Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 19
Association of Chronic Obstructive Pulmonary Disease with Risk of Psychiatric Disorders: A Two-Sample Mendelian Randomization Study
Authors Zhang Q, Zhang H, Xu Q
Received 1 October 2023
Accepted for publication 14 January 2024
Published 1 February 2024 Volume 2024:19 Pages 343—351
DOI https://doi.org/10.2147/COPD.S442725
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Qinxia Zhang,1,* Haifu Zhang,2,* Qinxing Xu1
1Department of Respiratory Medicine, The First People’s Hospital of Fuyang, Hangzhou, Zhejiang, 311400, People’s Republic of China; 2Department of Medicine, The First People’s Hospital of Fuyang, Hangzhou, Zhejiang, 311400, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Qinxing Xu, Department of Respiratory Medicine, The First People’s Hospital of Fuyang, Hangzhou, Zhejiang, 311400, People’s Republic of China, Email [email protected]
Background: Chronic obstructive pulmonary disease (COPD) is a prevalent respiratory disorder often accompanied by comorbidities. Although the past few years have witnessed significant scientific progress, the potential relationship between COPD and mental illness remains a subject of debate.
Materials and Methods: We retrieved COPD data from the genome-wide association studies (GWAS) directory and data on mental illnesses, including Alzheimer’s disease, schizophrenia, panic disorder, attention deficit hyperactivity disorder (ADHD), bipolar disorder, major depressive disorder, multiple disabilities, obsessive-compulsive disorder, post-traumatic stress disorder, and schizophrenia, from the Psychiatric Genomics Consortium. A two-sample Mendelian randomization (MR) approach was applied to explore the association between COPD and mental illnesses, with subgroup analyses based on smoking history.
Results: Our two-sample MR analysis revealed no causal link between overall COPD and the development of common psychiatric disorders. Subgroup analyses based on smoking history showed no causal association between never-smokers with COPD and the occurrence of psychiatric disorders. However, ever-smokers with COPD were associated with a significantly increased risk of ADHD (OR: 2.303, 95% CI: 1.558– 3.403, P = 0.001) and a modestly reduced risk of Alzheimer’s disease (OR: 0.994, 95% CI: 0.988– 0.999, P = 0.034).
Conclusion: COPD patients with a history of smoking face a higher risk of developing ADHD but may experience a slight reduction in the risk of Alzheimer’s disease. Conversely, there was no observed causal association between COPD and psychiatric disorders among patients who never smoked.
Keywords: chronic obstructive pulmonary disease, psychiatric disorders, Mendelian randomization
Introduction
Chronic obstructive pulmonary disease (COPD) is a progressive inflammatory respiratory condition characterized by symptoms like dyspnea, limited physical activity, and partially reversible airflow obstruction.1 While smoking is widely acknowledged as the primary contributor to COPD, other factors such as genetic abnormalities, abnormal lung development, and air pollution also play a role.2 Over the years, the global prevalence of COPD has been rising, making it the third most significant contributor to worldwide mortality and morbidity.3 Indeed, COPD places significant financial strain on governments, healthcare systems, societies, and economies worldwide.4
Clinically, COPD patients often present with comorbidities such as coronary heart disease, hypertension, cognitive impairment, psychological disorders, and other ailments, suggesting that COPD should no longer be considered exclusively as a pulmonary disorder.5 The coexistence of these chronic diseases mutually influences one another, complicating the diagnosis and treatment process and ultimately resulting in a poorer prognosis.6 Previous studies have reported a higher prevalence of anxiety and depression in COPD patients compared to the general population.7 Managing depression in patients with COPD has shown effectiveness through a diverse range of therapeutic interventions.8 Furthermore, several studies have demonstrated an association between COPD and cognitive impairment or dementia.9 Besides, Su et al found that COPD could independently increase the risk of bipolar disorder, especially in individuals regularly using short-acting beta-agonists (SABAs).10
Mendelian randomization (MR) is an approach employed to investigate causal relationships between exposures and outcomes of interest.11 This methodology utilizes single nucleotide polymorphisms (SNPs) as unconfounded proxies for exposures, thereby mitigating issues related to residual confounding and reverse causality often encountered in conventional observational studies.12 The MR design serves as a crucial strategy for inferring causality without relying on randomized clinical trials (RCTs), as genetic variants are randomly assorted during meiosis, simulating the principles of an RCT.13 Given the complexity of COPD’s pathogenesis and numerous confounding factors, conducting Mendelian randomization studies is essential to establish whether a causal relationship exists between COPD and psychiatric disorders at the genetic level.
