Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 17
Association between Inhaled β2-agonists Initiation and Risk of Major Adverse Cardiovascular Events: A Population-based Nested Case-Control Study
Authors Amegadzie JE , Gamble JM , Farrell J , Gao Z
Received 27 January 2022
Accepted for publication 3 April 2022
Published 20 May 2022 Volume 2022:17 Pages 1205—1217
DOI https://doi.org/10.2147/COPD.S358927
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Richard Russell
Joseph Emil Amegadzie,1 John-Michael Gamble,2 Jamie Farrell,1 Zhiwei Gao1
1Faculty of Medicine, Memorial University of Newfoundland, St. John’s, Newfoundland, Canada; 2Faculty of Science, School of Pharmacy, University of Waterloo, Waterloo, Ontario, Canada
Correspondence: Zhiwei Gao, Faculty of Medicine, Memorial University, 300 Prince Philip Drive, St. John’s, NL, A1B 3V6, Canada, Tel +17098646523, Email [email protected]
Purpose: Despite ample evidence underpinning the efficacy of β2-agonists in asthma and chronic obstructive pulmonary disease (COPD), the occurrence of β1- and β2-adrenoceptors in the heart suggests that β2-agonists may have deleterious cardiac effects. We investigated the association between new users of long-or short-acting β2-agonists (LABA or SABA) or ICS (inhaled corticosteroids)/LABA and major adverse cardiovascular events (MACE).
Methods: A nested case–control analysis was conducted using the UK Clinical Practice Research Datalink of patients with asthma, COPD or asthma–COPD overlap with initial treatment of LABA, SABA, ICS/LABA, ICS, long-or short-acting muscarinic antagonist (LAMA or SAMA) between 01 January 1998 and 31 July 2018. The primary outcome was MACE, defined as the first occurrence of stroke, heart failure, myocardial infarction, arrhythmia, or cardiovascular death. Each case was matched with up to 10 controls on age, sex, date of cohort-entry, and duration of follow-up. The risk of MACE associated with β2-agonists was estimated using conditional logistic regression after controlling for potential confounders.
Results: The cohort included 180,567 new users of β2-agonists, ICS, SAMA, or LAMA. Among asthmatics, β2-agonists were not associated with the risk of MACE (SABA vs ICS: HR 1.29 [0.96– 1.73]; ICS/LABA vs ICS, HR 0.75 [0.33– 1.73]). In contrast, among COPD patients, LABA (HR, 2.38 [1.04– 5.47]), SABA (HR, 2.02 [1.13– 3.59]) and ICS/LABA (HR, 2.08 [1.04– 4.16]) users had an increased risk of MACE compared with SAMA users. Among patients with asthma–COPD overlap, SABA (HR, 2.57 [1.26– 5.24]) was associated with an increased risk of MACE compared with ICS.
Conclusion: In conclusion, initiation of LABA, SABA, or ICS/LABA in COPD or SABA in asthma–COPD overlap is associated with increased risk of MACE. No associations were observed among patients with asthma.
Keywords: asthma, chronic obstructive pulmonary disease, nested case–control
Introduction
Asthma and chronic obstructive pulmonary diseases (COPD) are the two principal diagnoses of Obstructive Airways Diseases (OADs).1,2 Beta (β)2-adrenergic agonists are the frontline treatment for these diseases.3 They exert their pharmacologic effects via β2-adrenoceptors that are predominantly present on airway smooth muscles but also exist on cardiac muscles, vascular endothelium, eosinophils, and lymphocytes.4 β2-adrenoceptors agonists, including long-acting and short-acting β2-agonists (LABA and SABA), are typically designed to mimic the functions of epinephrine by producing autonomic responses within the airway smooth muscle and to limit the stimulation to β2-adrenoceptors outside the airway smooth muscles as much as possible to reduce adverse effects.3 However, a lack of selectivity for β2-adrenoceptors may result in “off-target” effects such as tachycardia, tremors, headaches, and an increase in cardiac ectopy.5–8 These effects are thought to be partially mediated by β2-adrenoceptors desensitization or exacerbation of airway inflammation and may lead to further cardiovascular sequelae.9
Epidemiological studies conducted on the effectiveness and safety of β2-agonists, including a large randomized controlled trial (RCT), report inconclusive findings because of significant drop-out rates, low events rates, the inclusion of patients with no record of OAD diagnosis or showed varied conclusions concerning their effectiveness and safety.10–17 Before these studies, studies of adverse cardiovascular events, including sudden cardiac death, fatal myocardial infarction, and arrhythmia resulting from β2-agonists use, have accumulated over the last six decades among patients with asthma and COPD.18–21 Furtherance to this, a recent combined analysis of safety trials of LABA by Busse et al. concluded that adding a LABA to an inhaled glucocorticoid (ICS) was safe.22 However, this study is limited on the premise that patients recruited were hesitant to participate in trials investigating the risk of death and fraught with the exclusion of patients with previous life-threatening events from their analysis.
