Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Association Between Trichoscopic Features and Serum Hormone Levels and Vitamin D Concentration in Patients with Androgenetic Alopecia in Eastern China: A Cross-Sectional Study
Authors Chen Q , Tao Q, Zhu Q, Zhu J, Du X
Received 11 July 2023
Accepted for publication 14 September 2023
Published 19 September 2023 Volume 2023:16 Pages 2547—2555
DOI https://doi.org/10.2147/CCID.S423177
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Qitao Chen, Qiuwei Tao, Qilin Zhu, Jing Zhu, Xufeng Du
Department of Dermatology, The Affiliated Wuxi People’s Hospital of Nanjing Medical University, Wuxi People’s Hospital, Wuxi Medical Center, Nanjing Medical University, Wuxi, People’s Republic of China
Correspondence: Xufeng Du, Department of Dermatology, The Affiliated Wuxi People’s Hospital of Nanjing Medical University, Wuxi People’s Hospital, Wuxi Medical Center, Nanjing Medical University, No. 299 Qingyang Road, Wuxi, People’s Republic of China, Tel +86 19952290906, Email [email protected]
Purpose: Androgenetic alopecia (AGA) is a common dermatological condition, with reported associations between serum vitamin D and sex hormone levels. However, the relationship between these factors and the severity of hair loss remains unclear.
Patients and Methods: Our cross-sectional study included 310 AGA patients who visited the dermatology clinic and underwent trichoscopy examinations throughout 2021. We collected data on serum sex hormones and 25(OH)D levels during their consultations. TrichoScan analysis was used to characterize and quantify AGA severity based on hair density and vellus hair proportions in the central scalp area, which were then correlated with the collected indicators.
Results: The study findings reflect the basic demographic characteristics of AGA patients in the Chinese population. We discovered a significant negative correlation between serum SHBG levels and AGA severity in women, but no association was found in men. Serum testosterone, estradiol, dehydroepiandrosterone sulfate, and 25(OH)D levels showed no significant correlation with the severity of androgenetic alopecia, regardless of gender.
Conclusion: To our knowledge, this is the first study to investigate the relationship between serum sex hormones, vitamin D, and AGA severity using trichoscopic scalp hair features.
Keywords: androgenetic alopecia, TrichoScan, vitamin D, sex hormones
Introduction
Androgenetic alopecia (AGA), also referred to as female pattern hair loss in women, is a prevalent type of hair loss encountered in dermatological outpatient clinics. In men, AGA typically manifests as a diminishing hair density on the vertex and frontal areas and a receding hairline, while in women, it is characterized by diffuse sparse hair over the central scalp with a widening of the part line. In all patients, hair thinning is a common feature. AGA significantly impacts the quality of life for sufferers by affecting their appearance, leading to social and psychological issues. In China, studies reveal that the prevalence of AGA in men is 21.3%, while it is 6% in women, constituting a considerable proportion of non-scarring alopecia cases.1,2
The pathogenesis of androgenetic alopecia was previously thought to be mainly influenced by two factors, endocrine and genetic susceptibility. It is mainly due to the action of dihydrotestosterone, a metabolite of testosterone, on androgen-sensitive hair follicles, which leads to follicular miniaturization and shedding. Additionally, a significant proportion of patients have a clear family history of the condition.3 The precise mechanism by which hormonal milieu influences the onset and progression of AGA remains unclear. It is generally believed that serum testosterone levels are significantly elevated in men with androgenetic hair loss compared to healthy controls, while only one-third of women in the affected population exhibit abnormal androgen levels.4 Furthermore, the relationship between serum testosterone levels and the severity of hair loss has yet to be elucidated. Vitamin D, a nutrient with a synthesis process intimately linked to the skin, has been proven to be involved in the regulation of skin immunity and homeostasis. Furthermore, it participates in the modulation of hair follicle cycling and is believed to be associated with various hair loss disorders.5,6 In cases of alopecia areata, the protective role of vitamin D has been substantiated, and numerous reports exist of successfully treating the condition with vitamin D supplements. 