Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 18
Association of Blood Inflammatory Biomarkers with Clinical Outcomes in Patients with AECOPD: An 8-Year Retrospective Study in Beijing
Authors Shao S, Zhang Z, Feng L, Liang L, Tong Z
Received 3 May 2023
Accepted for publication 4 August 2023
Published 17 August 2023 Volume 2023:18 Pages 1783—1802
DOI https://doi.org/10.2147/COPD.S416869
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jill Ohar
Shuai Shao,1,* Zhijin Zhang,1,* Lin Feng,2 Lirong Liang,2 Zhaohui Tong1
1Department of Respiratory and Critical Care Medicine, Beijing Institute of Respiratory Medicine, Beijing Chao-Yang Hospital, Capital Medical University, Beijing, 100020, People’s Republic of China; 2Department of Clinical Epidemiology, Beijing Institute of Respiratory Medicine and Beijing Chao-Yang Hospital, Capital Medical University, Beijing, 100020, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Zhaohui Tong, Department of Respiratory and Critical Care Medicine, Beijing Institute of Respiratory Medicine, Beijing Chao-Yang Hospital, Capital Medical University, NO. 8, Gong Ti South Road, Chao-Yang District, Beijing, 100020, People’s Republic of China, Email [email protected]
Purpose: To discover potential inflammatory biomarkers, which can compare favorably with traditional biomarkers, and their best cut-offs at first admission to predict clinical outcomes (short-term and long-term) and the risk of readmission among acute exacerbations of chronic obstructive pulmonary disease (AECOPD) patients.
Patients and Methods: Novel inflammatory biomarkers (such as the neutrophil–lymphocyte ratio [NLR], platelet–lymphocyte ratio [PLR], etc.) were compared with traditional biomarkers by Pearson’s correlation test. Logistic regression analysis and receiver operating characteristic (ROC) curves were applied to judge the accuracy of these novel biomarkers to predict in-hospital mortality.
Results: Surviving AECOPD patients had lower NLR, PLR, and lymphocyte-to-monocyte ratios than non-survival patients (all P < 0.001). According to Pearson’s correlation test, there was a linear correlation between novel and traditional biomarkers (all P < 0.05). In terms of a single biomarker, the AUC value of NLR was the largest, which was not inferior to C-reactive protein (Z-P = 0.064), and superior to erythrocyte sedimentation rate (Z-P = 0.002) and other novel single inflammatory biomarkers (all Z-P < 0.05). The mortality of patients with NLR ≥ 4.43 was 2.308-fold higher than that of patients with NLR < 4.43. After dividing patients into a higher or lower NLR group, pooled results showed that patients with NLR ≥ 4.43 had a higher rate of treatment failure, intensive care unit admission, longer hospital length of stay, one-year mortality after the index hospitalization, and overall mortality than patients with NLR < 4.43 (all P < 0.001). Patients with NLR ≥ 4.43 were associated with higher and earlier first readmission due to AECOPD than patients with lower NLR.
Conclusion: NLR was the best to forecast the clinical prognosis and readmission risk among AECOPD patients, which was not inferior to CRP, and the best cut-off value of NLR was 4.43.
Keywords: acute exacerbations of chronic obstructive pulmonary disease, neutrophil–lymphocyte ratio, mortality, inflammatory biomarkers, readmission rate, treatment failure rate
Background
Chronic obstructive pulmonary disease (COPD), characterized by persistent respiratory symptoms and airflow limitation,1 is an important public health threat with high mortality worldwide. In 2019, COPD accounted for 212.3 million prevalent cases and 3.3 million deaths globally.2 The World Health Organization (WHO) estimated that COPD will rise to the third leading cause of death in 2030, with the corresponding economic burden ranking fifth.3 COPD-caused mortality is largely related to acute exacerbation of COPD (AECOPD), which is defined as an acute worsening of respiratory symptoms in COPD (increased dyspnea, increased sputum purulence or volume together with worse cough or wheezing) and requiring additional therapy.1 According to previous studies, the short-term mortality of AECOPD patients in China ranged from 8.4%4 to 12.2%,5 and the mortality increased with the number of readmissions for AECOPD.6 Based on a post hoc analysis that included 120 patients, the overall mortality was 26 (37.7%), with 3.4 mean duration of follow-up.7 As a result, in order to provide more appropriate treatment and management of AECOPD patients, it is essential to find appropriate biomarkers that can be used for early and precise identification of patients’ short-term and long-term clinical prognosis and readmission risk.
Since the most common causes of AE are respiratory tract infections, the most frequently used forecast biomarkers in clinical work among AECOPD patients are C-reactive protein (CRP), procalcitonin (PCT) and erythrocyte sedimentation rate (ESR), with the support of previous studies and the experience of clinicians. According to previous studies, higher CRP was associated with higher noninvasive positive pressure ventilation (NIPPV) failure during hospitalization and readmission rates among patients with AECOPD.8,9 PCT and ESR can distinguish AECOPD patients from AECOPD patients with infection (such as community acquired pneumonia [CAP]).10 Among them, PCT had guiding value for the selection of ventilation switching points in sequential mechanical ventilation, predicting bacterial infection and the use of antibiotic prescriptions.5,11–14 Due to the lack of relevant laboratory inspection equipment and the relatively high cost, the application of traditional inflammatory biomarkers, such as PCT and ESR, was limited at some grass root levels of rural medical and health institutions in China. This pushed us to find more accessible and cost-effective biomarkers to predict the short- and long-term clinical outcomes for these patients. Recently, the ratio of some inflammatory biomarkers, such as the neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR), has drawn great attention from physicians, since they can be assessed using a simple complete blood count (CBC) test.5,10,15–19 Several studies have shown that the value of NLR might be the best biomarker to predict the prognosis of AECOPD patients,19,20 while other studies have reported that blood indicators such as PLR are the best.21,22 However, most of the studies just focused on limited kinds of inflammatory biomarkers and there is a paucity of studies to comprehensively confirm the best inflammatory biomarkers versus other traditional or novel inflammatory biomarkers. At the same time, direct comparisons between different areas under the curve (AUCs) were not confirmed by statistical methods. Moreover, due to the limited number of patients and different study designs in those studies, the cut-off values of NLR (ranging from 3.1823 to 16.8324) or other blood inflammatory biomarkers were inaccurate and inconsistent.25
Therefore, we performed the largest sample size clinical study to date to explore the best single or combined inflammatory biomarkers to predict the clinical prognosis of AECOPD patients and evaluate their best cut-off values. In this study, we proposed that the NLR is superior to other novel inflammatory biomarkers refined from the CBC test and was not inferior to traditional inflammatory biomarkers like CRP and ESR in predicting the short- and long-term clinical outcomes among AECOPD patients. We hope to provide more rigorous and sufficient evidence-based medical evidence for clinicians to quickly judge the prognosis of patients and guide further treatment.