It thus remains uncertain whether the new initiation of β2-agonists-based medications is associated with a differential risk of cardiovascular events. To advance our understanding of the cardio-respiratory safety of β2-agonists, we conducted a nested case–control study among new users of LABA, SABA, or ICS/LABA combination therapy on the risk of a major adverse cardiovascular event (MACE) among patients with asthma, COPD and asthma–COPD overlap using a large population-based cohort from a real-world setting.
Methods
Source Population
We conducted a nested case–control study using the United Kingdom (UK) Clinical Practice Research Datalink (CPRD). The CPRD is a database representative of the UK’s population that contains de-identified, longitudinal data, with approximately 700 contributing general practitioner (GP) primary care practices and more than 14 million acceptable (good quality) patients.23,24 The Patients’ data are available for demographic characteristics, symptoms, diagnoses, primary care prescriptions, laboratory tests, lifestyle information, and referrals to specialists and hospitals. Medications prescribed by GPs are automatically coded into computer records based on the British National Formulary.25 Approximately half of the source population is linked to the hospital (Hospital Episode Stats, HES) and death certificate (Office of National Statistics [ONS]) records.26 This dataset has been widely used to investigate a broad range of health-related topics, including the safety and effectiveness of respiratory medications.27–30 Our study was approved by the Health Research Ethics Board at Memorial University and the CPRD Independent Scientific Advisory Committee (ISAC 18_005RA).
Cohort Definition
Our source cohort included all HES/ONS-linked individuals in the CPRD aged ≥18 with a diagnosis of asthma, COPD, or asthma–COPD overlap and a new prescription for a LABA, SABA, ICS/LABA, ICS, or short- or long-acting muscarinic antagonist (SAMA, LAMA) between 1 January 1998 and 31 July 2018. To assess only new users, patients with a record of taking any inhaler drug within 365 days before their first medication prescription were excluded. The date of cohort entry was defined as the date of first prescriptions for any of these inhaled drugs. For subjects who initiated LAMA (tiotropium), cohort entry was defined as the date of the first prescription on or after 25 September 2003 (at least one year after the drug was available in the UK) separately for LAMA case events and their matched controls. This new-user design was adopted to assess time-varying hazards and intended effects related to treatment duration and reduce the likelihood of immortal time bias.
Patients were followed from the date of study-cohort entry until an event of MACE, emigration from a CPRD practice site, end of coverage in the database, end of the study period (31 July 2018), or whichever occurred first.
An incident readcode and gemscript codes in the CPRD database were used to define a patient with asthma and/or COPD (see Appendix 1). Patient definitions using both diagnostic codes (readcode) and prescriptions (gemscript codes) have shown that patients with asthma or COPD can be accurately identified from the CPRD database with reasonably good positive predictive values of 83.3% and 87.5%, respectively.31,32 Patients with asthma–COPD overlap were defined as having 1) asthma readcode and 2) COPD readcode, and 3) an ex/current smoker before the index date (the date of the first prescription). Detailed information on exclusion criteria is shown in Figure 1. Three separate sub-cohorts were created based on OAD diagnosis; namely asthma, COPD, or asthma–COPD overlap.
Nested Case–Control
For each of the three OAD sub-cohorts, a nested case–control study was carried out to investigate our primary endpoint of MACE. The nested case–control analytic approach was chosen because of the time-varying nature of exposure, the size of the cohort, and the long duration of follow-up.33 MACE was defined as the first occurrence of non-fatal heart failure, nonfatal myocardial infarction, non-fatal arrhythmia, non-fatal stroke, or any cardiovascular-related mortality after the date of cohort entry. To assess only incidents cases, patients with MACE events occurring before the date of cohort entry were excluded. Our event definitions were based on International Classification of Disease Version 10 codes (linked HES/ONS data). Thus, the index date for cases was the date of the event (ie, MACE). Please refer to Figure 2 for the schematic design of the nested case–control analysis adopted.
Consequently, for each event occurring during the follow-up, we used the risk-set sampling method to match the event with a random sample from the risk set; primarily, the (set of) cohort members who were being followed and were event-free at the index date of the case. These risk sets, which allow exposure to be measured at the time of the event occurrence, are identical to those used in a Cox proportional-hazards model. For asthma, COPD or asthma–COPD-overlap cohort, a case was randomly matched up to 10 (maximum 10) controls within each sub-cohort based on sex, age (±1 year), date of cohort entry (±180 days) and duration of follow-up. The index date of the cases was also assigned as index dates to their matched controls.