25-hydroxyvitamin D, also known as 25(OH)D, is used as a biomarker to assess the vitamin D status of an individual, as its levels in the blood reflect both dietary intake and synthesis in the skin. However, the protective effect of vitamin D supplementation on AGA remains unclear, with only studies indicating that serum 25(OH)D levels in patients with this condition are generally lower than those in healthy individuals.7–9 The relationship between vitamin D and sex hormones is a significant area of research in polycystic ovary syndrome (PCOS), and it is believed to be involved in the regulation of sex hormone production, contributing to the hyperandrogenemia associated with PCOS. However, it remains uncertain whether vitamin D participates in the process by which sex hormones affect hair follicles in AGA.10,11
Previously, the diagnosis of androgenetic alopecia relied mainly on clinical presentation and classification. However, with the application of machine learning-based trichoscope analysis tools in the clinic, clinicians can now measure the hair growth of hair loss patients using quantitative indicators.12 Yang et al13 validated the correlation between machine learning-based hair microscope analysis tool and the clinical classification system (Ludwig scale) by comparing the hair density and vellus hair ratio of various scalp regions. They found that the hair density and vellus hair ratio based on the TrichoScan analysis of the mid scalp area could to some extent reflect the severity of female pattern hair loss, and the resulting indicators were more statistically advantageous as quantitative data compared to the clinical classification.
Our cross-sectional study aimed to collect and analyze the hair microscope features, serum hormone indicators, and 25(OH)D concentrations of patients diagnosed with androgenetic alopecia in the central scalp area throughout the year 2021 in our hospital, exploring the correlations between these variables. To our knowledge, this is the first study to use trichoscope features to analyze the relationship between serum hormones, vitamin D, and the severity of AGA.
Methods
Study Subjects
We included 310 patients diagnosed with androgenetic alopecia who visited Wuxi People’s Hospital of Nanjing Medical University throughout the year 2021, divided into male and female groups. The inclusion criteria were as follows:
- No other systemic treatments for androgenetic alopecia before the visit.
- Presence of serum 25(OH)D, sex hormone level data, and trichoscopy test data.
Patients with the following criteria were excluded:
- Patients with incomplete data.
- Patients with other known conditions that may cause hair loss.
- Patients with other acute or chronic inflammation diseases.
- Patients with a history of cardiovascular disease, hyperlipidemia, endocrine disorders, and various malignancies.
- Patients who have taken steroids or dietary supplements such as vitamins, minerals, and other supplements within 3 months before the study.
Data
Blood samples were collected from all patients by venepuncture into plain Vacutainer® tubes prior to any treatment. For premenopausal women, blood samples were collected between 3 and 5 days after the end of menstruation (follicular phase). After collection, the blood samples were allowed to clot and then centrifuged at 2500 × g to separate the serum, which was aspirated and stored in aliquots at −80 °C until analysis. Serum levels of the biomarkers of interest, including 25(OH)D and sex hormones, were measured using a chemiluminescent microparticle immunoassay (Architect system, USA). The included serum hormone indicators are testosterone, estradiol, dehydroepiandrosterone sulfate (DHEAS), and sex hormone-binding globulin (SHBG). The assay performance was validated according to established protocols, and quality control samples were included in each batch of analyses to ensure accuracy and precision. All measurements were conducted by experienced laboratory technicians who were blinded to the clinical information of the patients. The collected raw data are displayed in Table S1 (females) and Table S2 (males).
