ratio; IRR, incidence rate ratio; HRQoL, health-related quality of life; SGRQ, St. George’s Respiratory Questionnaire.
Data Sharing Statement
The datasets underlying this article are available from the corresponding author upon reasonable request.
Acknowledgments
We thank Prof. Jiansheng Li and his team for authorizing us to use the Modified Patient-Reported Outcome Scale (mCOPD-PRO).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Funds for Cooperation Project of Nanchong City and North Sichuan Medical College, Grant No. 22SXQT0189, Nanchong Federation of Social Science Associations, Grant No. NC22B032, grant from China Scholarship Council, Grant No. 202208510018, and JST, the establishment of university fellowships towards the creation of science technology innovation, Grant No. JPMJFS2106.
Disclosure
The authors have no conflicts of interest to disclose in this work.
References
1. Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol Biol Sci Med Sci. 2001;56(3):M146–M156. doi:10.1093/gerona/56.3.m146
2. Ensrud KE, Ewing SK, Taylor BC, et al. Comparison of 2 frailty indexes for prediction of falls, disability, fractures, and death in older women. Arch Intern Med. 2008;168(4):382–389. doi:10.1001/archinternmed.2007.113
3. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381(9868):752–762. doi:10.1016/S0140-6736(12)62167-9
4. Veronese N, Sigeirsdottir K, Eiriksdottir G, et al. Frailty and risk of cardiovascular diseases in older persons: the age, gene/environment susceptibility-Reykjavik study. Rejuvenation Res. 2017;20(6):517–524. doi:10.1089/rej.2016.1905
5. Pollack LR, Litwack-Harrison S, Cawthon PM, et al. Patterns and predictors of frailty transitions in older men: the osteoporotic fractures in men study. J Am Geriatr Soc. 2017;65(11):2473–2479. doi:10.1111/jgs.15003
6. Marengoni A, Vetrano DL, Manes-Gravina E, Bernabei R, Onder G, Palmer K. The relationship between COPD and frailty: a systematic review and meta-analysis of observational studies. Chest. 2018;154(1):21–40. doi:10.1016/j.chest.2018.02.014
7. Yee N, Locke ER, Pike KC, et al. Frailty in chronic obstructive pulmonary disease and risk of exacerbations and hospitalizations. Int J Chron Obstruct Pulmon Dis. 2020;15:1967–1976. doi:10.2147/COPD.S245505
8. Wang L, Zhang X, Liu X. Prevalence and clinical impact of frailty in COPD: a systematic review and meta-analysis. BMC Pulm Med. 2023;23(1):164. doi:10.1186/s12890-023-02454-z
9. Luo J, Zhang D, Tang W, Dou LY, Sun Y. Impact of frailty on the risk of exacerbations and all-cause mortality in elderly patients with stable chronic obstructive pulmonary disease. Clin Interv Aging. 2021;16:593–601. doi:10.2147/CIA.S303852
10. Bernabeu-Mora R, García-Guillamón G, Valera-Novella E, Giménez-Giménez LM, Escolar-Reina P, Medina-Mirapeix F. Frailty is a predictive factor of readmission within 90 days of hospitalization for acute exacerbations of chronic obstructive pulmonary disease: a longitudinal study. Ther Adv Respir Dis. 2017;11(10):383–392. doi:10.1177/1753465817726314
11. Cesari M, Prince M, Thiyagarajan JA, et al. Frailty: an emerging public health priority. J Am Med Dir Assoc. 2016;17(3):188–192. doi:10.1016/j.jamda.2015.12.016
12. Agustí A, Celli BR, Criner GJ, et al. Global initiative for chronic obstructive lung disease 2023 report: GOLD executive summary. Eur Respir J. 2023;61(4):2300239. doi:10.1183/13993003.00239-2023
13. Mercieca-Bebber R, King MT, Calvert MJ, Stockler MR, Friedlander M. The importance of patient-reported outcomes in clinical trials and strategies for future optimization. Patient Relat Outcome Meas. 2018;9:353–367. doi:10.2147/PROM.S1562793
14. Machado A, Barusso M, De Brandt J, et al. Impact of acute exacerbations of COPD on patients’ health status beyond pulmonary function: a scoping review. Pulmonology. 2023;29(6):518–534. doi:10.1016/j.pulmoe.2022.04.004
15. Bourbeau J, Ford G, Zackon H, Pinsky N, Lee J, Ruberto G. Impact on patients’ health status following early identification of a COPD exacerbation. Eur Respir J. 2007;30(5):907–913. doi:10.1183/09031936.00166606
16. Casanova C, Marin JM, Martinez-Gonzalez C, et al. Differential effect of modified Medical Research Council dyspnea, COPD assessment test, and clinical COPD questionnaire for symptoms evaluation within the new GOLD staging and mortality in COPD. Chest. 2015;148:159–168. doi:10.1378/chest.14-2449