Materials and Methods
Data Source and Population
This was a single-center, retrospective study that included patients with AECOPD who were admitted to Beijing Chao-Yang Hospital from April 4th, 2013, to June 30th, 2021. All patient data were collected from the Hospital electronic medical record database (EMRD), which was operated by Beijing Chao-Yang Hospital, and the quality of the data was assured.26 The hospitalization records for patients aged ≥40 years old with a primary discharge diagnosis of AECOPD (ICD-10 codes J44.0–J44.9) were included in this study and were checked by two authors independently (SS and ZJZ). The diagnosis of COPD and AECOPD in Chao-Yang Hospital followed the global initiative for chronic obstructive lung disease (GOLD) guidelines over the years (detailed diagnostic criteria are listed in Supplementary Material 1). Hospitalized patients with corona virus disease 2019 (COVID-19) were not included during this study period, because the policy of Beijing Chao-Yang Hospital. Eventually, patients without CBC data or patients who did not undergo CBC testing within 48 hours after admission were excluded from this study. The clinical features and examination results in multiple hospitalizations of one patient could be correlated with each other. Thus, if one patient had several AECOPD hospitalization records, the first one was regarded as the index hospitalization and the subsequent records were considered as outcomes. This study was approved by the Research Ethics Board of Beijing Chao-Yang Hospital (project approval number: 2020-ke-544) and complied with the Declaration of Helsinki. All data extracted from the database were deidentified prior to analysis, making it impossible to identify individual-level data either in this study or in the retrieved database. Informed consent was waived due to the anonymous and mandatory nature of the data.
Data Collection and Quality Control
The data obtained in this study included patients’ demographic characteristics (age and sex, etc.), current smoking status, comorbidities, clinical symptoms, physical signs, laboratory tests of blood (CBC, arterial blood gas analysis, and blood chemistry, etc.), inflammatory markers (NLR, PLR, lymphocyte-to-monocyte ratio [LMR], platelet-to-mean platelet volume ratio [PMR], eosinophil-to-neutrophil ratio [ENR], eosinophil-to-lymphocyte ratio [ELR], CRP, PCT). Regarding the clinical outcomes, we included participant pharmacotherapy for AECOPD, respiratory support therapy (noninvasive mechanical ventilation [NIMV], invasive mechanical ventilation [IMV] or NIMV combined with IMV), length of stay (LOS), LOS > 14 days, admission to the intensive care unit (ICU), cost, first readmission due to AECOPD within 1 month/2 months/3 months/12 months/15 months/24 months after discharge (readmission data were obtained from Beijing Public Health Information Centre), organ failure, respiratory failure, treatment failure, in hospital mortality (during the index hospitalization period), one-year mortality after the index hospitalization, and overall mortality (the number of deaths during the last hospitalization based on the records from the Beijing Chao-Yang Hospital EMRD was divided by total cases). Spirometry was performed during the index hospitalization period among a limited population. The results of spirometry in other hospitals could not be found in the Hospital EMRD of Beijing Chao-Yang Hospital. Microbiological data of patients could not be obtained due to the lack of electronically available microbiological data.
Definitions
Organ failure was defined as either one of the following events: a) respiratory failure; b) liver failure; c) renal failure; or d) heart failure.
Treatment failure was defined as either one of the following events: a) IMV received during admission; b) admission to the ICU; c) organ failure; d) LOS > 14 days; or e) death during hospitalization.
Statistical Analysis
For continuous variables with a normal distribution, the mean ± standard deviation was employed to describe the presentation of data. The median and interquartile range (IQR) were employed for nonparametric continuous variables. Categorical variables are summarized using counts and percentages. Continuous variables were compared between two groups with t tests if the data were normally distributed; otherwise, the Mann–Whitney U-test was employed. Categorical variables were judged by using the chi-square test or Fisher’s exact test when the sample size was small. Spearman correlation analysis was used for correlation analysis, and the results were reported as correlation coefficients with corresponding P values. Receiver-operating characteristic (ROC) curves were applied to evaluate the ability of inflammatory biomarkers to predict in-hospital mortality. Because of the lack of established threshold values of inflammatory biomarkers, the best cut-off values were calculated in this study by using Youden’s index. The inflammation biomarkers with the largest area under the ROC curve were chosen to classify AECOPD patients to judge the clinical application value of the inflammation biomarkers. The Z test was used to compare the AUC value between each two indicators.27 Finally, to determine whether any of the clinical signs, symptoms, or laboratory test data were independently associated with in-hospital mortality, we performed logistic regression analyses by using a conditional forward stepwise regression model, and the results were reported as adjusted odds ratios (ORs), 95% confidence intervals (CIs) and P values. In addition, to make the results of this research feasible to clinicians, a nomogram was o established by the “rms” package in R software (version 3.6.0). A calibration curve was plotted to verify the relationship between the event rate predicted by the nomogram model and the actual situation. The bootstrap self-sampling method (the number of self-sampling times B=1000) was used for internal validation to reduce over-fitting deviation, and the concordance index (C-index) and ROC curve were used to evaluate the prediction accuracy of the nomogram. An AUC or C-Index less than 0.65 indicates poor model discrimination, 0.65 to 0.75 indicates that the model has some discriminatory ability, and a value greater than 0.75 indicates that the model has a good discriminatory ability.28 Finally, a Kaplan–Meier (K–M) curve was applied to visualize the data of the first readmission due to AECOPD after discharge among AECOPD patients who were included in this study. All values of p less than 0.05 were considered statistically significant. Statistical analysis was performed using SPSS 26.0 (IBM Corp., Armonk, NY) software, R software (version 3.6.0) and MedMalc software.