All included patients underwent trichoscope examination in our hospital. The selected location was the intersection point between the midline and the line connecting the two outer ear canal openings (Figure 1). Hair within a 2cm x 2cm area around the intersection point was trimmed to a length of 0.5–1cm. An examination was conducted using a computerized handheld USB camera with TrichoScan, PhotoMAX® Software, which was used to measure the local hair density and terminal/vellus hair ratio (Figure S1). The measurements were taken three times, and the average value was obtained.
 |
Figure 1 The selected location of the hair trichoscope. Note: *The red point shows (A) the line connecting bilateral ear canal openings; (B) the midline of the head. |
Statistical Analysis
R version 4.12 was used for descriptive statistical analysis of all variables and to test for normality. The “ppcor” package was used for Spearman partial correlation analysis between variables. A P-value of less than 0.05 indicated a significant difference according to the two-tailed test. The “lm” function is employed to conduct simple linear regression analysis on variables exhibiting significant correlations, and the “crPlots” function from the “car” package is utilized for linear regression diagnostics.
Results
Basic Characteristics
The descriptive analysis results for 137 male and 173 female patients are shown in Table 1. The age of the male and female groups in the patient population was similar, with early-onset AGA patients (aged under 30 years) accounting for 66.4% and 55.5% of male and female patients, respectively. The serum 25-hydroxyvitamin D levels were higher in males than in females (mean value 18.13 vs 13.99), and vitamin D deficiency was prevalent in the AGA population (<20ng/mL). Female patients had both lower hair density and higher vellus hair ratio at the time of their visit. Only three female patients were diagnosed with or had a history of PCOS.
 |
Table 1 Summary Statistics of Age, Plasma Sex Hormones, Vitamin D in AGA Patients |
The Severity of Female AGA is Negatively Correlated with Serum SHBG Levels
Table 2 shows the correlation between serum 25(OH)D, partial sex hormone levels, and TrichoScan parameters. In female AGA patients, SHBG was positively correlated with hair density and negatively correlated with vellus hair ratio. Linear regression analysis (Figure 2) supported the conclusion that SHBG was significantly negatively correlated with the severity of hair loss. There was no significant correlation between serum 25(OH)D, testosterone, estradiol, DHEAS, and hair density or vellus hair ratio in all AGA patients.
 |
Table 2 The Correlation Between Hair Parameters and Serological Parameters |
 |
Figure 2 Linear regression analysis of SHBG with hair density (A) and vellus ratio (B). |
The Correlation Between Serum Vitamin D Levels and Certain Hormone-Related Indicators
Table 3 illustrates that there was a minimal correlation between serum sex hormone levels and vitamin D in AGA patients.
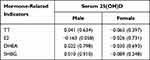 |
Table 3 The Correlation Between Serum 25(OH)D and Hormone-Related Indicators |
Discussion
Principal Results
In this study, we collected partial serum examination levels and TrichoScan data of patients diagnosed with androgenetic alopecia in the dermatology department of our hospital throughout the year 2021. We conducted a cross-sectional analysis of selected sex hormone indicators and vitamin D levels with hair density and vellus hair ratio in the mid-scalp area. The mean age of all patients at the time of their visit was under 30 years (males: 28.31, females: 29.44). This is noteworthy because, in other surveys on the prevalence of AGA among the Chinese population, the prevalence rate of AGA among individuals under 30 years old was no more than 5%,1,14 highlighting the urgent need for treatment and improvement for early-onset androgenetic alopecia patients.
Our study results suggest a significant negative correlation between serum sex hormone binding globulin (SHBG) levels and the severity of female pattern hair loss. SHBG is a homodimeric glycoprotein with two identical peptide chains and a molecular weight of 90–100 kDa. It is mainly produced by the liver and functions as a major protein that binds and transports testosterone, estradiol, and other sex steroids in plasma with high affinity. By regulating the levels of free hormones, SHBG affects their biological availability.15 The association between low serum SHBG levels and early-onset androgenetic alopecia (AGA) has been mentioned in several studies on polycystic ovary syndrome (PCOS) and is believed to be related to insulin resistance.16 However, of the 173 female patients included in our study, only three were clinically diagnosed with PCOS. In the existing literature, SHBG seems to mainly affect hair follicle papilla androgen receptors by modulating the bioavailability of sex hormones. Only SHBG was found to have a significant negative correlation with the severity of hair loss in female AGA patients, while also showing an increase in hair density and a decrease in the proportion of vellus hairs in our study. This suggests that free, unbound sex hormones may play an essential role in female pattern hair loss. Interestingly, a similar situation has not been observed in male AGA patients.