Materials and Methods
In this study, all data were sourced from the Genetic Alliance’s publicly accessible repository of statistical data obtained from genome-wide association studies (GWAS). All original studies received a specific ethical review and informed consent.
Study Design
Pooled data on COPD and common psychiatric disorders were collected from published GWAS. At the same time, the patients were divided into two groups according to whether they smoked. This study aimed to explore the causal relationship between COPD and the risk of psychiatric disorders using two-sample MR.
The MR Approach is predicated upon three fundamental assumptions: (1) genetic variants serving as instrumental variables (IVs) should exhibit robust associations with the risk factor under investigation; (2) the selected genetic variants should not be associated with potential confounding factors; and (3) the identified genetic variants solely influence the outcome risk through the risk factors, without involvement in alternative pathways (Figure 1).
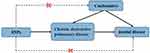 |
Figure 1 Overall design of Mendelian randomization analyses in the present study. |
Outcome and Exposure Data Source
COPD data were obtained from the GWAS database, and individuals were categorized into ever-smokers with COPD or never-smokers with COPD groups based on their smoking history. The GWAS Catalog database is publicly available for download at https://www.ebi.ac.uk/gwas/. Data on psychiatric disorders, including Alzheimer’s disease,14 anorexia nervosa,15 anxiety disorder,16 attention deficit hyperactivity disorder (ADHD),17 bipolar disorder,18 major depressive disorder (MDD),19 multiple disorders,20 obsessive-compulsive disorder (OCD),21 post-traumatic stress disorder (PTSD),22 and schizophrenia23 were obtained from the Psychiatric Genomics Consortium (https://pgc.unc.edu/). All participants were of European ancestry. Details of the GWAS outcome are provided in Table 1.
 |
Table 1 Details of Variance Explained by the Selected Instruments and F-Statistics for the MR Analysis Based on the Sample Size of Autoimmune Diseases |
MDD is diagnosed through questionnaires, and patients often present with pessimism, loss of interest, low mood, poor appetite, and even pessimism.24 In adults, ADHD is often characterized by impulsivity, impatience, chatter, hyperactivity and inattention.25
Genetic Variants Selection Criteria
Genetic instruments for each exposure trait or disease were selected at the genome-wide significance threshold (P < 5×108) from corresponding GWASs. Independent single nucleotide polymorphisms (SNPs) were defined by R2 < 0.001 and clump window > 10 kb, and correlated SNPs (linkage disequilibrium) with the lowest p-value were retained. Linkage disequilibrium among SNPs for each risk factor was calculated based on 1000 genomes LD reference panel (European population)26 using the PLINK clumping approach.27
Statistical Analysis
Cochran’s Q statistics were performed to assess the heterogeneity across individual SNPs. The random-effects inverse-variance-weighted (IVW-RE) model was used as the primary analytical method to examine the causal association.28 The MR-Egger method was used to determine whether the instrumental SNPs have pleiotropic effects.29 Several sensitivity analyses were conducted to explore potential pleiotropic bias. These included IVW-RE MR, MR-Egger regression, weighted median MR, funnel plots, and a leave-one-variant-out analysis for IVW-RE, wherein one variant was omitted at a time.
Results
Table 1 provides detailed information on the exposure groups and includes the results of heterogeneity and pleiotropy tests, all conducted in accordance with established academic standards.
