Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 16
Association of Chronic Obstructive Pulmonary Disease with Morbidity and Mortality in Patients with Peripheral Artery Disease: Insights from the EUCLID Trial
Authors Galani J , Mulder H, Rockhold FW , Weissler EH, Baumgartner I, Berger JS, Blomster JI, Fowkes FGR, Hiatt WR, Katona BG, Norgren L, Mahaffey KW, Quint JK , Patel MR, Jones WS
Received 21 November 2020
Accepted for publication 14 March 2021
Published 29 March 2021 Volume 2021:16 Pages 841—851
DOI https://doi.org/10.2147/COPD.S292978
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Richard Russell
Jemi Galani,1 Hillary Mulder,2 Frank W Rockhold,2 E Hope Weissler,2,3 Iris Baumgartner,4 Jeffrey S Berger,5 Juuso I Blomster,6 F Gerry R Fowkes,7 William R Hiatt,8,9 Brian G Katona,10 Lars Norgren,11 Kenneth W Mahaffey,12 Jennifer K Quint,13 Manesh R Patel,1,2 W Schuyler Jones1,2
1Department of Medicine, Duke University Medical Center, Durham, NC, USA; 2Duke Clinical Research Institute, Duke University School of Medicine, Durham, NC, USA; 3Department of Surgery, Division of Vascular Surgery, Duke University Health System, Durham, NC, USA; 4Department of Medicine, Swiss Cardiovascular Center, University of Bern, Bern, Switzerland; 5Departments of Medicine and Surgery, New York University School of Medicine, New York, NY, USA; 6University of Turku, Turku, Finland; 7Usher Institute of Population Health Sciences and Informatics, University of Edinburgh, Edinburgh, UK; 8Division of Cardiology, University of Colorado School of Medicine, Aurora, CO, USA; 9CPC Clinical Research, Aurora, CO, USA; 10AstraZeneca, Gaithersburg, MD, USA; 11Faculty of Medicine and Health, Örebro University, Örebro, Sweden; 12Stanford Center for Clinical Research, Stanford University School of Medicine, Stanford, CA, USA; 13Respiratory Epidemiology, Occupational Medicine and Public Health, National Heart and Lung Institute, Imperial College London, London, UK
Correspondence: W Schuyler Jones
Duke University Medical Center, Box 3330, Durham, NC, 27710, USA
Tel +1 919-668-8917
Fax +1 919-668-7026
Email [email protected]
Background: Patients with chronic obstructive pulmonary disease (COPD) are at increased risk of developing lower extremity peripheral artery disease (PAD) and suffering PAD-related morbidity and mortality. However, the effect and burden of COPD on patients with PAD is less well defined. This post hoc analysis from EUCLID aimed to analyze the risk of major adverse cardiovascular events (MACE) and major adverse limb events (MALE) in patients with PAD and concomitant COPD compared with those without COPD, and to describe the adverse events specific to patients with COPD.
Methods: EUCLID randomized 13,885 patients with symptomatic PAD to monotherapy with either ticagrelor or clopidogrel for the prevention of MACE. In this analysis, MACE, MALE, mortality, and adverse events were compared between groups with and without COPD using unadjusted and adjusted Cox proportional hazards model.
Results: Of the 13,883 patients with COPD status available at baseline, 11% (n=1538) had COPD. Patients with COPD had a higher risk of MACE (6.02 vs 4.29 events/100 patient-years; p< 0.001) due to a significantly higher risk of myocardial infarction (MI) (3.55 vs 1.85 events/100 patient-years; p< 0.001) when compared with patients without COPD. These risks persisted after adjustment (MACE: adjusted hazard ratio (aHR) 1.30, 95% confidence interval [CI] 1.11– 1.52; p< 0.001; MI: aHR 1.45, 95% CI 1.18– 1.77; p< 0.001). However, patients with COPD did not have an increased risk of MALE or major bleeding. Patients with COPD were more frequently hospitalized for dyspnea and pneumonia (2.66 vs 0.9 events/100 patient-years; aHR 2.77, 95% CI 2.12– 3.63; p< 0.001) and more frequently discontinued study drug prematurely (19.36 vs 12.54 events/100 patient-years; p< 0.001; aHR 1.34, 95% CI 1.22– 1.47; p< 0.001).