Result
Figure 1 shows the selection process of patients. There were 7059 hospitalization records for patients aged ≥40 years old with a primary discharge diagnosis of AECOPD (ICD-10 codes J44.0–J44.9) from April 4th, 2013 to June 30th, 2021. We deleted 2409 records of repeated admissions and retained 4650 initial admission records. A total of 265 patients without CBC data and 2 patients with abnormal data were excluded. In addition, 148 patients whose first CBC exceeded 48 h after admission were excluded from this study. Finally, 4235 eligible patients were included in this study. Among these patients, 64 cases were classified into the non-survival group, and 4171 cases were classified into the survival group. Four hundred and forty-nine patients had spirometry during the index hospitalization, the median forced expiratory volume in one second (FEV1) was 1.18 L (0.77–1.81 L), and the median FEV1/forced vital capacity was 48.15% (38.40–59.83%). Due to the limited cases reported for spirometry, they were not included in further analyses.
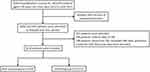 |
Figure 1 Patient flow chart. |
Baseline Characteristics of the Included Subjects
General characteristics are listed in Table 1. The median age of all patients was 71.00 (63.00–78.00) years, and the median body mass index (BMI) was 22.96 (20.07–26.02) kg/m2. Among all of these AECOPD patients, 74.1% were male, 23.7% were current smokers, and 49.9% were former smokers. Regarding comorbidities, cardiovascular disease was the most common disease (69.7%), followed by hypertension (45.2%) and diabetes mellitus (16.3%). Comparing the parameters of the non-survival and survival groups at admission, there were no significant differences between them for sex (p = 0.205), BMI (p = 0.435) or smoking history (p = 0.468). However, the pooled results showed that patients were older in the non-survival group than in the survival group (79.00 [74.25–85.75] vs 71.00 [63.00–78.00], P < 0.001). In terms of symptoms, the survival group was more likely to have breathe hard than the non-survival group (817 [19.6%] vs 5 [7.8%], P = 0.018). In addition, the total number of comorbidities in the non-survival group was significantly higher than that in the survival group (3.00 [2.00–4.00] vs 2.00 [0.00–3.00], P < 0.001). More details can be found in Table 1.
 |
Table 1 Baseline Characteristics of Patients |
Laboratory Tests, Treatments and Clinical Outcomes
Compared with the survival group, the non-survival group had a significantly elevated leukocyte count (8.91 [6.71–12.61] vs 6.76 [5.44–8.67], P < 0.001), neutrophil count (7.96 [5.02–11.39] vs 4.49 [3.32–6.31], P < 0.001), red blood cell distribution width (RDW) (14.40 [13.73–15.90] vs 13.20 [12.60–14.00], P < 0.001) and mean platelet volume (MPV) (10.70 [9.60–11.60] vs 10.20 [9.70–10.90], P = 0.043). However, the non-survival group showed markedly lower lymphocyte counts (0.75 [0.51–1.12] vs 1.40 [0.99–1.88], P < 0.001), eosinophil counts (0.02 [0.00–0.11] vs 0.11 [0.03–0.22]), platelet counts (179.00 [134.50–241.00] vs 206.00 [164.00–255.00]) and hemoglobin levels (101.50 [80.00–128.25] vs 129.00 [107.00–143.50]) than the survival group. Regarding biochemistry values, blood glucose, blood urea nitrogen (BUN), serum creatinine, serum glutamic-oxaloacetic transaminase (SGOT) and N-terminal pro-B-type natriuretic peptide (NT-proBNP) were significantly higher (p < 0.001) in the non-survival group versus survival group. In contrast, patients in the survival group had higher protein, albumin and low-density lipoprotein (LDL) levels (p < 0.001) than patients in the non-survival group. Differences in sodium, potassium, serum glutamic pyruvic transaminase (SGPT), monocyte count, mean corpuscular volume (MCV) and arterial blood gases were not significant between these two groups (Table 2).
 |
Table 2 Laboratory Parameters, Treatment and Clinical Outcome of Patients |
The inflammatory biomarkers that we were interested in were significantly different between these two groups. The non-survival group had significantly higher NLR (p < 0.001), PLR (p < 0.001), LMR (p < 0.001), CRP (p < 0.001) and ESR (p < 0.001) than the survival group. However, PMR (p = 0.005), ENR (p < 0.001) and ELR (p = 0.001) were markedly lower in the non-survival group compared with the survival group. More details are listed in Table 2. We also verified the correlation between these novel and traditional inflammatory biomarkers. NLR, PLR, LMR and PMR correlated positively with CRP (r = 0.467, r = 0.351, r = 0.437, r = 0.145, P < 0.001), as well as ESR (r = 0.269, r = 0.348, r = 0.243, r = 0.321, P < 0.001). ENR and ELR correlated negatively with CRP (r = −0.277, r = −0.076, P < 0.001) and ESR (r = −0.159, P < 0.001; r = −0.040, P = 0.011). All the differences in correlations reached statistical significance, which indicated that these novel inflammatory biomarkers have the potential to replace traditional inflammatory biomarkers. These results are listed in Supplementary Table 1.
In terms of treatment, there was no significant difference between the non-survival group and the survival group in the usage of systematic corticosteroids, antimicrobials and expectorants. Patients in the non-survival group had a significantly higher rate of using antifungal agents (19 [29.7%] vs 414 [9.9%], P < 0.001) and all terms of respiratory support (NIMV, IMV and NIMV+IMV) (p < 0.001) than those in the survival group. However, the application of bronchodilators (3659 [87.7%] vs 49 [76.6%], P = 0.007) and corticosteroid nebulization (2775 [66.5%] vs 33 [51.6%], P = 0.012) was higher in the survival group than in the non-survival group. Regarding clinical outcomes, the non-survival group had a longer LOS (10.00 [8.00–14.00] vs 15.00 [7.00–26.25], P = 0.03), higher risk of ICU admission (29 [45.3%] vs 139 [3.3%], P < 0.001) and more hospitalization expenses (13,595.32 [9969.10–19,154.57] vs 35,998.17 [19,460.16–89,472.84], P < 0.001) than the survival group (Table 2).