Exposure Assessment
All drug exposures were identified in the prescription files through the use of gemscript codes between cohort entry and index date and were classified into four mutually exclusive exposure groups of interest as;
- Current and new use of LABA was defined by a prescription duration plus a 30-day grace period overlapping the index date;
- Current and new use of SABA was defined by a prescription duration plus a 30-day grace period overlapping the index date;
- Current and new use of ICS/LABA was defined by a prescription duration plus a 30-day grace period overlapping the index date;
- Current and new use of inhaled non-β2-agonists-based drugs (ie, ICS, SAMA or LAMA) – reference category; was defined by a prescription duration plus a 30-day grace period overlapping the index date.
A 30-day grace period was added to the end of a prescription to allow for refill time. This 30-day grace period also accounted for non-adherence.
Potential Confounders
In addition to all matching factors, the models were further adjusted for several potential confounders measured at study-cohort entry, including body mass index, smoking status, alcohol abuse, blood pressure, material deprivation, the number of physician visits (before cohort entry), comorbidities, Charlson comorbidity index, cardiovascular drugs, important determinants of cardiovascular diseases (hyperlipidemia, hypertension, diabetes, atherosclerosis) and drugs prolonging QT interval.
We also adjusted in our final model the following: 1) the use of other respiratory or antibiotics drugs as a measure of COPD (also asthma or asthma–COPD overlap) severity, including the use of methylxanthines, oral corticosteroids, and respiratory antibiotics; 2) moderate or severe exacerbation which was defined by a new prescription for prednisolone or hospitalization for asthma, COPD or asthma–COPD overlap; 3) other drugs used (binary; yes/no) in the year before cohort entry including aspirin, acetaminophen, opioids and non-steroidal anti-inflammatory drugs in the year before cohort entry.
Statistical Analysis
Descriptive statistics to summarize the characteristics of the cohort, cases and matched controls were provided. For categorical variables with more than two categories, type 3 p-values (a p-value indicating all categorical variable levels) were also calculated. Conditional logistic regression was used to estimate odds ratios and 95% confidence interval for the risk of MACE associated with inhaled β2-agonists-based drugs (LABA, SABA or ICS/LABA) compared with ICS, LAMA or SAMA drugs after controlling for potential confounders. In the nested case–control analysis, conditional logistic regression is used to compute odds ratios that are unbiased estimators of hazard ratios (HRs), with little or no loss in precision.33,34
To investigate the possible modification effects of β-blocker use, an interaction term between a drug group variable and a variable indicating β-blocker type (cardioselective or non-cardioselective) was introduced into multiple regression models. Data analysis was conducted using SAS (version 9.4, SAS Institute Inc., Cary, NC).
Secondary Analysis
We also investigated the risk of each disease of the five major components of MACE, including HF, MI, stroke, arrhythmia and cardiovascular death. Risk-set sampling was done independently for each outcome.
Sensitivity Analysis
We conducted a series of sensitivity analyses to explore the robustness of our study design, potential mediating effects and results. It is common for GPs to prescribe as-needed (PRN) short-acting bronchodilators (i.e., SABAs) to be used alongside an ICS or an ICS/LABA or a LAMA for treating COPD or asthma. To test this possibility of potential contamination of our reference group with SABAs, the cohort population was restricted to add-ons or switchers therapy approach. Also, since the length of the grace period is uncertain, sensitivity analyses were conducted by varying the grace period to 0, 45 and 60 days.
Results
A total of 180,567 patients met the study inclusion criteria (see Figure 1), which included 2095, 111,829, 5614, 55,287, 2239 and 3502 new users of LABA, SABA, ICS/LABA, ICS, LAMA and SAMA respectively. During this time, 686 patients experienced MACE, of which 359, 239, and 88 cases were observed among patients with asthma, COPD, and asthma–COPD overlap, respectively. The mean age at cohort entry with MACE case-patients were 63.8, 74.3 and 72.3 years for asthma, COPD and asthma–COPD overlap, respectively (see Table 1 for detailed patient characteristics).