Conclusion: In patients with comorbid PAD and COPD, the risks of MACE, respiratory-related adverse events, and premature study drug discontinuation were higher when compared with patients without COPD.
Registration: ClinicalTrials.gov: NCT01732822.
Keywords: peripheral artery disease, chronic obstructive pulmonary disease, major adverse cardiovascular events
Chronic obstructive pulmonary disease (COPD) is a leading cause of death worldwide.1 Due to shared risk factors, particularly cigarette smoking, patients with COPD are at increased risk of developing cardiovascular disease.2–4 In patients with known COPD, peripheral artery disease (PAD) is more prevalent than in age-matched patients without COPD, affecting 8.8% of a cohort of patients with COPD with a mean age of 65 compared with 1.8% of those without COPD.5 In prospective studies of patients with COPD, a diagnosis of PAD was associated with lower exercise tolerance, a higher incidence of major adverse cardiovascular events (MACE), and higher all-cause mortality.6–8
In contrast, the prevalence of COPD among patients with PAD, as well as the added risk of MACE, major adverse limb events (MALE), and respiratory events associated with COPD, are less well defined. Understanding the additional burden associated with COPD concomitant with PAD has both clinical and research implications, including understanding the increased cardiovascular and limb risk, as well as adherence to medications, that may improve trial planning and patient management in this population. The EUCLID (Examining Use of Ticagrelor in Peripheral Artery Disease) study was a large cardiovascular outcomes trial in patients with symptomatic PAD that compared monotherapy between ticagrelor and clopidogrel, and it affords an opportunity to study the natural history of patients with PAD.9 Of note, ticagrelor carries the unique side effect of dyspnea, which may disproportionately affect medication adherence, and therefore clinical outcomes, among this cohort of patients with COPD.9 The primary aim of this analysis was to compare the occurrence of MACE and MALE in patients with PAD with and without concomitant COPD. The secondary aims were to compare all-cause mortality, Thrombolysis in Myocardial Infarction (TIMI) major bleeding, serious adverse events (all-cause and respiratory-related hospitalizations, premature study drug discontinuation), and non-serious adverse events (dyspnea, pneumonia, and malignancy) between groups in order to characterize the added burden of hospitalizations and adverse events associated with COPD. We hypothesized that patients with PAD and COPD have higher rates of MACE and MALE, are hospitalized for respiratory causes more often, prematurely discontinue study drug more frequently, and have dyspnea, pneumonia, and malignancy more often.
Methods
Study Design and Participants
Details of the EUCLID study design have been previously published.9,10 In brief, EUCLID was a multi-center, double-blind, active-comparator randomized controlled trial of 13,885 patients aged 50 years or older with lower extremity PAD comparing monotherapy with either ticagrelor (90 mg twice daily) or clopidogrel (75 mg once daily) in a 1:1 fashion. Enrollment criteria included either a history of lower extremity revascularization or an abnormal ankle-brachial index (ABI) ≤0.80. The presence or absence of COPD was assessed at the enrolling study visit on the basis of the documented medical history. For patients with a documented medical history of COPD, further data regarding the patients’ supplemental oxygen requirement and previous COPD-related hospitalizations was obtained (Figure 1). Information on COPD severity (i.e., Global Initiative for Obstructive Lung Disease [GOLD] criteria and pulmonary function testing) was not ascertained at any point in the study. The study was approved by all local Ethics Committees and Institutional Review Boards (Appendix Table I).