As a lipid-soluble vitamin and steroid hormone, Vitamin D is generally obtained through the skin or diet as ergocalciferol and cholecalciferol, then converted into 25-hydroxyvitamin D in the liver. It is then primarily converted into its active form, 1,25-hydroxyvitamin D, by the action of 1-α-hydroxylase (CYP27B1) in the kidneys. Various cell types, including skin keratinocytes and macrophages, can express CYP27B1 which participates in multiple physiological processes in conjunction with the Vitamin D receptor (VDR).5 Previous studies have observed that the levels of vitamin D in AGA patients are lower than those in healthy individuals. Based on the existing literature and the hypotheses we formulated when designing our study, vitamin D may affect the development of AGA through both direct and indirect mechanisms.17 The direct mechanisms in which vitamin D may affect AGA development is through its high expression in the hair follicle stem cells and the involvement of vitamin D receptor (VDR) in regulating hair cycling in all but the first phase. In VDR knockout mice, hair follicle stem cells are reduced and their activity is weakened, resulting in hair loss.6,18,19 On the other hand, since VDR and CYP27B1 are expressed in gonadal tissues including the testes and ovaries, vitamin D can indirectly affect the occurrence and development of AGA by regulating the expression of some sex hormones through receptor-mediated steroidogenic enzyme activation.20 Our study showed that, compared to another large-scale study on vitamin D in the Chinese population, although a considerable proportion of our patients exhibited vitamin D deficiency compared to the general population (males: 21.3% vs 59.1%, females: 43.6% vs 87.9%, <20ng/mL),21 there was no significant correlation between serum vitamin D levels and the severity of androgenetic alopecia, and it was not associated with the levels of the included sex hormones. These research findings suggest that serum vitamin D may not be involved in the pathological development of androgenetic alopecia.
Comparison with Prior Work
Studies with similar designs are already listed in Table 4. Overall, although androgen-related indicators generally increase in the patient group compared to normal controls, and SHBG decreases significantly, it is generally believed that there is no significant correlation between the severity of hair loss grading and these related indicators. However, our study reflects a significant correlation between SHBG and female androgenetic alopecia progression that differs from other studies. The association between vitamin D and the severity of androgenetic alopecia (AGA) remains controversial. Only one study using the Hamilton-Norwood scale and trichogram data to investigate the effect of vitamin D on 50 male patients with androgenic alopecia came to a conclusion similar to our study. D’Andrea et al10 after conducting a meta-analysis, proposed that this association has high heterogeneity, and the results may vary in cross-sectional studies with changes in study populations.
 |
Table 4 Related Research Compilation |
Limitations and Future Directions
Limitations
Cross-sectional design: As mentioned earlier, our study’s cross-sectional nature limits our ability to establish causality and investigate long-term trends. This design also hinders our ability to examine how the variables might change over time and whether the observed associations persist or alter as time progresses.
Lack of mechanistic exploration: Our study did not delve into the specific mechanisms underlying the observed associations, leaving unanswered questions about the factors that drive these relationships. This limitation highlights the need for further research to elucidate the underlying processes and mechanisms.
We call for the implementation of large-scale prospective cohort studies to further explore the associations between vitamin D, sex hormones, and the severity of androgenetic alopecia. This will help provide evidence for vitamin D supplementation and hormonal regulation therapies in the treatment of AGA.
Conclusion
Our study has revealed important findings regarding the relationship between trichoscopic features and serum hormone levels and vitamin D concentration in patients with Androgenetic Alopecia in Eastern China. The data highlights the prevalence of early-onset AGA, with most patients being under 30 years old. Moreover, it was found that vitamin D deficiency was common among AGA patients. Interestingly, in female patients, a correlation was observed between SHBG levels, hair density, and vellus hair ratio. These findings emphasize the urgent need for improved treatments for early-onset AGA patients. Further research is needed to explore the underlying mechanisms of these observed correlations and to develop targeted treatments based on these findings.