Table 2 presents the results of the primary analyses for the association between the new use of inhaled β2-agonists-based drugs and the incidence of MACE. Among patients with asthma alone or asthma–COPD overlap, new use of ICS/ LABA was not significantly associated with risk of MACE in comparison to a new use of ICS. Also, the new use of SABA was not significantly associated with an increased risk of MACE compared to a new use of ICS. However, among COPD patients, in comparison to the new use of SAMA, an increased risk of major adverse cardiovascular events was observed among new use of the SABA (adjusted HR, 2.02 [1.13–3.59]), ICS/LABA (adjusted HR, 2.08 [1.04–4.16]), and LABA (adjusted HR, 2.38 [1.04–5.47]). Similarly, among patients with asthma–COPD overlap, new use of SABA was associated with an increased risk of MACE (adjusted HR, 2.57 [1.26–5.24]) compared with new use of ICS. However, there was no association between new use of β2-agonists-based drugs compared with new users of LAMA or ICS among COPD patients. Thus, significant associations between inhaled β2-agonists and MACE were observed for all inhaled β2-agonists (vs SAMA) in COPD patients and SABA (vs ICS) in Asthma–COPD Overlap patients. Subsequently, no interaction term between a β-blockers use variable and a drug group variable was statistically significant in the multiple regression models of MACE. Notably, not all exposure or treatment groups were displayed in our analyses of associations. Thus, cells with fewer than five events are not shown per the confidentiality policies of the CPRD.
 |
Table 2 Association Between Use of Inhaled β2-Agonists-Based Drugs and the Incidence of Major Adverse Cardiovascular Events |
Secondary Analysis
Individual analyses of the five major components of MACE revealed no association between the new use of SABA compared to the new use of ICS for all the major components for patients who are asthmatics, as shown in Table 3. We obtained similar results concerning the new use of SABA among patients with COPD. Similarly, SABA use was not associated with an increased risk of heart failure or arrhythmia compared with the new use of ICS.
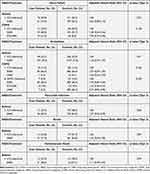 |
Table 3 Secondary Outcomes of Individual Components of Major Adverse Cardiovascular Events |
Sensitivity Analysis
The results of our sensitivity analyses (see Appendix 2) for MACE were overall consistent with those of our primary analyses among asthma patients when the analyses were repeated by varying the grace period by 0 days (adjusted HR 1.28 [0.96–1.71]), 45 days (adjusted HR, 1.33 [1.02–1.74]), 60 days (adjusted HR, 1.47 [1.14–1.89]) and with add-on or switchers (adjusted HR, 1.17 [0.92–1.49]). The HR generated in our primary analyses (adjusted HR, 1.29 [0.96–1.73]) for asthma was similar to the one generated in our fixed-effect analysis (adjusted HR, 1.24 [1.11–1.37]). Similarly, the HR generated in our primary analyses (adjusted HR, 1.07 [0.67–1.70]) for COPD was similar to the one generated in our fixed-effect analysis (adjusted HR, 1.09 [0.91–1.31]).
Discussion
Our findings suggest that using SABA or ICS/LABA combination therapy was not associated with an increased risk of MACE compared with ICS among patients with asthma. On the other hand, our findings suggest that new use of SABA, LABA, or ICS/LABA is associated with more than twofold increased risk of MACE compared with SAMA among patients diagnosed with COPD. Similarly, patients with asthma–COPD overlap who are new users of SABA are approximately 2.6 times more likely to suffer MACE than patients using ICS. In contrast, our findings suggest that using SABA or ICS/LABA combination therapy was not associated with an increased risk of MACE compared with ICS among patients with asthma. Furthermore, we did not observe any significant association between the use of β2-agonists-based drugs and incidences of the individual components of MACE.
Regarding differentiating the risk of MACE concerning either SABA or LABA, our study reported that among patients with COPD, in comparison to new users of SAMA, new users of SABA, ICS/LABA and LABA were significantly associated with more than two times the risk of MACE. However, the differences in risk of MACE associated with SABA, ICS/LABA, and LABA were not statistically significant. It is also true that among patients with asthma–COPD overlap, in comparisons to new users of ICS, the differences in the risk of MACE associated with either SABA or ICS/LABA were not statistically significant.
Our findings are consistent with results from a recent meta-analysis of RCTs showing an increased risk of cardiac failure among patients with stable COPD and receiving LABA therapy than patients receiving placebo (odds ratio, OR 1.70, 95% CI, 1.00–2.90).35 Additional network meta-analysis results indicated that LABAs combined with LAMAs resulted in an increased risk of cardiac failure in patients with COPD compared with placebo (OR 2.32, 95% CI, 1.10–5.09).35 Also, in another meta-analysis of 43 randomized trials, the authors reported that inhaled LABA increased the risk of cardiac failure (relative risk [RR] 1.71, 95% CI 1.04–2.84, I2 27.5%).36 Furthermore, a recent nested-case control study indicated that new use of LABAs or LAMAs in patients with COPD is associated with an approximately 1.5-fold increase in severe cardiovascular risk, irrespective of prior CVD status and history of exacerbation.37 With the increased risks associated with LABA use, GPs need to be very mindful and monitor for cardiovascular symptoms within 30 days of new initiations of LABA treatment for COPD.