Overall COPD
Heterogeneity was detected between COPD and some psychiatric disorders as determined by the Cochran heterogeneity test using the IVW method. Importantly, the MR-Egger method did not reveal significant horizontal pleiotropy in the association between COPD and psychiatric disorders. The IVW-RE MR analysis demonstrated no significant association between COPD and the risk of psychiatric disorders consistent with multiple MR methods, including MR Egger, weighted median, simple mode, and weighted mode. Our study did not identify any significant association between COPD and major depressive disorder (OR: 1.001, 95% CI: 0.998–1.005, P = 0.451) (Figure 2). Further verification using MR Egger, weighted median, simple mode, and weighted mode methods yielded consistent results (Supplement Table 1).
 |
Figure 2 Mendelian randomization of overall chronic obstructive pulmonary disease and psychiatric disorders in the primary analysis. |
Smoking-Related COPD
Utilizing the IVW method, the Cochran heterogeneity test revealed significant heterogeneity between individuals with a smoking history and certain psychiatric disorders in the context of COPD. Interestingly, the association between COPD and psychiatric disorders did not exhibit significant horizontal pleiotropy, except for anxiety disorders. The IVW-RE MR analysis demonstrated a significant association between ever-smokers with COPD and an increased risk of ADHD (OR: 2.303, 95% CI: 1.558–3.403, P = 0.001), along with a potential protective effect against Alzheimer’s disease to some extent (OR: 0.994, 95% CI: 0.988–0.999, P = 0.034) (Figure 3). However, no significant correlations were observed with other psychiatric disorders. Validation using MR Egger, weighted median, simple mode, and weighted mode methods yielded consistent results (Supplement Table 2).
 |
Figure 3 Mendelian randomization of ever smoking chronic obstructive pulmonary disease and psychiatric disorders in the primary analysis. |
Never-Smokers with COPD
After applying the IVW method, the Cochran heterogeneity test indicated significant heterogeneity between COPD individuals who never smoked and specific psychiatric disorders. The MR-Egger method did not provide significant evidence of horizontal pleiotropy in the association between COPD and psychiatric disorders. The IVW-RE MR analysis indicated no significant association between never smoking COPD and psychiatric disorders (Figure 4). Validation using MR Egger, weighted median, simple mode, and weighted mode methods yielded similar findings (Supplement Table 3).
 |
Figure 4 Mendelian randomization of never smoking chronic obstructive pulmonary disease and psychiatric disorders in the primary analysis. |
Discussion
Previous studies have explored the potential association between COPD and the occurrence of various mental disorders. However, controversy surrounds this association, prompting us to conduct a two-sample MR analysis with subgroup analysis based on smoking history. Our findings revealed that COPD patients with a smoking history exhibited a significantly elevated risk of ADHD while potentially experiencing a reduced risk of Alzheimer’s disease to some extent. Conversely, we found no causal relationship between COPD patients without a smoking history and common psychiatric disorders.
Cognitive impairment is a common issue, observed in approximately 10–61% of COPD patients, with conversion rates to dementia ranging from 50% to 70% within 5 to 7 years after its onset.30 Around 25% of individuals diagnosed with Alzheimer’s disease also have COPD.31 The association between COPD and cognitive decline is still under debate, as it remains unclear whether the decline in lung function or the shared risk factor of smoking plays a predominant role.32 A previous MR study suggested no significant association between lung function decline in COPD patients and the development of Alzheimer’s disease.33 Smoking, as another risk factor, seems to exert a more substantial influence on the initiation and progression of both diseases. It is widely acknowledged that tobacco smoke contains high levels of oxidants, inducing oxidative stress and affecting the normal functioning of brain tissues.34 Cataldo et al identified smoking as a significant risk factor for the development of Alzheimer’s disease.35 However, a neuropathological analysis found no significant increase in neuroinflammatory plaques among heavy smokers with dementia compared to non-smokers.36 Our current study suggests that individuals with COPD and a smoking history may experience a reduced susceptibility to Alzheimer’s disease (OR: 0.994, 95% CI: 0.988–0.999, P = 0.034). Smoking has long been recognized as a prominent risk factor for COPD, and the most effective intervention in managing COPD is the cessation of this habit.37 Consequently, upon diagnosis of COPD, patients should be offered smoking cessation intervention therapy.38 Smoking may emerge as a pivotal modifiable risk factor in the pathogenesis of Alzheimer’s disease.39 Among older adults, successful quitters exhibited a significantly slower rate of cognitive decline than those who continued smoking during the subsequent 2-year period.40 Based on the above findings, it can be inferred that quitting smoking following COPD diagnosis may confer a protective effect against the development of Alzheimer’s disease in previous smokers.