Predictive Power of NLR and Other Novel Inflammatory Biomarkers for in-Hospital Mortality
Table 3 shows that the NLR had a maximum area under the AUC (0.847; 95% CI, 0.810–0.884; P < 0.001) compared with other single inflammatory biomarkers (with Z-P value < 0.05). The AUCs of RDW, ELR and PMR were less than 0.7 (Figure 2). Although the Z-P value between NLR and CRP was 0.064, it was shown that NLR as a new inflammatory biomarker was not inferior to traditional biomarker, and it was even better than ESR, which was one of the traditional biomarkers (Z-P = 0.002). The cut-off values for predicting in-hospital mortality were NLR ≥ 4.43, LMR ≥ 0.38, ENR ≤ 0.01 and PLR ≥ 176.49. Next, we investigated the predictive value of different biomarker combinations. The results showed that NLR + PLR + LMR increased the predictive sensitivity, with a maximum AUC of 0.860 (95% CI 0.821–0.898; P < 0.001), sensitivity of 89.1%, and specificity of 69.8%. However, compared with NLR, the predictive power of NLR + PLR + LMR was not superior to it (Z-P = 0.167). Similarly, regardless of whether combined biomarkers or multiple biomarkers were used, the predictive effect of the NLR was not inferior to them (with Z-P > 0.05). As a result, the application of the NLR alone rather than combined with other novel inflammatory biomarkers can be considered in clinical work to predict the clinical outcomes of patients.
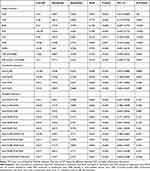 |
Table 3 ROC Curve Data |
According to multivariate logistic regression analysis, NLR ≥ 4.43 (OR, 3.308; 95% CI, 1.235–8.856; P = 0.017), LMR ≥ 0.38 (OR, 2.343; 95% CI, 1.080–5.080; P = 0.031), ELR ≤ 0.01 (OR, 2.343; 95% CI, 1.198–4.577; P = 0.013), age (OR, 1.066; 95% CI, 1.028–1.107; P = 0.001), comorbidities ≥ 2 (OR, 3.916; 95% CI, 1.752–8.751; P = 0.001), blood glucose (OR, 1.095; 95% CI, 1.028–1.166; P = 0.005), albumin (OR, 0.876; 95% CI, 0.834–0.919; P < 0.001) and RDW (OR, 1.317; 95% CI, 1.176–1.475; P < 0.001) were independent risk factors for in-hospital mortality (Table 4). We also tried to include erythrocyte count, hemoglobin, MPV, serum creatinine, SGOT, protein and LDL, but they were excluded from the equation (p > 0.05).
 |
Table 4 Logistic Regression Analysis of Risk Factors for Hospital Mortality |
Patient Outcomes Grouped by the NLR Cut-off Value
Table 5 shows the baseline characteristics, laboratory tests, treatments and clinical outcomes of the patients grouped by the NLR cut-off value. Three patients without NLR data were excluded. Compared to patients with NLR<4.43, patients in the group with NLR≥4.43 were older (74.00 [66.00–80.00] vs 69.00 [62.00–77.00], P < 0.001) and had more males (1137 [76.8%] vs 1999 [72.6%], P = 0.003) and comorbidities (2.00 [1.00–3.00] vs 2.00 [0.00–3.00], P < 0.001). In terms of clinical outcomes, the NLR ≥ 4.43 group had a longer LOS (12.00 [9.00–15.00] vs 10.00 [8.00–13.00], P < 0.001), higher rate of ICU admission (136 [9.2%] vs 32 [1.2%], P < 0.001), higher IMV application (34 [2.3%] vs 6 [0.2%], P < 0.001) and higher rate of treatment failure (1099 [42.0%] vs 381 [23.6%], P < 0.001) versus NLR < 4.43 group. In addition, patients with NLR ≥ 4.43 had more hospitalization expenses (17,215.64 [12,105.60–26,498.85] vs 12,397.97 [9310.04–16,622.39], P < 0.001), and more patients were readmitted earlier due to AECOPD within 1 month, 2 months, 3 months, 12 months and 15 months after discharge than patients with NLR < 4.43. Based on the last recording at Chao-Yang Hospital, 537 patients died in the hospital and the median time between the first index hospitalization and the last hospitalization was 2 years (IQR 1.0 year-3.0 years). Higher one-year mortality after the index hospitalization (34 [2.3%] vs,22 [0.8%] P < 0.001) and overall mortality (222 [15.0%] vs 215 [7.8%], P < 0.001) were found among patients with NLR ≥ 4.43 versus patients with NLR < 4.43 (Table 5). Based on the K–M curve, the patients with an NLR ≥ 4.43 had a higher first readmission rate due to AECOPD after discharge than patients with NLR < 4.43 (Figure 3). The pooled data revealed that patients in the higher NLR group had a higan her rate of all kinds of therapies included in this study (bronchodilator, corticosteroid nebulization, chemotherapeutic corticosteroid therapy, antimicrobial, antifungal agents, expectorant and respiratory support). All the differences reached statistical significance and are listed in Table 5. Through visual analysis of the first readmission data of all eligible patients due to AECOPD after discharge (2925 patients without related data were excluded from this analysis), the number of patients readmitted within one month after discharge due to AECOPD was the highest, reaching 166 (12.7%) (Figure 4).
 |
Table 5 Patient Data Grouped Based on NLR Cut-off Value |
 |
Figure 3 K–M curve of first readmissions due to AECOPD within 3 years after discharge. |
 |
Figure 4 Number of initial readmissions due to AECOPD per month after discharge. |
Nomogram and Calibration Curve
Based on logistic regression analysis, the combination of age, ≥2 comorbidities, blood glucose, RDW, albumin, NLR ≥ 4.43, LMR ≥ 0.38 or ELR ≤ 0.01 was considered an independent risk indicator to predict the risk of in-hospital mortality in AECOPD patients. Therefore, a nomogram was constructed to incorporate selected biomarkers (Figure 5). The calibration curve showed that the model was well calibrated. The AUC of the nomogram model and the C-index of the internal validation results were 0.914 (95% CI: 0.883 to 0.945) and 0.914, respectively (detailed results of the nomogram model validation are listed in Supplementary Figures 1, 2 and Supplementary Material 2).
Discussion
Main Findings in This Study
This study provided a number of novel inflammatory biomarkers that can predict the in-hospital mortality of AECOPD patients and verified their correlation with traditional biomarkers. The NLR, with the maximum AUC value, was considered to be the best single inflammatory biomarker compared with other novel inflammatory biomarkers in this study. In addition, the NLR was not inferior to CRP, a traditional biomarker, and combined inflammatory biomarkers and was even superior to traditional biomarkers such as ESR and RDW according to the results of the present study. This study revealed that the best cut-off value of NLR was 4.43, and patients with NLR ≥4.43 were more likely to have more comorbidities (such as chronic renal failure, heart failure, cardiovascular disease and malignancy), higher treatment failure rate, organ failure rate, ICU admission, longer LOS and poorer long-term prognosis, such as one-year mortality after the index hospitalization and overall mortality, than patients with NLR <4.43. Moreover, patients with a higher NLR (≥4.43) were more likely to be associated with higher and earlier AECOPD-related readmission within 15 months after discharge than patients with a lower NLR (<4.43). The first readmission due to AECOPD was highest within one month after discharge, suggesting that it is essential for physicians to improve the management and follow-up strength of AECOPD patients after discharge, especially during the early period.