Another meta-analysis of RCTs also reported an increased risk of a cardiovascular event associated with β2-agonists-based drugs (RR 2.54, 95% CI, 1.59–4.05) compared to placebo.38 In this study, initiation of β2-agonists treatment increases heart rate and reduces potassium concentrations compared to placebo. β2-agonists such as SABAs are recommended as the initial treatment to relieve symptoms and exercise limitations in COPD.2 Nonetheless, the efficacy of SABAs in COPD is lower than in asthma due to limited or no reversibility observed in COPD patients. For patients with asthma–COPD overlap, we observed that patients on rescue medication of SABA are 2.6 times more likely to experience MACE than ICS. Given the lack of randomized intervention studies of asthma–COPD overlap, it is difficult to provide firm treatment guidance for these patients. It is indicated that treatment with inhaled glucocorticoids should be continued in patients with long-standing asthma, even if a component of irreversible airway obstruction develops.39
Previous studies of the risk of cardiovascular diseases associated with β2-agonists mainly included prevalent users,40 limited to elderly patients only14,16 or lacked statistical power.41–43 Our study is novel in that we examined a single prescription drug use after the new initiation of β2-agonists therapy and the risk of MACE since the advent of β2-agonists on the market. A thorough discussion on the potential mechanism of cardiovascular effects associated with β2-agonist use is beyond the scope of our study; the use of SABAs or LABAs, acting as autonomous nervous system agents, is suspected of causing sympathetic overstimulation by activating both β2-adrenoceptors and cardiac muscle.44,45 Also, the presence of a moderate amount of β2-adrenoreceptors with percentages ranging from 14% to 40% in ventricular myocardium and from 20% to 55% in atrial tissue explain why β2-agonists can induce many adverse effects on cardiac function.46 Although the biological mechanisms underlying increased risk of MACE associated with LABAs are still not fully understood; however, it is believed that LABA can significantly increase interleukin-8 (IL-8) production,47 and IL-8 is a solid predictor to predict cardiovascular events among patients with coronary artery disease,48 to predict of cardiovascular and overall mortality among patients with end-stage renal disease,49 and to predict the development of heart failure in patients with acute myocardial infarction.50 Another potential mechanism is since LABAs are autonomous nervous system agents. LABAs are believed to cause over-activation of the sympathetic nervous system, associated with increased risk of many MACE, including ischemic heart disease, chronic heart failure, and hypertension.51,52 Conversely, β1- adrenergic receptors are the dominant receptor in the heart, whereas the β2- adrenergic receptor (including agonists such as LABAs and SABAs) is responsible for the relaxation of vascular, uterine, and airway smooth muscle.53 However, many of these agonists showed poor pharmacokinetics (PK) profiles and cardiovascular adverse effects due to a lack or partial selectivity for the β2- adrenergic receptor.5–8,53
The present study has several strengths. First, we assessed/used a large population-based cohort of patients initiating β2-agonist-based drugs, ICS, SAMA, or LAMA therapies. Even though our study is observational in nature and thus susceptible to potential confounding, we used rigorous matching and statistical adjustment to minimize residual confounding and control for many potential confounders. Second, using a new-user design eliminated biases related to the inclusion of prevalent users. Third, consistent results with varied time-window in our sensitivity analyses further confirmed the robustness of our results. Another strength is the generalizability of the results from our cohort, which is representative of the overall United Kingdom population of patients diagnosed with asthma, COPD, and asthma–COPD overlap.
Among limitations, the first is the possibility that patients using SAMA would be healthier and have the less severe disease than those receiving ICS/LABA or LABA. However, we addressed this issue by including multiple disease severity-related covariates in our final models. A second limitation is the inability to examine individual agents of β2-agonist agents to preclude which agents may be responsible for CV events. However, in a study that compared the cardiac effects of formoterol, salbutamol and fenoterol, Bremmer et al. found that all β2-agonist agents increased heart rate and Corrected-QT interval, decreased Q-S2 interval and plasma potassium levels compared with placebo.54 Residual confounding may be introduced into the analysis due to incomplete adjustment for unmeasured baseline covariates. However, in particular, the CPRD GOLD database measures numerous confounders that are not available in a typical electronic health records database.
Conclusion
New initiation of β2-agonist therapy, including SABA, LABA or ICS/LABA combination therapy, was associated with an increased risk of major adverse cardiovascular events among patients diagnosed with COPD. Similarly, patients with asthma–COPD overlap who are new users of SABA were more likely to suffer major adverse cardiovascular events. Our study confirms available evidence in the literature. The decision to prescribe β2-agonist-based drugs should be premised on consideration of patient benefit and increased risk of major adverse cardiovascular events.