The prevalence of mild cognitive impairment in patients with moderate-to-severe COPD is reported to be as high as 36%, primarily affecting executive function, memory, and attention.41 Additionally, a cohort study by Dodd et al demonstrated that severe COPD and heavy smoking are risk factors for executive dysfunction in patients.42 Notably, the core manifestation of ADHD is executive dysfunction, with behavioral features encompassing inattention, hyperactivity, and impulsivity.43,44 Our study revealed a significantly elevated risk of ADHD among individuals with COPD who were former smokers compared to non-smokers. Smoking induces oxidative stress and upregulates the generation of superoxide free radicals, which play a pivotal role in the pathogenesis of COPD.45 Therefore, quitting smoking is a crucial intervention for all patients diagnosed with COPD and has the potential to significantly enhance their prognosis.46 At the same time, ADHD is a significant risk factor for persistent smoking, with the severity of its symptoms exhibiting a significantly positive correlation with both lifetime smoking risk and daily smoking volume.47 Additionally, adult smokers with ADHD exhibit a higher propensity for challenges in quitting compared to individuals in the general population.48 Therefore, the oxidative stress of smoking and the withdrawal effects of smoking cessation may be responsible for this phenomenon.
Previous studies have commonly observed that patients suffering from COPD exhibit varying degrees of anxiety symptoms, with a prevalence ranging from 6% to 33%, and in advanced COPD cases, the prevalence can exceed 25%.49 However, the clinical manifestations of anxiety closely resemble the respiratory challenges associated with COPD, making it challenging to differentiate between the two.50 This study’s findings indicate no observed correlation between COPD and severe anxiety disorders, regardless of individuals’ smoking history. It is highly conceivable that in previous studies, the symptoms of dyspnea associated with COPD were misinterpreted as manifestations of anxiety, potentially leading to false positive outcomes. Furthermore, this study solely encompassed data pertaining to major depressive disorder while excluding mild depressive disorder, resulting in a selection bias and yielding inconclusive findings. Consequently, future investigations necessitate additional GWAS data for validation.
This study represents the first attempt to investigate the causal association between COPD and psychiatric disorders using two-sample MR Analysis and GWAS-level summary data, effectively mitigating potential confounding factors and reverse causation through comprehensive aggregation of substantial genetic data. However, it is important to acknowledge the limitations of this study. Firstly, the sample population was restricted to individuals of European descent; thus, caution should be exercised when generalizing these findings to broader populations. To ensure the validity of our MR study, we selected independent SNPs that achieved genome-wide significance (P < 5×108), reducing the potential impact of weak instrumental variables. Consequently, only a limited number of SNPs remained after rigorous screening. However, future GWAS studies with larger sample sizes are warranted to identify additional SNPs. Substantial heterogeneity was observed among certain SNPs, which may still introduce potential bias into the results despite employing a random-effects model.
Conclusions
In conclusion, ever-smokers with COPD have a greater risk of developing ADHD but may reduce the risk of Alzheimer’s disease to some extent. No causal association with psychiatric disorders was observed in COPD patients without a smoking history. Therefore, attention needs to be paid to preventing psychiatric disorders in COPD patients with a smoking history.
Ethics Approval and Consent to Participate
As per the regulations outlined in People’s Republic of China’s “Notice on the Implementation of Ethical Review Measures for Life Science and Medical Research”, our study falls under the exemption criteria specified in Discussion of the regulation. Therefore, ethics approval was not required for this research, as it met the following conditions:
- Exemption Premise: This study used only publicly available data, especially summary level data from GWAS, did not involve sensitive personal information, did not cause harm to individuals, and did not compromise their privacy.
- Exemption Provision: Our research adheres to the exemption circumstances outlined in Discussion of the regulation: We utilized lawfully obtained publicly available data for our analysis. The data used in this study were fully anonymized, ensuring the privacy and confidentiality of individuals. Our research focuses on analyzing existing data and does not involve interventions, human biological samples, or activities related to reproductive cloning, genetic manipulation, or germ cells.
Due to the nature of our study and its compliance with the exemption criteria, we did not require explicit ethics approval. And we affirm that this research was conducted in accordance with the applicable laws, regulations, and ethical standards.
Acknowledgments
We thank Home for Researchers editorial team for language editing service.