Abbreviations
AGA, Androgenetic Alopecia; 25(OH)D, 25-hydroxyvitamin D3; TT, Testosterone; E2, Estradiol; DHEA, Dehydroepiandrosterone Sulfate; SHBG, Sex Hormone-binding Globulin; PCOS, Polycystic Ovary Syndrome.
Data Sharing Statement
The authors confirm that the data supporting the findings of this study are available within the article and its Supplementary Materials.
Ethics Approval and Consent to Participate
The research was conducted ethically in accordance with the World Medical Association Declaration of Helsinki and was approved by Wuxi People’s Hospital Research Ethics Committee, Wuxi, China (KY23077). All participants have confirmed the informed consent statement, acknowledging that their examination data may be used in the study.
Consent for Publication
The patient image shown in the schematic has been partially obscured and has been approved by the patient for publication.
Acknowledgments
We would like to express our gratitude to OpenAI’s GPT-4 for its assistance in refining the language of this manuscript.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported in part by grants from Investigation into the Molecular Mechanisms Underpinning Lymphocytic Scarring Alopecia with Inflammatory Fibrosis, HBBJ-012 and Cohort and Clinical Research Program of Wuxi Medical Center, Nanjing Medical University, WMCC202306.
Disclosure
The authors declare that they have no competing interests.
References
1. Wang TL, Zhou C, Shen YW, et al. Prevalence of androgenetic alopecia in China: a community-based study in six cities. Br J Dermatol. 2010;162(4):843–847. doi:10.1111/J.1365-2133.2010.09640.X
2. Huang CH, Fu Y, Chi CC. Health-related quality of life, depression, and self-esteem in patients with androgenetic alopecia: a systematic review and meta-analysis. JAMA Dermatol. 2021;157(8):963–970. doi:10.1001/JAMADERMATOL.2021.2196
3. Lolli F, Pallotti F, Rossi A, et al. Androgenetic alopecia: a review. Endocrine. 2017;57(1):9–17. doi:10.1007/S12020-017-1280-Y
4. Starace M, Orlando G, Alessandrini A, Piraccini BM. Female androgenetic alopecia: an update on diagnosis and management. Am J Clin Dermatol. 2020;21(1):69–84. doi:10.1007/S40257-019-00479-X
5. Mostafa WZ, Hegazy RA. Vitamin D and the skin: focus on a complex relationship: a review. J Adv Res. 2015;6(6):793–804. doi:10.1016/J.JARE.2014.01.011
6. Saini K, Mysore V. Role of vitamin D in hair loss: a short review. J Cosmet Dermatol. 2021;20(11):3407–3414. doi:10.1111/JOCD.14421
7. Zhu B, Zhang L, Wang J, Tan G. Vitamin D supplementation for patients with alopecia areata: a protocol for systematic review and meta-analysis. Medicine. 2022;101(42):E31089. doi:10.1097/MD.0000000000031089
8. Gerkowicz A, Chyl-Surdacka K, Krasowska D, Chodorowska G. The role of vitamin D in non-scarring alopecia. Int J Mol Sci. 2017;18(12):2653. doi:10.3390/IJMS18122653
9. Rasheed H, Mahgoub D, Hegazy R, et al. Serum ferritin and vitamin d in female hair loss: do they play a role? Skin Pharmacol Physiol. 2013;26(2):101–107. doi:10.1159/000346698
10. D’Andrea S, Martorella A, Coccia F, et al. Relationship of vitamin D status with testosterone levels: a systematic review and meta-analysis. Endocrine. 2021;72(1):49–61. doi:10.1007/S12020-020-02482-3
11. Morgante G, Darino I, Spanò A, et al. PCOS physiopathology and vitamin D deficiency: biological insights and perspectives for treatment. J Clin Med. 2022;11(15):4509. doi:10.3390/JCM11154509