17. Nishimura K, Kusunose M, Sanda R, Mori M, Shibayama A, Nakayasu K. Comparison of predictive properties between tools of patient-reported outcomes: risk prediction for three future events in subjects with COPD. Diagnostics. 2023;13(13):2269. doi:10.3390/diagnostics13132269
18. Afroz N, Gutzwiller FS, Mackay AJ, Naujoks C, Patalano F, Kostikas K. Patient-reported outcomes (PROs) in COPD clinical trials: trends and gaps. Int J Chron Obstruct Pulmon Dis. 2020;15:1789–1800. doi:10.2147/COPD.S235845
19. Scarlata S, Finamore P, Laudisio A, et al. Association between frailty index, lung function, and major clinical determinants in chronic obstructive pulmonary disease. Aging Clin Exp Res. 2021;33(8):2165–2173. doi:10.1007/s40520-021-01878-z
20. Kusunose M, Oga T, Nakamura S, Hasegawa Y, Nishimura K. Frailty and patient-reported outcomes in subjects with chronic obstructive pulmonary disease: are they independent entities? BMJ Open Respir Res. 2017;4(1):e000196. doi:10.1136/bmjresp-2017-000196
21. Abellan van Kan G, Rolland YM, Morley JE, Vellas B. Frailty: toward a clinical definition. J Am Med Dir Assoc. 2008;9(2):71–72. doi:10.1016/j.jamda.2007.11.005
22. Li J, Wang J, Xie Y, Feng Z. Development and validation of the modified patient-reported outcome scale for chronic obstructive pulmonary disease (mCOPD-PRO). Int J Chron Obstruct Pulmon Dis. 2020;15:661–669. doi:10.2147/COPD.S240842
23. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management and prevention of COPD; 2023. Available from: http://www.goldcopd.org/.
24. Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD Assessment Test. Eur Respir J. 2009;34(3):648–654. doi:10.1183/09031936.00102509
25. Mahler DA, Wells CK. Evaluation of clinical methods for rating dyspnea. Chest. 1988;93(3):580–586. doi:10.1378/chest.93.3.580
26. Kondrup J, Allison SP, Elia M, et al. ESPEN guidelines for nutrition screening 2002. Clin Nutr. 2003;22(4):415–421. doi:10.1016/s0261-5614(03)00098-0
27. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi:10.1016/0021-9681(87)90171-8
28. Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67(6):361–370. doi:10.1111/j.1600-0447.1983.tb09716.x
29. Hanlon P, Guo X, McGhee E, Lewsey J, McAllister D, Mair FS. Systematic review and meta-analysis of prevalence, trajectories, and clinical outcomes for frailty in COPD. NPJ Prim Care Respir Med. 2023;33(1):1. doi:10.1038/s41533-022-00324-5
30. Agustí A, Faner R. COPD beyond smoking: new paradigm, novel opportunities. Lancet Respir Med. 2018;6(5):324–326. doi:10.1016/S2213-2600(18)30060-2
31. Natanek SA, Gosker HR, Slot IG, et al. Heterogeneity of quadriceps muscle phenotype in chronic obstructive pulmonary disease (COPD); implications for stratified medicine? Muscle Nerve. 2013;48(4):488–497. doi:10.1002/mus.23784
32. MacNee W. Accelerated lung aging: a novel pathogenic mechanism of chronic obstructive pulmonary disease (COPD). Biochem Soc Trans. 2009;37(4):819–823. doi:10.1042/BST0370819
33. Chin M, Kendzerska T, Inoue J, et al. Comparing the hospital frailty risk score and the clinical frailty scale among older adults with chronic obstructive pulmonary disease exacerbation. JAMA Network Open. 2023;6(2):e2253692. doi:10.1001/jamanetworkopen.2022.53692
34. Cazzola M, MacNee W, Martinez FJ, et al. Outcomes for COPD pharmacological trials: from lung function to biomarkers. Eur Respir J. 2008;31(2):416–469. doi:10.1183/09031936.00099306
35. Weldam SW, Schuurmans MJ, Liu R, Lammers JW. Evaluation of quality of life instruments for use in COPD care and research: a systematic review. Int J Nurs Stud. 2013;50(5):688–707. doi:10.1016/j.ijnurstu.2012.07.017
36. Oga T, Nishimura K, Tsukino M, Sato S, Hajiro T, Mishima M. Longitudinal deteriorations in patient reported outcomes in patients with COPD. Respir Med. 2007;101(1):146–153. doi:10.1016/j.rmed.2006.04.001
37. Engström CP, Persson LO, Larsson S, Sullivan M. Health-related quality of life in COPD: why both disease-specific and generic measures should be used. Eur Respir J. 2001;18(1):69–76. doi:10.1183/09031936.01.00044901
38. Chen PJ, Yang KY, Perng WC, Lin KC, Wang KY. Effect of dyspnea on frailty stages and related factors in Taiwanese men with COPD. Int J Chron Obstruct Pulmon Dis. 2018;13:2463–2469. doi:10.2147/COPD.S172694