 |
Figure 1 Inclusion criteria for EUCLID trial. This figure highlights the inclusion criteria of patients for both the EUCLID trial and this COPD-focused post hoc analysis of the EUCLID trial. |
Outcomes
The primary efficacy and safety endpoints in EUCLID were MACE (a composite of cardiovascular death, myocardial infarction, or ischemic stroke) and major bleeding according to TIMI criteria. For the present analysis, our primary efficacy outcomes were MACE and MALE (a composite of hospitalization for acute limb ischemia or major lower extremity amputation). Secondary endpoints of interest were all-cause mortality, serious adverse events (all-cause hospitalization, hospitalization for dyspnea or pneumonia, and premature study drug discontinuation), and non-serious adverse events of special interest (dyspnea, pneumonia, malignancy, and any respiratory-related adverse event). Serious adverse events were defined as adverse events resulting in death or hospitalization. Each endpoint and adverse event was collected via the case report form at every clinic visit. The efficacy and safety endpoints of myocardial infarction (MI), cardiovascular death, stroke, acute limb ischemia, and bleeding were adjudicated according to the EUCLID Clinical Endpoints Classification Charter by board-certified cardiologists or neurologists, depending on the event type. Adverse events were systematically collected in all participants in the EUCLID trial. Those adverse events that were serious, including hospitalizations and events that led to study drug discontinuation, were reviewed by the Duke Clinical Research Institute Safety Surveillance Group, and these adverse events were cross-referenced with the Clinical Endpoints Classification database to ensure that clinical endpoints were not double counted.
Statistical Analysis
The analytic cohort included all patients randomized in EUCLID who received at least one dose of study drug and had information on COPD status at baseline (n=13,883). For baseline characteristics, continuous variables were reported as medians with associated 25th and 75th percentiles and were compared between patients with and without COPD using the Wilcoxon rank sum test. Categorical variables were reported as counts with percentages and were assessed using the chi-square test.
The efficacy and safety outcomes (MACE, MALE, bleeding, and all-cause mortality) and serious adverse events (all-cause and respiratory-related hospitalizations and premature study drug discontinuation) were evaluated as time-to-event outcomes. Incidence rates were calculated as the number of events per 100 patient-years of follow-up. The unique number of patients who experienced non-serious adverse events and the mean and median number of adverse events per patient are reported by COPD status.
The relationships between baseline COPD and efficacy and safety outcomes and serious adverse events were examined using unadjusted and adjusted Cox proportional hazards models. The models were adjusted for randomized treatment and differing sets of baseline clinical characteristics including age, sex, weight, body mass index, tobacco use, prior medical history, medications, limb symptoms at study entry, and prior revascularizations/amputations (Appendix Table II). The proportional hazards assumption was assessed using weighted Schoenfield residuals and no major violations were found. The linearity assumption for continuous adjustment variables was also examined, and natural cubic splines were used when necessary. Hazard ratios (HR) with 95% confidence intervals (CI) comparing patients with COPD with those without COPD at baseline and the associated p-values are presented. The analysis of non-serious adverse events did not undergo adjustment.
To evaluate the interaction between treatment arm and COPD, the unadjusted Cox proportional hazards models from above were additionally fit with randomized treatment and the interaction between treatment and COPD status. HRs with 95% CIs comparing ticagrelor with clopidogrel for those with and without COPD are presented with interaction p-values.
All analyses were performed by the Duke Clinical Research Institute (Durham, NC) using SAS version 9.4 (SAS Institute, Inc. Cary, NC).
Results
Study Population and Clinical Characteristics
Of the 13,883 patients enrolled in EUCLID who received at least one dose of study drug and had COPD status available, a total of 1538 patients (11%) had COPD at baseline (Table 1). Patients with COPD were similarly distributed between the ticagrelor (50.9% [n=783]) and clopidogrel (49.1% [n=755]) treatment groups. Of those with COPD, 5.7% (n=87) required daily oxygen therapy and 12.4% (n=191) had a COPD hospitalization in the year prior to enrollment. Patients with baseline COPD were older than those without COPD (median 67 vs 66 years, p<0.001), weighed more (median 78 kg vs 76kg, p=0.012), and were more likely to be current or former smokers (92.5% vs 76.6%, p<0.001). Patients with COPD more frequently had a history of transient ischemic attack (TIA) (5.3% vs 3.4%), carotid artery disease (26.6% vs 18.3%), coronary artery disease (CAD) (35.6% vs 28.2%), MI (21.4% vs 17.8%), hypertension (83.4% vs 77.6%), hyperlipidemia (82.3% vs 74.6%), and prior malignancy (12.9% vs 7.3%) compared with patients without COPD (all p-values <0.001). Interestingly, patients with PAD and COPD had lower rates of diabetes when compared with those without COPD (32.6% vs 39.2%, p<0.001).
 |
Table 1 Baseline Clinical Characteristics by COPD at Baseline |
Patients with PAD and COPD had a higher rate of statin (77.5% vs 72.8%, p<0.001) and antiplatelet use prior to study entry, specifically aspirin (71.3% vs 66.2%, p<0.001) and clopidogrel (39.4% vs 31.3%, p<0.001). Patients with PAD and COPD had more frequently undergone a previous lower extremity revascularization (63.6% vs 55.9%, p<0.001) and experienced more severe limb symptoms (severe claudication and ischemic rest pain) at study entry when compared with the remaining study population.