Discussion of the Most Important Differences in the Present Study
To our knowledge, this is the largest retrospective study to date focused on the clinical predictive efficacy of novel inflammatory biomarkers among patients with AECOPD. The NLR is a cell inflammatory biomarker that can be easily derived from CBC tests. Previous studies revealed that the NLR was increased in several malignancies compared with patients with benign malignancies or healthy individuals.29–31 At the same time, the NLR was correlated with disease activity and clinical outcome in some chronic inflammatory diseases, such as hypertension, diabetes, and inflammatory bowel disease.32 Stable COPD patients with malignancies or metabolic syndrome, which might cause patients to be at risk of AECOPD, had higher NLR values than patients without these comorbidities.32,33 Most previous related studies focused on short-term outcomes, such as 28-day mortality,24,34 90-day mortality,21,35 in-hospital mortality,36,37 LOS,34,38 ICU admission rate,34,38 etc. A recent meta-analysis demonstrated that the NLR of AECOPD patients was significantly associated with the risk of adverse outcomes (mortality, LOS, and ICU admission rate, need for IMV, pulmonary hypertension, etc., [OR = 1.054, 95% CI 1.016–1.093, P = 0.005]), which was consistent with our study.25 However, the heterogeneity of the meta-analysis was significant, possibly because of the significant heterogeneity of the AECOPD patients contained in each study and the small sample size, which might cause higher sampling error.25 Yao et al enrolled 146 AECOPD patients with heart failure, and pooled results showed that non-survival patients had significantly higher levels of NLR than survival patients, and the cut-off value of NLR was 16.83, although NLR did not have the maximum value of AUC compared with CRP/albumin.24 While another study recruited 80 AECOPD patients and demonstrated that the cut-off value of NLR was 3.4, NLR was not the best biomarker to predict the diagnosis of AECOPD patients compared with pressure of carbon dioxide (PaCO2).39 Yao et al reported that the combination of NLR, PLR, and CRP could increase the sensitivity of prognosis compared with NLR.5 Although the NLR combined with the LMR and PLR had the largest AUC value in this study, the predictive effect of the NLR was inferior to that of the NLR combined with the LMR and PLR, thus the combination of multiple inflammatory biomarkers would inevitably increase the burden on physicians.
Several studies have focused on short-term outcomes, and relatively little is known regarding the prognostic ability of the NLR for long-term outcomes, such as the risk of readmission after discharge, one-year mortality after the index hospitalization and overall mortality. In this study, patients with NLR ≥ 4.43 were more likely readmitted due to AECOPD within 1 month, 2 months, 3 months, 12 months and 15 months after discharge than patients with NLR < 4.43. However, this difference did not exist in the relevant data within 24 months after discharge. Another study in Turkey found that outpatients with an NLR greater than 4.50 with a non-eosinophilic exacerbation on admission had an increased risk of readmission in the first month, which was similar to the findings of this study.40 However, a study published in 2022 enrolled 170 AECOPD patients and revealed that patients in the readmission group (>1 readmission) had a lower NLR than patients in the non-readmission group (≤1 readmission) (7.17 ± 8.95 vs 14.42 ± 31.17, P = 0.001), which was contrary to our study.41 This may be because the patients in the non-readmission group had a higher proportion of systematic corticosteroid application, which might increase the number of neutrophils and cause lymphocytes to be killed and dissolved, leading to an increase in NLR.41 At the same time, the different definitions of readmission groups might cause the different outcomes.41 In addition to the NLR, peripheral blood eosinophil levels were used to evaluate the long-term outcomes among AECOPD patients. A previous study revealed that AE of COPD during 1 year was greater among COPD patients with blood eosinophil counts higher than or equal to 300 cells/mm3 (rate ratio [RR] 1.25; 95% CI 1.10–1.43), higher than or equal to 400 cells/mm3 (RR 1.48; 95% CI 1.26–1.75), and higher than or equal to 500 cells/mm3 (RR 1.76; 95% CI 1.45–2.14), respectively, versus patients with blood eosinophil counts less than those cut-offs.42 Another study contained 811 AECOPD patients and reported that patients in the eosinophilic group (blood eosinophil counts ≥ 0.30×109 cells/L) had lower 3-year mortality than patients in the non-eosinophilic group (blood eosinophil counts < 0.30×109 cells/L) (40% vs 54%, P = 0.006).43 Although eosinophil was not assessed in this study, the novel inflammatory biomarkers such as ENR and ELR were inversely associated with in-hospital mortality. Moreover, this study showed that NLR could also predict long-term clinical prognosis, which provided an alternative clinical assessment method among AECOPD patients.
Besides, the pooled results of this study showed that nearly one-quarter of patients were readmitted for AECOPD for the first time within 3 months, and this proportion reached two-thirds within 1 year. In addition, the first readmission due to AECOPD in patients was more likely to occur within 1 month after discharge than in any subsequent month, reaching 12.7%. A study in China showed that the readmission rate of patients with COPD presenting with acute exacerbation within 30 days was 21.52%.44 A systematic review and meta-analysis published in 2020 including 57 studies indicated that the prevalence of COPD-related readmission varied from 2.6 to 82.2% at 30 days and 25.0–87.0% at 12 months post-discharge.45 The significant heterogeneity of the readmission rate may reflect variations in study methodology and the local context, such as ethnicity, disease diagnosis method, and health care quality. However, overall, the relatively high readmission rates and a large proportion of early readmissions in COPD patients after discharge were undoubted, which indicated the need for improving the quality of treatment and health care during hospitalization and post-discharge. Improving access to primary health care and providing care bundles (including consults, inpatient interventions, education, transitions of care, and after discharge care) are effective strategies to decrease hospital readmissions.46 Furthermore, the impact of COVID-19 among AECOPD patients should be interpreted. According to a meta analysis including 13 studies assessing the influence of COVID-19 on readmission rates among AECOPD patients, age is the most dominant risk factor for readmission rate, followed by diabetes, high LOS, COPD, chronic kidney disease, liver disease, metastatic disease and coronary artery disease, and COVID-19 was not superior to them.47 Donnelly et al compared the readmission rate between survival patients with COVID-19, pneumonia, or heart failure. And the pooled results revealed that COVID-19 survivors had lower rates of 60-day readmission and death than patients with pneumonia (26.1 vs 31.7%; p = 0.006), or heart failure (27.0% vs 37.0%; p < 0.001).48 As a result, management of chronic diseases and comorbidities remains the key to preventing readmission among patients with COPD.