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Guo P, Li R, Piao TH, Wang CL, Wu XL, Cai HY. Pathological mechanism and targeted drugs of COPD. Int J Chron Obstruct Pulmon Dis. 2022;17:1565–1575. doi:10.2147/COPD.S366126
2. Tashkin DP, Goodin T, Bowling A, et al. Effect of smoking status on lung function, patient-reported outcomes, and safety among COPD patients treated with glycopyrrolate inhalation powder: pooled analysis of GEM1 and GEM2 studies. Respir Res. 2019;20(1):135. doi:10.1186/s12931-019-1112-0
3. Burkow TM, Vognild LK, Johnsen E, Bratvold A, Risberg MJ. Promoting exercise training and physical activity in daily life: a feasibility study of a virtual group intervention for behaviour change in COPD. BMC Med Inform Decis Mak. 2018;18(1):136. doi:10.1186/s12911-018-0721-8
4. van Boven JF, van Raaij JJ, van der Galien R, et al. Impact of multiple-dose versus single-dose inhaler devices on COPD patients’ persistence with long-acting beta(2)-agonists: a dispensing database analysis. NPJ Prim Care Respir Med. 2014;24:14069. doi:10.1038/npjpcrm.2014.69
5. Ge H, Liu X, Gu W, et al. Distribution of COPD comorbidities and creation of acute exacerbation risk score: results from SCICP. J Inflamm Res. 2021;14:3335–3348. doi:10.2147/JIR.S315600
6. Bel EH. Obesity and asthma control: do gender, age, and ethnicity really matter? Am J Respir Crit Care Med. 2013;187(7):667–668. doi:10.1164/rccm.201302-0333ed
7. Yohannes AM, Alexopoulos GS. Depression and anxiety in patients with COPD. Eur Respir Rev. 2014;23(133):345–349. doi:10.1183/09059180.00007813
8. Pollok J, van Agteren JEM, Carson KV, Esterman AJ, Smith BJ, Licinio J. Psychological therapies for the treatment of depression in chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2019;3(3):CD012347. doi:10.1002/14651858.Cd012347
9. Bonnevie T, Medrinal C, Combret Y, et al. Mid-term effects of pulmonary rehabilitation on cognitive function in people with severe chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2020;15:1111–1121. doi:10.2147/COPD.S2494093
10. Su VY, Hu LY, Yeh CM, et al. Chronic obstructive pulmonary disease associated with increased risk of bipolar disorder. Chron Respir Dis. 2017;14(2):151–160. doi:10.1177/1479972316680846
11. Davies NM, Holmes MV, Davey Smith G. Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ. 2018;362:k601. doi:10.1136/bmj.k601
12. Smith GD, Lawlor DA, Harbord R, Timpson N, Day I, Ebrahim S. Clustered environments and randomized genes: a fundamental distinction between conventional and genetic epidemiology. PLoS Med. 2007;4(12):e352. doi:10.1371/journal.pmed.0040352
13. Pingault JB, O’Reilly PF, Schoeler T, Ploubidis GB, Rijsdijk F, Dudbridge F. Using genetic data to strengthen causal inference in observational research. Nat Rev Genet. 2018;19(9):566–580. doi:10.1038/s41576-018-0020-3
14. Bellenguez C, Kucukali F, Jansen IE, et al. New insights into the genetic etiology of Alzheimer’s disease and related dementias. Nat Genet. 2022;54(4):412–436. doi:10.1038/s41588-022-01024-z
15. Watson HJ, Yilmaz Z, Thornton LM, et al. Genome-wide association study identifies eight risk loci and implicates metabo-psychiatric origins for anorexia nervosa. Nat Genet. 2019;51(8):1207–1214. doi:10.1038/s41588-019-0439-2
16. Schoeler T, Speed D, Porcu E, Pirastu N, Pingault JB, Kutalik Z. Participation bias in the UK biobank distorts genetic associations and downstream analyses. Nat Hum Behav. 2023;7(7):1216–1227. doi:10.1038/s41562-023-01579-9
17. Demontis D, Walters GB, Athanasiadis G, et al. Genome-wide analyses of ADHD identify 27 risk loci, refine the genetic architecture and implicate several cognitive domains. Nat Genet. 2023;55(2):198–208. doi:10.1038/s41588-022-01285-8