Abbreviation
COPD, chronic obstructive pulmonary disease; CPRD, Clinical Practice Research Datalink; ICS, inhaled corticosteroid; SAMA, short-acting muscarinic antagonist; LABA, long-acting β2-agonists; LAMA, long-acting muscarinic antagonist; MACE, major adverse cardiovascular events; SABA, short-acting β2-agonists; HR, hazard ratio.
Acknowledgments
This study is based on data from the Clinical Practice Research Datalink (CPRD-GOLD) obtained under license from the UK Medicines and Healthcare products Regulatory Agency. However, the interpretation and conclusions contained in this study are those of the authors alone.
Funding
This work was supported by a research grant from Canada Research Respiratory Network (CRRN), Ottawa, Canada (Young Investigator Award, 2017). The study funder was not involved in the study design or the writing of the protocol.
Disclosure
The authors report no conflicts of interest in this work.
References
1. GINA. Global initiative for Asthma; 2020. Available from: https://ginasthma.org/wp-content/uploads/2020/06/GINA-2020-report_20_06_04-1-wms.pdf.
2. GOLD. COPD gold guidelines; 2020. Available from: https://goldcopd.org/wp-content/uploads/2020/03/GOLD-2020-POCKET-GUIDE-ver1.0_FINAL-WMV.pdf.
3. Barisione G, Baroffio M, Crimi E, et al. Beta-adrenergic agonists. Pharmaceuticals. 2010;3(4):1016–1044. doi:10.3390/ph3041016
4. Abosamak NER, Shahin MH. Beta 2 receptor agonists/ antagonists. In: StatPearls. Treasure Island FL: StatPearls Publishing LLC; 2021.
5. Cazzola M, Page CP, Rogliani P, et al. β 2 -agonist therapy in lung disease. Am J Respir Crit Care Med. 2013;187(7):690–696. doi:10.1164/rccm.201209-1739PP
6. Cazzola M, Matera MG. Tremor and β(2)-adrenergic agents: is it a real clinical problem? Pulm Pharmacol Ther. 2012;25(1):4–10. doi:10.1016/j.pupt.2011.12.004
7. Sears MR. Adverse effects of beta-agonists. J Allergy Clin Immunol. 2002;110(6 Suppl):S322–S328. doi:10.1067/mai.2002.129966
8. Taylor DR, Sears MR, Cockcroft DW. The beta-agonist controversy. Med Clin North Am. 1996;80(4):719–748. doi:10.1016/S0025-7125(05)70465-X
9. Billington CK, Penn RB, Hall IP. β(2) agonists. Handb Exp Pharmacol. 2017;237:23–40.
10. Dong YH, Chang C-H, Gagne JJ, et al. Comparative cardiovascular and cerebrovascular safety of inhaled long-acting bronchodilators in patients with chronic obstructive pulmonary disease: a Population-Based Cohort Study. Pharmacotherapy. 2016;36(1):26–37. doi:10.1002/phar.1684
11. Calverley PM, Anderson JA, Celli B, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356(8):775–789. doi:10.1056/NEJMoa063070
12. Xia N, Wang H, Nie X, Bhattacharya S. Inhaled long-acting β2-agonists do not increase fatal cardiovascular adverse events in COPD: a Meta-Analysis. PLoS One. 2015;10(9):e0137904. doi:10.1371/journal.pone.0137904
13. Wilchesky M, Ernst P, Brophy JM, et al. Bronchodilator use and the risk of arrhythmia in COPD: part 1: Saskatchewan cohort study. Chest. 2012;142(2):298–304. doi:10.1378/chest.10-2499
14. Wilchesky M, Ernst P, Brophy JM, et al. Bronchodilator use and the risk of arrhythmia in COPD: part 2: reassessment in the larger Quebec cohort. Chest. 2012;142(2):305–311. doi:10.1378/chest.11-1597
15. Juniper EF, O′byrne PM, Guyatt GH, et al. Development and validation of a questionnaire to measure asthma control. Eur Respir J. 1999;14(4):902–907. doi:10.1034/j.1399-3003.1999.14d29.x
16. Gershon A, Croxford R, Calzavara A, et al. Cardiovascular safety of inhaled long-acting bronchodilators in individuals with chronic obstructive pulmonary disease. JAMA Intern Med. 2013;173(13):1175–1185. doi:10.1001/jamainternmed.2013.1016
17. Tashkin DP, Celli B, Senn S, et al. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008;359(15):1543–1554. doi:10.1056/NEJMoa0805800
18. Salpeter SR. Cardiovascular safety of beta(2)-adrenoceptor agonist use in patients with obstructive airway disease: a systematic review. Drugs Aging. 2004;21(6):405–414. doi:10.2165/00002512-200421060-00005