Strengths and Limitations
To the best of our knowledge, this study was the most comprehensive study covering novel inflammatory biomarkers and had the largest sample size thus far. In addition to exploring the predictive ability of single indicators, the predictive ability of the combinations of multiple inflammatory biomarkers for clinical outcomes was also analyzed in this study. At the same time, this study used a more scientific method to compare the different AUCs between traditional and novel inflammatory biomarkers to find the predictors with real statistical differences. In addition, a nomogram was established to visualize the pooled results and offer help to clinicians for a more intuitive assessment of mortality risk among AECOPD patients. Furthermore, treatment failure (a composite index), time of first readmission after discharge, one-year mortality after the index hospitalization and overall mortality were first assessed, which further proved the predictive value of the NLR for the treatment effect of AECOPD patients during hospitalization and after discharge. This study still has several limitations. First, it was a single-center retrospective observational study, and the biases inherent in this type of study should not be ignored. Second, some relevant data, such as stable COPD severity and spirometry data of all patients and microbiological data, were not recorded in the EMRD. Then, the generalization of our nomogram should be interpreted with caution due to the absence of external validation. Considering the possibilities of error coding of ICD among patients, prospective studies and experienced respiratory physicians will be needed to recruit eligible patients.
Unanswered Questions and Future Research
Because of the limitations mentioned above, more high-quality prospective studies with large sample sizes are warranted in the future to further investigate the predictive ability of the NLR and the clinical feasibility of the nomogram in this study. At the same time, further studies should pay more attention to biomarkers that can predict long-term outcomes, such as the readmission rate and long-term mortality, among AECOPD patients and establish the potential clinical use of the NLR alone or in combination with other inflammatory biomarkers. Finally, the stability and test time of the NLR in AECOPD patients should also be noted and analyzed in the future.
Conclusion
The NLR was the best single inflammatory biomarker to predict in-hospital and long-term mortality, treatment failure, organ failure and the first readmission due to AECOPD (within 15 months) among AECOPD patients. The appropriate cut-off value of NLR was 4.43. AECOPD should be monitored carefully, especially within the first month after discharge, which requires better quality, personalized and convenient follow-up methods to be proposed for patients with AECOPD post-discharge.
Abbreviations
AECOPD, Acute exacerbation of chronic obstructive pulmonary disease; NLR, Neutrophil–lymphocyte ratio; PLR, Platelet–lymphocyte ratio; LMR, Lymphocyte-to-monocyte ratio; ENR, Eosinophil-to-neutrophil ratio; ELR, Eosinophil-to-lymphocyte ratio; CRP, C-reactive protein; ESR, Erythrocyte sedimentation rate; PMR, Platelet to mean platelet volume ratio; ROC, Receiver-operating characteristic; KM, Kaplan–Meier; WHO, World Health Organization; PCT, Procalcitonin; CRP, C-reactive protein; ESR, Erythrocyte sedimentation rate; NIPPV, Non-invasive positive pressure ventilation; CAP, Community acquired pneumonia; CBC, Complete blood count; EMRD, Electronic medical record database; NIMV Non-invasive mechanical ventilation; IMV, Invasive mechanical ventilation; LOS, Length of stay; ICU, Intensive care unit; IQR, Interquartile range; ROC, Receiver-operating characteristic; OR, Odds ratios; CIs, Confidence intervals; K–M, Kaplan–Meier; BMI, Body mass index; RDW, Red blood cell distribution width; MPV, Mean platelet volume; BUN, Blood urea nitrogen; SGOT, Serum glutamic-oxaloacetic transaminase; NT-proBNP, N-terminal pro-B-type natriuretic peptide; LDL, Low-density lipoprotein; SGPT, Serum glutamic pyruvic transaminase; MCV, Mean corpuscular volume; PLT, Platelet; PaCO2, Pressure of carbon dioxide; HCO3, Bicarbonate; ALB, Albumin; RR, Rate ratio; GOLD, Global initiative for chronic obstructive lung disease; AUC, Area under curve; COVID-19, Corona Virus Disease 2019; C-index, Concordance index.
Data Sharing Statement
Data about individual deidentified participants of this trial will be available from the corresponding author Zhaohui Tong (Email: [email protected]) on reasonable request after the main results of the study have been published.
Ethics Statement
This study was approved by the Research Ethics Board of Beijing Chao-Yang Hospital (project approval number: 2020-ke-544). This study complies with the declaration of Helsinki.
Informed Consent Statement
Informed consent was waived due to the anonymous and mandatory nature of the data.
Author Contributions
Shuai Shao developed the initial idea of this study. Shuai Shao, Zhijing Zhang made their contributions to study design, execution, acquisition of data, analysis, interpretation and writing of this article. Zhaohui Tong, Lin Feng and Lirong Liang also made significantly contributed to this work in study execution, acquisition of data, interpretation of this article. Lirong Liang and Lin Feng critically reviewed the article. Zhaohui Tong substantially revised this article and provided revision suggestions for it to make it better. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by grants from the Beijing Municipal Science & Technology Commission (No. Z201100005520028) and the Beijing Municipal Administration of Hospitals Incubating Program (PX2020014). The funders had no role in the study design, data collection, data analysis and interpretation, writing of the report, or the decision to submit the article for publication.
Disclosure
The authors declare that there are no conflicts of interest in this work.