Unadjusted Outcomes
Efficacy and Safety Outcomes
The risk of occurrence of the primary composite endpoint (cardiovascular death, MI, or ischemic stroke) is shown in Figure 2 and Appendix Figure I. There was a significantly higher risk of MACE in patients with PAD and COPD compared with those without COPD (6.02 vs 4.29 events/100 patient-years, p<0.001). This was primarily driven by a higher occurrence of MI (3.55 vs 1.85 events/100 patient-years, p<0.001), with no statistically significant difference in the number of cardiovascular deaths (2.35 vs 2.01 events/100 patient-years, p=0.059) or ischemic strokes (0.85 vs 0.89 events/100 patient-years, p=0.691). Specifically, 50.0% (n=65) of the MIs experienced by patients with concomitant PAD and COPD were type 1 and 43.8% (n=57) were type 2, compared with 61.5% (n=340) type 1 and 32.4% (n=179) type 2 MIs in patients without COPD. After excluding type 2 MI from the endpoint definitions, the higher risk of MACE in patients with PAD and COPD compared with those without COPD persisted (4.78 vs 3.79 events/100 patient-years, p=0.004), as did risk of MI (2.05 vs 1.28 events/100 patient-years, p<0.001). The occurrence of MALE was 1.10 events per 100 patient-years in patients with COPD and 1.19 events per 100 patient-years in patients without COPD (p=0.54). Patients with COPD did not have any increase in major bleeding (0.90 vs 0.74 events/100 patient-years, p=0.34), but did have higher all-cause mortality (5.01 vs 3.46 events/100 patient-years, p<0.001) compared with those without COPD.
Serious Adverse Events
All-cause hospitalizations were significantly higher among patients with COPD when compared with patients without COPD (33.30 vs 21.53 events/100 patient-years, p<0.001). Specifically, patients with PAD and COPD were hospitalized for dyspnea and pneumonia more frequently when compared with patients without COPD (2.66 vs 0.90 events/100 patient-years, p<0.001). Patients with COPD had a significantly greater rate of premature study drug discontinuation (19.36 vs 12.54 events/100 patient-years, p<0.001) than those without COPD. Table 2 further specifies serious adverse events by history of COPD.
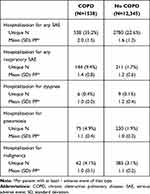 |
Table 2 Serious Adverse Events by History of COPD |
Non-Serious Adverse Events
Table 3 details the non-serious adverse events by history of COPD. The frequency of all adverse events and respiratory-related adverse events was higher in patients with PAD and COPD compared with those without COPD. Among patients with baseline COPD, more patients experienced at least one respiratory-related adverse event than patients without baseline COPD (22.8% vs 8.9%). However, the number of respiratory-related adverse events experienced per patient was similar between the two groups (mean 1.4 vs 1.2 adverse events per patient). When examining specific adverse events of interest, patients with PAD and COPD were more likely to report dyspnea (14.6% vs 7.1%), pneumonia (6.7% vs 2.9%), and malignancy (6.1% vs 4.3%) when compared with patients without baseline COPD. Patients with PAD and COPD were also more likely to require hospitalization for a serious adverse event (35.2% vs 22.6%) than those without COPD.