18. Stahl EA, Breen G, Forstner AJ, et al. Genome-wide association study identifies 30 loci associated with bipolar disorder. Nat Genet. 2019;51(5):793–803. doi:10.1038/s41588-019-0397-8
19. Howard DM, Adams MJ, Clarke TK, et al. Genome-wide meta-analysis of depression identifies 102 independent variants and highlights the importance of the prefrontal brain regions. Nat Neurosci. 2019;22(3):343–352. doi:10.1038/s41593-018-0326-7
20. Cross-Disorder Group of the Psychiatric Genomics Consortium. Electronic address pmhe, Cross-Disorder Group of the Psychiatric Genomics C. Genomic relationships, novel loci, and pleiotropic mechanisms across eight psychiatric disorders. Cell. 2019;179(7):1469–1482 e11. doi:10.1016/j.cell.2019.11.020
21. International Obsessive Compulsive Disorder Foundation Genetics C, Studies OCDCGA. Revealing the complex genetic architecture of obsessive-compulsive disorder using meta-analysis. Mol Psychiatry. 2018;23(5):1181–1188. doi:10.1038/mp.2017.154
22. Nievergelt CM, Maihofer AX, Klengel T, et al. International meta-analysis of PTSD genome-wide association studies identifies sex- and ancestry-specific genetic risk loci. Nat Commun. 2019;10(1):4558. doi:10.1038/s41467-019-12576-w
23. Trubetskoy V, Pardinas AF, Qi T, et al. Mapping genomic loci implicates genes and synaptic biology in schizophrenia. Nature. 2022;604(7906):502–508. doi:10.1038/s41586-022-04434-5
24. Christensen KS, Oernboel E, Nielsen MG, Bech P. Diagnosing depression in primary care: a rasch analysis of the major depression inventory. Scand J Prim Health Care. 2019;37(2):256–263. doi:10.1080/02813432.2019.1608039
25. Zalsman G, Shilton T. Adult ADHD: a new disease? Int J Psychiatry Clin Pract. 2016;20(2):70–76. doi:10.3109/13651501.2016.1149197
26. Abecasis GR, Auton A, Brooks LD, et al. An integrated map of genetic variation from 1092 human genomes. Nature. 2012;491(7422):56–65. doi:10.1038/nature11632
27. Chang CC, Chow CC, Tellier LC, Vattikuti S, Purcell SM, Lee JJ. Second-generation PLINK: rising to the challenge of larger and richer datasets. Gigascience. 2015;4:7. doi:10.1186/s13742-015-0047-8
28. Hemani G, Bowden J, Davey Smith G. Evaluating the potential role of pleiotropy in Mendelian randomization studies. Hum Mol Genet. 2018;27(R2):R195–R208. doi:10.1093/hmg/ddy163
29. Zhu Z, Zhang F, Hu H, et al. Integration of summary data from GWAS and eQTL studies predicts complex trait gene targets. Nat Genet. 2016;48(5):481–487. doi:10.1038/ng.3538
30. Drago V, Babiloni C, Bartres-Faz D, et al. Disease tracking markers for Alzheimer’s disease at the prodromal (MCI) stage. J Alzheimers Dis. 2011;26(Suppl 3):159–199. doi:10.3233/JAD-2011-0043
31. Dodd JW. Lung disease as a determinant of cognitive decline and dementia. Alzheimers Res Ther. 2015;7(1):32. doi:10.1186/s13195-015-0116-3
32. Barnes PJ, Celli BR. Systemic manifestations and comorbidities of COPD. Eur Respir J. 2009;33(5):1165–1185. doi:10.1183/09031936.00128008
33. Higbee D, Granell R, Walton E, Korologou-Linden R, Davey Smith G, Dodd J. Examining the possible causal relationship between lung function, COPD and Alzheimer’s disease: a Mendelian randomisation study. BMJ Open Respir Res. 2021;8(1):e000759. doi:10.1136/bmjresp-2020-000759
34. Durazzo TC, Mattsson N, Weiner MW. Alzheimer’s disease neuroimaging i. smoking and increased Alzheimer’s disease risk: a review of potential mechanisms. Alzheimers Dement. 2014;10(3 Suppl):S122–S145. doi:10.1016/j.jalz.2014.04.009