References
1. Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am J Respir Crit Care Med. 2017;195(5):557–582. doi:10.1164/rccm.201701-0218PP
2. Safiri S, Carson-Chahhoud K, Noori M, et al. Burden of chronic obstructive pulmonary disease and its attributable risk factors in 204 countries and territories, 1990–2019: results from the global burden of disease study 2019. BMJ. 2022;378:e069679. doi:10.1136/bmj-2021-069679
3. Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO global initiative for chronic obstructive lung disease (GOLD) workshop summary. Am J Respir Crit Care Med. 2001;163(5):1256–1276. doi:10.1164/ajrccm.163.5.2101039
4. Zhang Y, Lin YX. 慢性阻塞性肺疾病急性加重住院患者一年及长期死亡风险因素分析 [Risk factors analysis for one-year and long-term mortality in patients hospitalized for acute exacerbation of chronic obstructive pulmonary disease]. Zhonghua Jie He He Hu Xi Za Zhi. 2019;42(12):895–900. Chinese. doi:10.3760/cma.j.issn.1001-0939.2019.12.004
5. Yao C, Liu X, Tang Z. Prognostic role of neutrophil-lymphocyte ratio and platelet-lymphocyte ratio for hospital mortality in patients with AECOPD. Int J Chron Obstruct Pulmon Dis. 2017;12:2285–2290. doi:10.2147/COPD.S141760
6. Liu SF, Lin KC, Chin CH, et al. Factors influencing short-term re-admission and one-year mortality in patients with chronic obstructive pulmonary disease. Respirology. 2007;12(4):560–565. doi:10.1111/j.1440-1843.2007.01110.x
7. Patout M, Meira L, D’Cruz R, et al. Neural respiratory drive predicts long-term outcome following admission for exacerbation of COPD: a post hoc analysis. Thorax. 2019;74(9):910–913. doi:10.1136/thoraxjnl-2018-212074
8. Ruiz-González A, Lacasta D, Ibarz M, et al. C-reactive protein and other predictors of poor outcome in patients hospitalized with exacerbations of chronic obstructive pulmonary disease. Respirology. 2008;13(7):1028–1033. doi:10.1111/j.1440-1843.2008.01403.x
9. Jing Z, Chun C, Ning S, et al. Systemic inflammatory marker CRP was better predictor of readmission for AECOPD than sputum inflammatory markers. Arch Bronconeumol. 2016;52(3):138–144. doi:10.1016/j.arbres.2015.01.011
10. Gao S, Duan Y, Chen J, Wang J. Evaluation of blood markers at admission for predicting community acquired pneumonia in chronic obstructive pulmonary disease. COPD. 2021;18(5):557–566. doi:10.1080/15412555.2021.1976739
11. Ding F, Liu W, Wang H, Wang W, Yang C. Guidance value of procalcitonin detection in selecting switching points for sequential therapy in patients with acute exacerbation of chronic obstructive pulmonary disease complicated by respiratory failure. Int J Chron Obstruct Pulmon Dis. 2022;17:2693–2699. doi:10.2147/COPD.S366028
12. Ye YP, Zhao H, Kang T, et al. Optimal cut-off value of serum procalcitonin in predicting bacterial infection induced acute exacerbation in chronic obstructive pulmonary disease: a prospective observational study. Chron Respir Dis. 2022;19:14799731221108516. doi:10.1177/14799731221108516
13. Wang J, Shang H, Yang X, Guo S, Cui Z. Procalcitonin C-reactive protein, PaCO2, and noninvasive mechanical ventilation failure in chronic obstructive pulmonary disease exacerbation. Medicine. 2019;98(17):e15171. doi:10.1097/MD.0000000000015171
14. Liu L, Luan Y, Xiao L, et al. The predictive value of serum procalcitonin for non-invasive positive pressure ventilation in the patients with acute exacerbation of chronic obstructive pulmonary disease. Medicine. 2021;100(16):e25547. doi:10.1097/MD.0000000000025547
15. Jimeno S, Ventura PS, Castellano JM, et al. Prognostic implications of neutrophil-lymphocyte ratio in COVID-19. Eur J Clin Invest. 2021;51(1):e13404. doi:10.1111/eci.13404
16. Ellingsen J, Janson C, Bröms K, et al. Neutrophil-to-lymphocyte ratio, blood eosinophils and COPD exacerbations: a cohort study. ERJ Open Res. 2021;7(4):00471–2021. doi:10.1183/23120541.00471-2021
17. Tanrıverdi H, Örnek T, Erboy F, et al. Comparison of diagnostic values of procalcitonin, C-reactive protein and blood neutrophil/lymphocyte ratio levels in predicting bacterial infection in hospitalized patients with acute exacerbations of COPD. Wien Klin Wochenschr. 2015;127(19–20):756–763. doi:10.1007/s00508-014-0690-6
18. Duman D, Aksoy E, Agca MC, et al. The utility of inflammatory markers to predict readmissions and mortality in COPD cases with or without eosinophilia. Int J Chron Obstruct Pulmon Dis. 2015;10:2469–2478. doi:10.2147/COPD.S90330
19. Sun W, Luo Z, Jin J, Cao Z, Ma Y. The neutrophil/lymphocyte ratio could predict noninvasive mechanical ventilation failure in patients with acute exacerbation of chronic obstructive pulmonary disease: a retrospective observational study. Int J Chron Obstruct Pulmon Dis. 2021;16:2267–2277. doi:10.2147/COPD.S320529
20. Luo Z, Zhang W, Chen L, Xu N. Prognostic value of neutrophil: lymphocyte and platelet: lymphocyte ratios for 28-Day mortality of patients with AECOPD. Int J Gen Med. 2021;14:2839–2848. doi:10.2147/IJGM.S312045
21. Kumar P, Law S, Sriram KB. Evaluation of platelet lymphocyte ratio and 90-day mortality in patients with acute exacerbation of chronic obstructive pulmonary disease. J Thorac Dis. 2017;9(6):1509–1516. doi:10.21037/jtd.2017.05.77
22. Alparslan BS, Tuncay E, Gungor S, et al. Can red blood cell distribution width (RDW) level predict the severity of acute exacerbation of chronic obstructive pulmonary disease (AECOPD)? Int J Clin Pract. 2021;75(11):e14730. doi:10.1111/ijcp.14730
23. Yilmaz G, Salihoglu Z. Does mean platelet volume/platelet count ratio and red blood cell distribution width predict in-hospital mortality in patients admitted for acute exacerbation of chronic obstructive pulmonary disease? J Immunol Clin Microbiol. 2019;4(2):18–25.