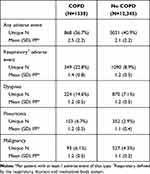 |
Table 3 Adverse Events by History of COPD |
Adjusted Outcomes
Efficacy and Safety Outcomes
Figure 3 details the risk of occurrence of the efficacy and safety endpoints after adjustment for baseline characteristics. The adjusted hazard ratio (aHR) for MACE was 1.30 (95% CI 1.11–1.52, p<0.001) and for MI was 1.45 (95% CI 1.18–1.77, p<0.001). After excluding type 2 MI, the aHR for MACE was 1.21 (95% CI 1.02–1.43, p=0.030) and for MI was 1.26 (95% CI 0.97–1.62, p=0.083). Patients with PAD and COPD did not have a higher risk of adverse limb events (aHR 0.98, 95% CI 0.71–1.37, p=0.92) than those without COPD. Patients with PAD and COPD had a higher risk of all-cause mortality (aHR 1.50, 95% CI: 1.27–1.76, p<0.001) compared with those without COPD. However, there was no increased risk of bleeding observed in patients with PAD and COPD when compared with patients without baseline COPD (aHR 1.04, 95% CI 0.69–1.56, p=0.866), and there was no interaction between COPD status and response to ticagrelor for any clinical outcome.
Serious Adverse Events
Patients with COPD were at higher risk of all-cause hospitalization (aHR 1.41, 95% CI 1.31–1.53, p<0.001) after adjustment, and specifically at risk of hospitalization for dyspnea and pneumonia (aHR 2.77, 95% CI 2.12–3.63, p<0.001) compared with those without COPD. Patients with PAD and COPD were more likely to prematurely discontinue their assigned study drug when compared with patients without baseline COPD, regardless of whether it was ticagrelor or clopidogrel (19.36 vs 12.54 discontinuations/100 patient-years; aHR 1.34, 95% CI 1.22–1.47, p<0.001). In patients with PAD both with and without COPD, there was an increased risk of premature study drug discontinuation with ticagrelor compared with clopidogrel (PAD with COPD: aHR 1.22, 95% CI 1.04–1.44; PAD without COPD: aHR 1.21, 95% CI 1.13–1.30). However, there was no significant difference in the hazard ratios comparing ticagrelor with clopidogrel discontinuation between patients with and without COPD (interaction p=0.913).
Discussion
We evaluated the past medical history, clinical outcomes and adverse events in patients with concomitant PAD and COPD using data from the EUCLID study, yielding four major findings. First, patients with PAD and COPD had more comorbidities at baseline and had more severe PAD at the time of study entry than those without COPD. Second, patients with PAD and COPD had a higher risk of MACE (primarily driven by a significantly higher rate of MI) and a higher risk of all-cause mortality and all-cause hospitalization, findings that persisted after adjustment for baseline characteristics. Third, patients with PAD and COPD did not have a higher risk of MALE compared with patients with PAD without COPD, despite more severe PAD at baseline. Finally, patients with PAD and COPD more frequently experienced respiratory-related serious adverse events, including respiratory-related hospitalizations, and were more likely to discontinue the study drug prematurely, regardless of whether it was ticagrelor or clopidogrel.
The few prior studies of patients with concomitant PAD and COPD have focused on mortality, which they reported was higher in this population, consistent with our data.11,12 Our analysis adds depth and nuance to these findings, showing that the increase in all-cause mortality is not due to cardiovascular mortality, while patients with COPD are also at significantly greater risk of other clinically-important events. Patients with COPD in addition to PAD carry an increased risk of MACE driven by significantly higher risks of MI, specifically type 2 MI, as well as increased risks of all-cause and respiratory-related hospitalizations. Patients with PAD who were hospitalized for acute pulmonary events have been shown to be at increased risk of MACE following the hospitalization, and the same has been observed in patients both with and without COPD.13–16 This is hypothesized to be due to the inflammatory state associated with pulmonary hospitalizations.13,15,16 It is possible that the increased risk of MI we observed in EUCLID participants with PAD and COPD is attributable to the more frequent respiratory-related adverse events experienced by the patients with PAD and baseline COPD. Furthermore, these pulmonary exacerbations may lead to hypoxia, creating an imbalance between myocardial oxygen demand and delivery and an increased frequency of type 2 MI seen in patients with concomitant PAD and COPD when compared with patients without COPD.17 Although a higher frequency of type 2 MI is expected in patients with COPD, our data shows persistence of the higher risk of MACE in patients with concomitant PAD and COPD even after exclusion of type 2 MI from the primary endpoints, suggesting that type 2 MI may not be the sole cause of increased mortality in this population. Interestingly, while MALE is also associated with pro-inflammatory states such as those that might be experienced during COPD exacerbations, there was no increased risk for MALE in patients with COPD.