39. Medina-Mirapeix F, Bernabeu-Mora R, Giménez-Giménez LM, Escolar-Reina P, Gacto-Sánchez M, de Oliveira-Sousa SL. Physical frailty characteristics have a differential impact on symptoms as measured by the CAT score: an observational study. Health Qual Life Outcomes. 2018;16(1):140. doi:10.1186/s12955-018-0969-9
40. Park SK, Richardson CR, Holleman RG, Larson JL. Frailty in people with COPD, using the National Health and Nutrition evaluation survey dataset (2003–2006). Heart Lung. 2013;42(3):163–170. doi:10.1016/j.hrtlng.2012.07.004
41. Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic airflow limitation. The St. George’s respiratory questionnaire. Am Rev Respir Dis. 1992;145(6):1321–1327. doi:10.1164/ajrccm/145.6.1321
42. Yan LC, Lu HY, Wang XY, et al. Prevalence and risk factors of frailty in patients with chronic obstructive pulmonary disease: systematic review and meta-analysis. Eur Geriatr Med. 2023;14(4):789–802. doi:10.1007/s41999-023-00800-2
43. Tanabe N, Sato S. Narrative review of current COPD status in Japan. J Thorac Dis. 2021;13(6):3878–3887. doi:10.21037/jtd-20-2263
44. Brighton LJ, Evans CJ, Farquhar M, et al. Integrating comprehensive geriatric assessment for people with COPD and frailty starting pulmonary rehabilitation: the Breathe Plus feasibility trial protocol. ERJ Open Res. 2021;7(1):00717–2020. doi:10.1183/23120541.00717-2020
45. Andrianopoulos V, Klijn P, Franssen FM, Spruit MA. Exercise training in pulmonary rehabilitation. Clin Chest Med. 2014;35(2):313–322. doi:10.1016/j.ccm.2014.02.013
46. Troosters T, Gosselink R, Janssens W, Decramer M. Exercise training and pulmonary rehabilitation: new insights and remaining challenges. Eur Respir Rev. 2010;19(115):24–29. doi:10.1183/09059180.00007809
47. Gloeckl R, Marinov B, Pitta F. Practical recommendations for exercise training in patients with COPD. Eur Respir Rev. 2013;22(128):178–186. doi:10.1183/09059180.00000513
48. Gloeckl R, Schneeberger T, Jarosch I, Kenn K. Pulmonary rehabilitation and exercise training in chronic obstructive pulmonary disease. Dtsch Ärztebl Int. 2018;115(8):117–123. doi:10.3238/arztebl.2018.0117
49. Zeng Y, Jiang F, Chen Y, Chen P, Cai S. Exercise assessments and trainings of pulmonary rehabilitation in COPD: a literature review. Int J Chron Obstruct Pulmon Dis. 2018;13:2013–2023. doi:10.2147/COPD.S167098
50. Centers for Disease Control and Prevention. Urban-rural differences in chronic obstructive pulmonary disease (COPD). Available from: https://www.cdc.gov/ruralhealth/copd/index.html.
51. Croft JB, Wheaton AG, Liu Y, et al. Urban-rural county and state differences in chronic obstructive pulmonary disease–United States, 2015. MMWR Morb Mortal Wkly Rep. 2018;67(7):205–211. doi:10.15585/mmwr.mm6707a1
52. Raju S, Brigham EP, Paulin LM, et al. The burden of rural chronic obstructive pulmonary disease: analyses from the National health and nutrition examination survey. Am J Respir Crit Care Med. 2020;201(4):488–491. doi:10.1164/rccm.201906-1128LE
53. Barreto P, Greig C, Ferrandez AM. Detecting and categorizing frailty status in older adults using a self-report screening instrument. Arch Gerontol Geriatr. 2012;54(3):e249–e254. doi:10.1016/j.archger.2011.08.003
54. Tazzeo C, Rizzuto D, Calderón-Larrañaga A, et al. Multimorbidity patterns and risk of frailty in older community-dwelling adults: a population-based cohort study. Age Ageing. 2021;50(6):2183–2191. doi:10.1093/ageing/afab138
55. Divo M, Celli BR. Multimorbidity in patients with chronic obstructive pulmonary disease. Clin Chest Med. 2020;41(3):405–419. doi:10.1016/j.ccm.2020.06.002
56. Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol a Biol Sci Med Sci. 2004;59(3):255–263. doi:10.1093/gerona/59.3.m255
57. Hogan D, Lan LT, Diep DT, Gallegos D, Collins PF. Nutritional status of Vietnamese outpatients with chronic obstructive pulmonary disease. J Hum Nutr Diet. 2017;30(1):83–89. doi:10.1111/jhn.12402
58. Nguyen HT, Collins PF, Pavey TG, Nguyen NV, Pham TD, Gallegos DL. Nutritional status, dietary intake, and health-related quality of life in outpatients with COPD. Int J Chron Obstruct Pulmon Dis. 2019;14:215–226. doi:10.2147/COPD.S181322
59. Hoong JM, Ferguson M, Hukins C, Collins PF. Economic and operational burden associated with malnutrition in chronic obstructive pulmonary disease. Clin Nutr. 2017;36(4):1105–1109. doi:10.1016/j.clnu.2016.07.008
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.