24. Yao C, Wang L, Shi F, et al. Optimized combination of circulating biomarkers as predictors of prognosis in AECOPD patients complicated with heart failure. Int J Med Sci. 2021;18(7):1592–1599. doi:10.7150/ijms.52405
25. Zinellu A, Zinellu E, Pau MC, et al. A comprehensive systematic review and meta-analysis of the association between the neutrophil-to-lymphocyte ratio and adverse outcomes in patients with acute exacerbation of chronic obstructive pulmonary disease. J Clin Med. 2022;11(12):3365. doi:10.3390/jcm11123365
26. Qianqian Z, Jiachen L, Lirong L. Construction and application of data quality control system of big data integrated application platform for respiratory diseases based on electronic medical records. J Med Inform. 2022;43(7):55–60.
27. Nan LB, Yin XT, Gao JP. Significant diagnostic value of free-serum PSA (FPSA)/Prostate-Specific Antigen Density (PSAD) and (F/T)/PSAD for prostate cancer of the Chinese population in a single institution. Med Sci Monit. 2019;25:8345–8351. doi:10.12659/MSM.916900
28. Iasonos A, Schrag D, Raj GV, Panageas KS. How to build and interpret a nomogram for cancer prognosis. J Clin Oncol. 2008;26(8):1364–1370. doi:10.1200/JCO.2007.12.9791
29. Bourdin A, Burgel PR, Chanez P, et al. Recent advances in COPD: pathophysiology, respiratory physiology and clinical aspects, including comorbidities. Eur Respir Rev. 2009;18(114):198–212. doi:10.1183/09059180.00005509
30. Chen N, Liu S, Huang L, et al. Prognostic significance of neutrophil-to-lymphocyte ratio in patients with malignant pleural mesothelioma: a meta-analysis. Oncotarget. 2017;8(34):57460–57469. doi:10.18632/oncotarget.15404
31. Cossu A, Budroni M, Paliogiannis P, et al. Epidemiology of thyroid cancer in an area of epidemic thyroid goiter. J Cancer Epidemiol. 2013;2013:584768. doi:10.1155/2013/584768
32. Paliogiannis P, Fois AG, Sotgia S, et al. The neutrophil-to-lymphocyte ratio as a marker of chronic obstructive pulmonary disease and its exacerbations: a systematic review and meta-analysis. Eur J Clin Invest. 2018;48(8):e12984. doi:10.1111/eci.12984
33. Paliogiannis P, Fois AG, Sotgia S, et al. Neutrophil to lymphocyte ratio and clinical outcomes in COPD: recent evidence and future perspectives. Eur Respir Rev. 2018;27(147):170113. doi:10.1183/16000617.0113-2017
34. Teng F, Ye H, Xue T. Predictive value of neutrophil to lymphocyte ratio in patients with acute exacerbation of chronic obstructive pulmonary disease. PLoS One. 2018;13(9):e0204377. doi:10.1371/journal.pone.0204377
35. Liu J, Liu J, Zou Y. Relationship between neutrophil-lymphocyte ratio and short-term prognosis in the chronic obstructive pulmonary patients with acute exacerbation. Biosci Rep. 2019;39(5): BSR20190675.
36. Rahimirad S, Ghaffary MR, Rahimirad MH, Rashidi F. Association between admission neutrophil to lymphocyte ratio and outcomes in patients with acute exacerbation of chronic obstructive pulmonary disease. Tuberk Toraks. 2017;65(1):25–31. doi:10.5578/tt.27626
37. Emami AM, Alavi-Naeini N. Evaluation of the relationship of neutrophil-to lymphocyte ratio and platelet-to-lymphocyte ratio with in-hospital mortality in patients with acute exacerbation of chronic obstructive pulmonary disease. Clin Respir J. 2021;15(4):382–388. doi:10.1111/crj.13312
38. Lu FY, Chen R, Li N, et al. Neutrophil-to-lymphocyte ratio predicts clinical outcome of severe acute exacerbation of COPD in frequent exacerbators. Int J Chron Obstruct Pulmon Dis. 2021;16:341–349. doi:10.2147/COPD.S290422
39. Esmaeel HM, Ahmed HA. The refined ABCD assessment and non-costly laboratory parameters are outcome predictors in acute exacerbation of COPD. Egypt J Chest Dis Tuberc. 2017;66(4):599–603. doi:10.1016/j.ejcdt.2017.06.004
40. Çoban AM, Aksoy E, Duman D, et al. Does eosinophilia and neutrophil to lymphocyte ratio affect hospital re-admission in cases of COPD exacerbation? Tuberk Toraks. 2017;65(4):282–290. doi:10.5578/tt.57278
41. Dai L, Bin-Miao L, Xue-Mei O. Predictive value of neutrophil-to-lymphocyte ratio and bilirubin levels in the readmission of acute exacerbation of chronic obstructive pulmonary disease. Am J Med Sci. 2022;365(2):169–175. doi:10.1016/j.amjms.2022.05.026
42. Zeiger RS, Tran TN, Butler RK, et al. Relationship of blood eosinophil count to exacerbations in chronic obstructive pulmonary disease. J Allergy Clin Immunol Pract. 2018;6(3):944–954.e5. doi:10.1016/j.jaip.2017.10.004
43. Jabarkhil A, Moberg M, Janner J, et al. Elevated blood eosinophils in acute COPD exacerbations: better short- and long-term prognosis. Eur Clin Respir J. 2020;7(1):1757274. doi:10.1080/20018525.2020.1757274
44. Lu HY, Zhang R, Chang Y, et al. A structural equation model-based study on the status and influencing factors of acute exacerbation readmission of elderly patients with chronic obstructive pulmonary disease within 30 days. BMC Pulm Med. 2022;22(1):299. doi:10.1186/s12890-022-02093-w
45. Njoku CM, Alqahtani JS, Wimmer BC, et al. Risk factors and associated outcomes of hospital readmission in COPD: a systematic review. Respir Med. 2020;173:105988. doi:10.1016/j.rmed.2020.105988
46. Kendra M, Mansukhani R, Rudawsky N, et al. Decreasing hospital readmissions utilizing an evidence-based COPD care bundle. Lung. 2022;200(4):481–486. doi:10.1007/s00408-022-00548-9
47. Loo WK, Hasikin K, Suhaimi A, et al. Systematic review on COVID-19 readmission and risk Factors: future of machine learning in COVID-19 readmission studies. Front Public Health. 2022;10:898254. doi:10.3389/fpubh.2022.898254
48. Donnelly JP, Wang XQ, Iwashyna TJ, Prescott HC. Readmission and death after initial hospital discharge among patients with COVID-19 in a large multihospital system. JAMA. 2021;325(3):304–306. doi:10.1001/jama.2020.21465
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