19. Tricco AC, Strifler L, Veroniki -A-A, et al. Comparative safety and effectiveness of long-acting inhaled agents for treating chronic obstructive pulmonary disease: a systematic review and network meta-analysis. BMJ Open. 2015;5(10):e009183. doi:10.1136/bmjopen-2015-009183
20. Lulich KM, Goldie RG, Ryan G, Paterson JW, et al. Adverse reactions to beta 2-agonist bronchodilators. Med Toxicol. 1986;1(4):286–299. doi:10.1007/BF03259844
21. Lockett MF. Dangerous effects of isoprenaline in myocardial failurE. Lancet. 1965;2(7403):104–106. doi:10.1016/S0140-6736(65)92221-X
22. Busse WW, Bateman ED, Caplan AL, et al. Combined analysis of asthma safety trials of long-acting β(2)-agonists. N Engl J Med. 2018;378(26):2497–2505. doi:10.1056/NEJMoa1716868
23. Herrett E, Gallagher AM, Bhaskaran K, et al. Data resource profile: Clinical Practice Research Datalink (CPRD). Int J Epidemiol. 2015;44(3):827–836. doi:10.1093/ije/dyv098
24. Quint JK, Moore E, Lewis A, et al. Recruitment of patients with Chronic Obstructive Pulmonary Disease (COPD) from the Clinical Practice Research Datalink (CPRD) for research. NPJ Prim Care Respir Med. 2018;28(1):21. doi:10.1038/s41533-018-0089-3
25. Hamilton W. Using CPRD data for public health research. Br J Gen Pract. 2018;68(672):334. doi:10.3399/bjgp18X697745
26. McDonald L, Schultze A, Carroll R, et al. Performing studies using the UK clinical practice research datalink: to link or not to link? Eur J Epidemiol. 2018;33(6):601–605. doi:10.1007/s10654-018-0389-5
27. Price DB, Carter V, Martin J, et al. Comparative safety profile of the fixed-dose combination corticosteroid and long-acting β(2)-agonist fluticasone propionate/formoterol fumarate: a 36-month longitudinal cohort study in UK primary care. Drugs. 2020;80(1):47–60. doi:10.1007/s40265-019-01224-8
28. Afonso A, Schmiedl S, Becker C, et al. A methodological comparison of two European primary care databases and replication in a US claims database: inhaled long-acting beta-2-agonists and the risk of acute myocardial infarction. Eur J Clin Pharmacol. 2016;72(9):1105–1116. doi:10.1007/s00228-016-2071-8
29. Amegadzie JE, Gamble J-M, Farrell J, et al. Gender differences in inhaled pharmacotherapy utilization in patients with Obstructive Airway Diseases (OADs): a Population-Based Study. Int J Chron Obstruct Pulmon Dis. 2020;15:2355–2366. doi:10.2147/COPD.S264580
30. Suissa S, Dell’Aniello S, Ernst P. Comparative effectiveness of initial LAMA versus LABA in COPD: real-World Cohort Study. COPD. 2021;18(1):1–8. doi:10.1080/15412555.2021.1877649
31. Nissen F, Morales DR, Mullerova H, et al. Validation of asthma recording in the Clinical Practice Research Datalink (CPRD). BMJ Open. 2017;7(8):e017474. doi:10.1136/bmjopen-2017-017474
32. Quint JK, Mullerova H, DiSantostefano RL, et al. Validation of chronic obstructive pulmonary disease recording in the Clinical Practice Research Datalink (CPRD-GOLD). BMJ Open. 2014;4(7):e005540. doi:10.1136/bmjopen-2014-005540
33. Essebag V, Platt RW, Abrahamowicz M, et al. Comparison of nested case-control and survival analysis methodologies for analysis of time-dependent exposure. BMC Med Res Methodol. 2005;5(1):5. doi:10.1186/1471-2288-5-5
34. Suissa S. The Quasi-cohort approach in pharmacoepidemiology: upgrading the nested case-control. Epidemiology. 2015;26(2):242–246. doi:10.1097/EDE.0000000000000221
35. Wu J, Ye Y, Li C, et al. Correlation of inhaled long-acting bronchodilators with adverse cardiovascular outcomes in patients with stable COPD: a Bayesian Network Meta-Analysis of Randomized Controlled Trials. J Cardiovasc Pharmacol. 2019;74(3):255–265. doi:10.1097/FJC.0000000000000705
36. Li C, Cheng W, Guo J, et al. Relationship of inhaled long-acting bronchodilators with cardiovascular outcomes among patients with stable COPD: a meta-analysis and systematic review of 43 randomized trials. Int J Chron Obstruct Pulmon Dis. 2019;14:799–808. doi:10.2147/COPD.S198288