This study provides new information on adverse events unique to patients with concomitant PAD and COPD. One noteworthy observation was an increased rate of premature study drug discontinuation in patients with PAD and COPD, with a higher risk of discontinuing ticagrelor compared with clopidogrel in patients both with and without COPD. Ticagrelor carries the unique side effect of dyspnea, which may be particularly problematic for patients with COPD.9 In a meta-analysis of four randomized clinical trials of ticagrelor, including EUCLID, premature ticagrelor discontinuation was seen in 25% of patients, and the highest risk of discontinuation was among patients who experienced dyspnea, a more common adverse event in patients with PAD and COPD compared with those without COPD.18 Ticagrelor-related dyspnea may compound COPD-related dyspnea, complicating clinicians’ efforts to monitor patients with COPD for cardiac and pulmonary dysfunction.
While establishing worse clinical outcomes and a higher rate of adverse events for patients with concomitant PAD and COPD, these findings also demonstrate the need for further research in this area. For instance, given the association between pulmonary hospitalizations and cardiovascular events, as well as the possibility of undiagnosed COPD among patients with PAD (given the overlapping risk factors), evaluation of enhanced monitoring strategies for cardiovascular events following pulmonary hospitalizations in patients with PAD should be considered. While the higher risk of MACE within this population was driven solely by a significantly higher risk of MI, there is a dearth of evidence about the causes of non-cardiovascular death within this population. Additionally, further clarification is needed regarding the causes of premature study drug discontinuation, which may be attributable to dyspnea or to decreased medication adherence associated with polypharmacy, hospitalizations, and increasing COPD symptoms in this population.19,20
There were limitations to this analysis. First, this was a post hoc analysis of a subgroup of patients that represented 5% of the overall study population. While this analysis focused only on patients with symptomatic PAD, COPD is more prevalent among patients with asymptomatic PAD, a group not included in this study.7,12 Second, discrete data on COPD severity (i.e., GOLD criteria, pulmonary function testing results, medications for COPD) were not ascertained at any point in the study, thereby making it difficult to correlate severity of baseline COPD with clinical outcomes. We relied on investigator reported history of COPD rather than any formal testing at time of enrollment. As a result, we may have failed to capture undiagnosed or less severe cases of COPD in the PAD alone group, as well as overreported cases of COPD in the COPD and PAD group due to misclassification of other lung diseases such as asthma.21 Additionally, other comorbid chronic conditions in patients with COPD, such as heart failure, a highly possible alternative diagnosis in the EUCLID cohort, may be misdiagnosed as a COPD exacerbation.22 This may provide a falsely elevated rate of respiratory-related adverse events. Third, we were not able to ascertain whether the rate of ticagrelor discontinuation among patients with PAD and COPD for the reason of dyspnea was higher than in patients without COPD. Therefore, it is unclear if the decreased pulmonary reserve in patients with COPD was driving the increased risk of premature study drug discontinuation within that subgroup.
Conclusions
In patients with PAD and baseline COPD, the risk of major adverse cardiovascular events, all-cause mortality, all-cause hospitalizations, and respiratory-related adverse events was higher when compared with patients with PAD without COPD. Surprisingly, the rate of major adverse limb events was similar between patients with PAD with and without COPD. Patients with COPD were also more likely to discontinue study medication (either ticagrelor or clopidogrel) when compared with patients without COPD; however, after adjustment, the rate of discontinuation of ticagrelor was similar in patients with and without COPD. The results from this study suggest that the increased risk of multiple respiratory- and non-respiratory-related clinical events among patients with both COPD and PAD warrant increased efforts to investigate and prevent cardiovascular and respiratory events in this population.
Data Sharing Statement
The authors do not plan to share individual deidentified participant data at this time.
Funding
The EUCLID study was funded by AstraZeneca. This analysis was funded by independently by the authors.