12. Hoffmann R. TrichoScan: a novel tool for the analysis of hair growth in vivo. J Investig Dermatol Symp Proc. 2003;8(1):109–115. doi:10.1046/J.1523-1747.2003.12183.X
13. Yang X, Yu W, Qiao R, Sun J, Jiang Y. The predictive value of midscalp hair density and terminal hair percentage in the severity evaluation of FPHL assessed by trichoscan in a sample of Chinese Population. Clin Cosmet Investig Dermatol. 2022;15:2675–2684. doi:10.2147/CCID.S390148
14. Xu F, Sheng YY, Mu ZL, et al. Prevalence and types of androgenetic alopecia in Shanghai, China: a community-based study. Br J Dermatol. 2009;160(3):629–632. doi:10.1111/J.1365-2133.2008.08909.X
15. Xing C, Zhang J, Zhao H, He B. Effect of sex hormone-binding globulin on polycystic ovary syndrome: mechanisms, manifestations, genetics, and treatment. Int J Womens Health. 2022;14:91. doi:10.2147/IJWH.S344542
16. Arias-Santiago S, Gutiérrez-Salmerón MT, Buendía-Eisman A, Girón-Prieto MS, Naranjo-Sintes R. Sex hormone-binding globulin and risk of hyperglycemia in patients with androgenetic alopecia. J Am Acad Dermatol. 2011;65(1):48–53. doi:10.1016/J.JAAD.2010.05.002
17. Zubair Z, Kantamaneni K, Jalla K, et al. Prevalence of low serum vitamin d levels in patients presenting with androgenetic alopecia: a review. Cureus. 2021;13(12). doi:10.7759/CUREUS.20431
18. Bikle D, Christakos S. New aspects of vitamin D metabolism and action - addressing the skin as source and target. Nat Rev Endocrinol. 2020;16(4):234–252. doi:10.1038/S41574-019-0312-5
19. Kise S, Iijima A, Nagao C, et al. Functional analysis of vitamin D receptor (VDR) using adenovirus vector. J Steroid Biochem Mol Biol. 2023:230. doi:10.1016/J.JSBMB.2023.106275
20. Muscogiuri G, Altieri B, de Angelis C, et al. Shedding new light on female fertility: the role of vitamin D. Rev Endocr Metab Disord. 2017;18(3):273–283. doi:10.1007/S11154-017-9407-2
21. Bai K, Dong H, Liu L, et al. Serum 25-hydroxyvitamin D status of a large Chinese population from 30 provinces by LC-MS/MS measurement for consecutive 3 years: differences by age, sex, season and province. Eur J Nutr. 2023;62(3):1503–1516. doi:10.1007/S00394-023-03094-Z
22. Odhaib SA, Al Hamdi K, Mansour AA. Is there any association between clinical and biochemical hyperandrogenism in women with female pattern hair loss? Cureus. 2020;12(11). doi:10.7759/CUREUS.11732
23. Zhang Y, Xu J, Jing J, Wu X, Lv Z. Serum levels of androgen-associated hormones are correlated with curative effect in Androgenic alopecia in young men. Med Sci Monit. 2018;24:7770–7777. doi:10.12659/MSM.913116
24. Sanke S, Samudrala S, Yadav A, Chander R, Goyal R. Study of serum vitamin D levels in men with premature androgenetic alopecia. Int J Dermatol. 2020;59(9):1113–1116. doi:10.1111/IJD.14982
25. Kondrakhina IN, Verbenko DA, Zatevalov AM, et al. A cross-sectional study of plasma trace elements and vitamins content in androgenetic alopecia in men. Biol Trace Elem Res. 2021;199(9):3232–3241. doi:10.1007/S12011-020-02468-2
26. Costanzo PR, Suárez SM, Kozak AE, Knoblovits P. Seasonal variations in sex steroids in a young male population and their relationship with plasma levels of vitamin D. World J Mens Health. 2022;40(2):308. doi:10.5534/WJMH.200156
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