35. Cataldo JK, Prochaska JJ, Glantz SA. Cigarette smoking is a risk factor for Alzheimer’s disease: an analysis controlling for tobacco industry affiliation. J Alzheimers Dis. 2010;19(2):465–480. doi:10.3233/JAD-2010-1240
36. Tyas SL, White LR, Petrovitch H, et al. Mid-life smoking and late-life dementia: the Honolulu-Asia Aging Study. Neurobiol Aging. 2003;24(4):589–596. doi:10.1016/s0197-4580(02)00156-2
37. Zeng H, Li T, He X, et al. Oxidative stress mediates the apoptosis and epigenetic modification of the Bcl-2 promoter via DNMT1 in a cigarette smoke-induced emphysema model. Respir Res. 2020;21(1):229. doi:10.1186/s12931-020-01495-w
38. Candela M, Costorella R, Stassaldi A, Maestrini V, Curradi G. Treatment of COPD: the simplicity is a resolved complexity. Multidiscip Respir Med. 2019;14:18. doi:10.1186/s40248-019-0181-8
39. Wadhwa R, Paudel KR, Mehta M, et al. Beyond the obvious: smoking and respiratory infection implications on Alzheimer’s disease. CNS Neurol Disord Drug Targets. 2020;19(9):698–708. doi:10.2174/1871527319999200817112427
40. Almeida OP, Garrido GJ, Alfonso H, et al. 24-month effect of smoking cessation on cognitive function and brain structure in later life. Neuroimage. 2011;55(4):1480–1489. doi:10.1016/j.neuroimage.2011.01.063
41. Campman CA, Sitskoorn MM. Better care for patients with COPD and cognitive impairment. Lancet Respir Med. 2013;1(7):504–506. doi:10.1016/S2213-2600(13)70163-2
42. Dodd JW, Novotny P, Sciurba FC, Benzo RP. Executive function, survival, and hospitalization in chronic obstructive pulmonary disease. a longitudinal analysis of the National Emphysema Treatment Trial (NETT). Ann Am Thorac Soc. 2015;12(10):1473–1481. doi:10.1513/AnnalsATS.201506-373OC
43. Hadanny A, Catalogna M, Yaniv S, et al. Hyperbaric oxygen therapy in children with post-concussion syndrome improves cognitive and behavioral function: a randomized controlled trial. Sci Rep. 2022;12(1):15233. doi:10.1038/s41598-022-19395-y
44. Connaughton M, Whelan R, O’Hanlon E, McGrath J. White matter microstructure in children and adolescents with ADHD. NeuroImage Clin. 2022;33:102957. doi:10.1016/j.nicl.2022.102957
45. Maremanda KP, Sundar IK, Rahman I. Role of inner mitochondrial protein OPA1 in mitochondrial dysfunction by tobacco smoking and in the pathogenesis of COPD. Redox Biol. 2021;45:102055. doi:10.1016/j.redox.2021.102055
46. Qin R, Liu Z, Zhou X, et al. Adherence and efficacy of smoking cessation treatment among patients with COPD in China. Int J Chron Obstruct Pulmon Dis. 2021;16:1203–1214. doi:10.2147/COPD.S301579
47. Fuemmeler BF, Kollins SH, McClernon FJ. Attention deficit hyperactivity disorder symptoms predict nicotine dependence and progression to regular smoking from adolescence to young adulthood. J Pediatr Psychol. 2007;32(10):1203–1213. doi:10.1093/jpepsy/jsm051
48. Kollins SH, McClernon FJ, Fuemmeler BF. Association between smoking and attention-deficit/hyperactivity disorder symptoms in a population-based sample of young adults. Arch Gen Psychiatry. 2005;62(10):1142–1147. doi:10.1001/archpsyc.62.10.1142
49. Recio Iglesias J, Díez-Manglano J, López García F, Díaz Peromingo JA, Almagro P, Varela Aguilar JM. Management of the COPD patient with comorbidities: an experts recommendation document. Int J Chron Obstruct Pulmon Dis. 2020;15:1015–1037. doi:10.2147/copd.S242009
50. Fuentes-Alonso M, Lopez-Herranz M, Lopez-de-andres A, et al. Prevalence and determinants of mental health among COPD patients in a population-based sample in Spain. J Clin Med. 2021;10(13):2786. doi:10.3390/jcm10132786
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