37. Wang M-T, Liou J-T, Lin CW, et al. Association of cardiovascular risk with inhaled long-acting bronchodilators in patients with chronic obstructive pulmonary disease: a Nested Case-Control Study. JAMA Intern Med. 2018;178(2):229–238. doi:10.1001/jamainternmed.2017.7720
38. Salpeter SR, Ormiston TM, Salpeter EE. Cardiovascular effects of beta-agonists in patients with asthma and COPD: a meta-analysis. Chest. 2004;125(6):2309–2321. doi:10.1378/chest.125.6.2309
39. Postma DS, Rabe KF. The Asthma-COPD overlap syndrome. N Engl J Med. 2015;373(13):1241–1249. doi:10.1056/NEJMra1411863
40. Lee C-H, Choi S, Jang EJ, et al. Inhaled bronchodilators and the risk of tachyarrhythmias. Int J Cardiol. 2015;190:133–139. doi:10.1016/j.ijcard.2015.04.129
41. De Vries F, Pouwels S, Bracke M, et al. Use of β 2 agonists and risk of acute myocardial infarction in patients with hypertension. Br J Clin Pharmacol. 2008;65(4):580–586. doi:10.1111/j.1365-2125.2007.03077.x
42. Suissa S, Assimes T, Ernst P. Inhaled short acting beta agonist use in COPD and the risk of acute myocardial infarction. Thorax. 2003;58(1):43–46. doi:10.1136/thorax.58.1.43
43. Martin RM, Dunn NR, Freemantle SN, et al. Risk of non-fatal cardiac failure and ischaemic heart disease with long acting beta 2 agonists. Thorax. 1998;53(7):558–562. doi:10.1136/thx.53.7.558
44. Cazzola M, Matera MG, Donner CF. Inhaled beta2-adrenoceptor agonists: cardiovascular safety in patients with obstructive lung disease. Drugs. 2005;65(12):1595–1610. doi:10.2165/00003495-200565120-00001
45. Wood-Baker R, Cochrane B, Naughton MT. Cardiovascular mortality and morbidity in chronic obstructive pulmonary disease: the impact of bronchodilator treatment. Intern Med J. 2010;40(2):94–101. doi:10.1111/j.1445-5994.2009.02109.x
46. Bristow MR, Ginsburg R, Umans V, et al. Beta 1- and beta 2-adrenergic-receptor subpopulations in nonfailing and failing human ventricular myocardium: coupling of both receptor subtypes to muscle contraction and selective beta 1-receptor down-regulation in heart failure. Circ Res. 1986;59(3):297–309. doi:10.1161/01.RES.59.3.297
47. Kavelaars A, van de Pol M, Zijlstra J, Heijnen CJ. Beta 2-adrenergic activation enhances interleukin-8 production by human monocytes. J Neuroimmunol. 1997;77:211–216. doi:10.1016/S0165-5728(97)00076-3
48. Inoue T, Komoda H, Nonaka M, Kameda M, Uchida T, Node K. Interleukin-8 as an independent predictor of long-term clinical outcome in patients with coronary artery disease International. J Cardiol. 2008;124:319–325.
49. Panichi V, Taccola D, Rizza GM, et al. Interleukin-8 is a powerful prognostic predictor of all-cause and cardiovascular mortality in dialytic patients. Nephron Clin Pract. 2006;102:c51–c58. doi:10.1159/000088923
50. Dominguez-Rodriguez A, Abreu-Gonzalez P, Garcia-Gonzalez M, Ferrer J. Prognostic value of interleukin-8 as a predictor of heart failure in patients with myocardial infarction and percutaneous intervention. Int J Cardiol. 2006;111:158–160. doi:10.1016/j.ijcard.2005.05.063
51. Graham LN, Smith PA, Huggett RJ, Stoker JB, Mackintosh AF, Mary DA. Sympathetic drive in anterior and inferior uncomplicated acute myocardial infarction. Circulation. 2004;109:2285–2289. doi:10.1161/01.CIR.0000129252.96341.8B
52. Leimbach WN
53. Wada Y, Nakano S, Morimoto A, et al. Discovery of novel indazole derivatives as orally available β3-adrenergic receptor agonists lacking off-target-based cardiovascular side effects. J Med Chem. 2017;60(8):3252–3265. doi:10.1021/acs.jmedchem.6b01197
54. Bremner P, Woodman K, Burgess C, et al. A comparison of the cardiovascular and metabolic effects of formoterol, salbutamol and fenoterol. Eur Respir J. 1993;6(2):204–210.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.