Disclosure
FWR: Grants from AstraZeneca during the conduct of the study; personal fees from Merck Research Labs, GSK, Sanofi, Novartis, Lilly, Rhythm, Tolerion, NovoNordisk, UCB, AstraZeneca, Merck KGaA, Janssen, Sarepta, Eidos, Phathom, Amgen, honoraria from Athira Pharma, Spencer Health Solutions, and DataVant outside the submitted work; and ownership in HunterRockhold, Inc, equity in GlaxoSmithKline, Athira, Spencer Health Solutions, DataVant. JSB: Institutional research grants from Astra Zeneca, National Heart, Lung, and Blood Institute, and American Heart Association; Consulting fees from Amgen, Janssen, Merck, Takeda. JIB: Consultation for and prior employment with AstraZeneca. FGRF: Member of advisory boards for AstraZeneca, Bayer, Merck. WRH: Grant support from Amgen, grant support from NIH and grants to CPC from Bayer, Janssen, Amgen. BGK: Employee of AstraZeneca. LN: Honorarium/other from Pluristem, AnGes, Bayer. KWM: Personal fees from Abbott, grants from Afferent, AHA, grants, personal fees from Amgen, personal fees from Anthos, grants from Apple, Inc, grants, personal fees from AstraZeneca, personal fees from Baim Institute, grants, personal fees from Bayer, personal fees from Boehringer Ingelheim, grants from Cardiva Medical, Inc, personal fees from CSL Behring, grants from Eidos, personal fees from Elsevier, grants from Ferring, grants from Gilead, grants from Google (Verily), personal fees from Inova, personal fees from Intermountain Health, grants, personal fees from Johnson & Johnson, grants from Luitpold, personal fees from Medscape, grants from Medtronic, grants from Merck, personal fees from Mount Sinai, personal fees from Mundi Pharma, personal fees from Myokardia, grants, personal fees from NIH, grants, personal fees from Novartis, personal fees from Novo Nordisk, personal fees from Otsuka, personal fees from Portola, personal fees from Regeneron, grants from Sanifit, grants, personal fees from Sanofi, personal fees from SmartMedics, grants from St. Jude, personal fees from Theravance; more disclosure information is available at https://profiles.stanford.edu/kenneth-mahaffey?tab=research-and-scholarship. JKQ: Research grants from The Health Foundation, MRC, GlaxoSmithKline, Bayer, Boehringer Ingelheim, British Lung Foundation, Chiesi, AstraZeneca, Insmed and Asthma UK outside the submitted work; Honorarium/personal fees from GlaxoSmithKline, Boehringer Ingelheim, AstraZeneca, Bayer, Insmed. MRP: Institutional research grants from AstraZeneca, CSL, HeartFlow, Janssen Research; Personal and grants from Bayer. WSJ: Research Grants from Boehringer Ingelheim, Doris Duke Charitable Foundation, National Institutes of Health, Patient-Centered Outcomes Research Institute; Honorarium/other from Bayer, Bristol-Myers Squibb, Janssen Pharmaceuticals. The authors report no other conflicts of interest in this work.
References
1. Lozano R, Naghavi M, Foreman K. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2013;380:2095–2128. doi:10.1016/S0140-6736(12)61728-0
2. Negewo N, Gibson P, McDonald V. COPD and its comorbidities: impact, measurement and mechanisms. Respirology. 2015;20(8):1160–1171. doi:10.1111/resp.12642
3. Fabbri L, Rabe K. From COPD to chronic systemic inflammatory syndrome? Lancet. 2007;370(9589):797–799. doi:10.1016/S0140-6736(07)61383-X
4. Fowkes F, Rudan D, Rudan I, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013;382(9901):1329–1340. doi:10.1016/S0140-6736(13)61249-0
5. Houben-Wilke S, Jorres R, Bals R, et al. Peripheral artery disease and its clinical relevance in patients with chronic obstructive pulmonary disease in the COPD and systemic consequences - comorbidities network study. Am J Respir Crit Care Med. 2017;195:189–197. doi:10.1164/rccm.201602-0354OC
6. Castagna O, Boussuges A, Nussbaum E, Marqueste L, Brisswalter J. Peripheral arterial disease: an underestimated aetiology of exercise intolerance in chronic obstructive pulmonary disease patients. Eur J Cardiovasc Prev Rehabil. 2008;15(3):270–277. doi:10.1097/HJR.0b013e3282f009a9
7. Crisafulli E, Scelfo C, Tzani P, Aiello M, Bertorelli G, Chetta A. Asymptomatic peripheral artery disease can limit maximal exercise capacity in chronic obstructive pulmonary disease patients regardless of airflow obstruction and lung hyperinflation. Eur J Prev Cardiol. 2017;24(9):990–999. doi:10.1177/2047487317695629
8. de Liefde II, Van Domburg R, Bax J, Klein J, Verhagen H, Poldermans D. A decline in walking distance predicts long-term outcome in patients with known or suspected peripheral artery disease. Eur J Cardiovasc Prev Rehabil. 2009;38:482–487.
9. Hiatt WR, Fowkes FG, Heizer G, et al. Ticagrelor versus clopidogrel in symptomatic peripheral artery disease. N Engl J Med. 2017;376(1):32–40. doi:10.1056/NEJMoa1611688
10. Berger J, Katona B, Jones W, et al. Design and rationale for the effects of ticagrelor and clopidogrel in patients with peripheral artery disease (EUCLID) trial. Am Heart J. 2016;175:86–93. doi:10.1016/j.ahj.2016.01.018
11. Kaszuba M, Śliwka A, Piliński R, et al. The comorbidity of chronic obstructive pulmonary disease and peripheral artery disease–a systematic review. J Chronic Obstruct Pulmon Dis. 2019;16:292–302. doi:10.1080/15412555.2019.1653271
12. Terzikhan N, Lahousse L, Verhamme K, et al. COPD is associated with an increased risk of peripheral artery disease and mortality. ERJ Open Res. 2018;4(4):00086–2018. doi:10.1183/23120541.00086-2018
13. Kunisaki K, Dransfield M, Anderson J, et al. Exacerbations of chronic obstructive pulmonary disease and cardiac events. A post hoc cohort analysis from the SUMMIT randomized clinical trial. Am J Respir Crit Care Med. 2018;198(1):51–57. doi:10.1164/rccm.201711-2239OC
14. McDermott M, Tian L, Wunderink R, et al. Pulmonary hospitalizations and ischemic heart disease events in patients with peripheral artery disease. Vasc Med. 2017;22(3):218–224. doi:10.1177/1358863X16680461
15. Corrales-Medina VF, Alvarez KN, Weissfeld LA, et al. Association between hospitalization for pneumonia and subsequent risk of cardiovascular disease. JAMA. 2015;313(3):264–274. doi:10.1001/jama.2014.18229
16. Rothnie K, Mullerova H, Smeeth L, Pearce N, Douglas I, Quint J. Risk of myocardial infarction associated with acute exacerbations of COPD: effects of exacerbation frequency. ERJ. 2016;48:PA3107.
17. Saaby L, Poulsen T, Hosbond S, et al. Classification of myocardial infarction: frequency and features of type 2 myocardial infarction. Am J Med. 2013;126(9):789–797. doi:10.1016/j.amjmed.2013.02.029
18. Arora S, Shemisa K, Vaduganathan M, et al. Premature ticagrelor discontinuation in secondary prevention of atherosclerotic CVD. J Am Coll Cardiol. 2019;73(19):2454–2464. doi:10.1016/j.jacc.2019.03.470
19. Bender B. Nonadherence in chronic obstructive pulmonary disease patients: what do we know and what should we do next? Curr Opin Pulm Med. 2014;20(2):132–137. doi:10.1097/MCP.0000000000000027
20. Rogliani P, Ora J, Puxeddu E, Matera M, Cazzola M. Adherence to COPD treatment: myth and reality. Respir Med. 2017;129:117–123. doi:10.1016/j.rmed.2017.06.007
21. Diab N, Gershon AS, Sin DD, et al. Underdiagnosis and overdiagnosis of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2018;198(9):1130–1139. doi:10.1164/rccm.201804-0621CI
22. Beghe B, Verduri A, Roca M, Fabbri LM. Exacerbation of respiratory symptoms in COPD patients may not be exacerbations of COPD. Eur Respir J. 2013;41(4):993–995. doi:10.1183/09031936.00180812
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


