Back to Journals » Journal of Multidisciplinary Healthcare » Volume 15
Association Between Symptoms and Severity of Disease in Hospitalised Novel Coronavirus (COVID-19) Patients: A Systematic Review and Meta-Analysis
Authors Talukder A , Razu SR, Alif SM, Rahman MA, Islam SMS
Received 11 January 2022
Accepted for publication 25 March 2022
Published 12 May 2022 Volume 2022:15 Pages 1101—1110
DOI https://doi.org/10.2147/JMDH.S357867
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Ashis Talukder,1 Shaharior Rahman Razu,2 Sheikh Mohammad Alif,3 Muhammad Aziz Rahman,4,5 Sheikh Mohammed Shariful Islam6
1Statistics Discipline, Khulna University, Khulna, 9208, Bangladesh; 2Sociology Discipline, Khulna University, Khulna, 9208, Bangladesh; 3School of Public Health and Preventive Medicine, Monash University, Melbourne, Australia; 4Institute of Health and Wellbeing, Federation University Australia, Berwick, Australia; 5Faculty of Public Health, Universitas Airlangga, Surabaya, Indonesia; 6Institute for Physical Activity and Nutrition, Deakin University, Melbourne, Australia
Correspondence: Ashis Talukder, Statistics Discipline, Khulna University, Khulna, 9208, Bangladesh, Tel +8801772063507, Email [email protected] Muhammad Aziz Rahman, Institute of Health and Wellbeing, Federation University Australia, Berwick, Australia, Tel +61343137908, Email [email protected]
Background: Symptoms of the novel coronavirus disease (COVD-19) are well known, although asymptomatic cases were also reported due to this rapidly evolving viral disease. However, there has been limited research with inconsistent findings on symptoms of COVID-19 and disease severity. We aimed to evaluate the association between symptoms and severity of disease in adult patients with confirmed COVID-19 by performing a meta-analysis.
Methods: We conducted this study by searching four online databases (Medline, Web of Science, EMBASE and Cochrane library) of published studies that included symptoms of COVID-19 cases and severity of the disease between January 1, 2020, and October 31, 2021. PRISMA and MOOSE guidelines were followed, and only articles published in English were selected. We performed meta-analysis using Mantel-Haenszel random-effects model. Note that we included peer-reviewed studies conducted in Wuhan and published in the English language that reported the clinical characteristics of COVID-19, particularly the symptoms of novel coronavirus patients with their prevalence and distribution of patients based on the severity of the disease.
Results: Out of 255 articles identified, a total of twenty articles, including 5390 participants, met the inclusion criteria and were included. Among the participants, 2997 (55.60%) were males, and 974 (18.07%) reported severe conditions. Fever was the most commonly reported symptom in the reported COVID-19 confirmed cases (88.47%, 95% CI: 80.74– 93.35%), which was followed by cough, fatigue, and less proportionally dyspnea and myalgia. Dyspnea was the only symptom, which was associated with severity of COVID-19 (OR 2.43, 95% CI: 1.52– 3.89).
Conclusion: Dyspnoea was found to be associated with severity of COVID-19. People with existing respiratory illnesses, such as chronic obstructive pulmonary diseases need to be careful about the onset of such symptom and should seek medical attention.
Keywords: coronavirus, COVID-19, symptoms, respiratory, severity
Introduction
The novel coronavirus disease 19 (COVID-19) is a pathogenic viral infection caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The condition has received enormous worldwide attention due to its high human-to-human transmissibility following the first cases reported in Wuhan, China, in December 2019.1,2 COVID-19 belongs to the same subgroup of coronavirus diseases. However, it is homologically far different from its predecessors like severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV).3 Although the disease is mild in most people, it may progress to severe illness like pneumonia, acute respiratory distress syndrome (ARDS) and multi-organ dysfunction causing a fatality.
The clinical spectrum of COVID-19 varies from asymptomatic or presenting few symptoms to symptomatic forms as the first symptoms usually appear after five to six days, according to the World Health Organization (WHO) reports.4 Common symptoms include fever, fatigue, dry cough, sore throat and dyspnea while pneumonia, sneezing, malaise, diarrhea, headache and conjunctivitis,5–9 loss of taste and smell10 have also been reported. Due to its typical progressive and aggravation process, COVID-19 has been classified from mild to severe types based on the severity of the disease by the Beijing Centers for Diseases Control and Prevention (NHC China, 2020).11 Globally, 80% of the reported COVID-19 cases presented with mild respiratory symptoms, 15% of cases required hospitalization and 5% cases were critical in nature.12
COVID-19 affects human body in a number of ways. Although the virus is known for affecting the respiratory systems drastically,13 other parts of the body including heart, CNS, renal, liver, and gastrointestinal functions.14–16 Persons with immunosuppressed and underlying conditions are often at a high risk of severe COVID-19 outcome despite vaccinations.17 However, the severity of COVID-19 outcomes varied among different age, sex, race, gender and other subgroups. Besides biological factors, social determinants such as income, education, social class, etc., are found to be associated with COVID-19 outcomes.13
Health researchers are ceaselessly working on understanding which symptoms are more closely associated with severe cases as the pandemic evolves. To get a more convincing idea about symptoms are associated severity of COVID-19, we attempt to provide a systematic and detailed evaluation from the recent data through our meta-analysis. The study will not only estimate the prevalence of symptoms of COVID-19 among the patients, but also assess the risk of severity in patients compared to non-severe patients. This analysis will help patient management and strategic interventions including policy measures to prevent and combat COVID-19 in coming days.
Materials and Methods
Search Strategy and Selection Criteria
This systematic review and meta-analysis were conducted according to PRISMA and MOOSE guidelines.18,19 An extensive search strategy was designed to retrieve all published articles from January 1, 2020 to October 31, 2021, in PubMed (Medline), Web of Science, EMBASE, and Cochrane Library databases. Search results were compiled using the bibliographic software Endnote™ X9.2. Based on the criteria of different databases, we used the following search terms: “2019-nCoV”, or “2019 novel coronavirus”, or “COVID-19”, or “clinical characteristics of COVID-19” or ‘symptoms of coronavirus’. We additionally screened the list of references for each selected article to identify studies that may have been missed during the initial search. Two independent researchers (AT and SRR) screened retrieved articles. The same investigators independently assessed full texts of records deemed eligible for inclusion. Any discrepancies were resolved by discussion and consensus with a senior investigator (SMSI).
We included peer-reviewed studies conducted in Wuhan and published in the English language that reported the clinical characteristics of COVID-19, particularly the symptoms of novel coronavirus patients with their prevalence and distribution of patients based on the severity of the disease. All the included studies’ patients were hospitalised cases of COVID-19, confirmed by the laboratory-based Real-Time Reverse Transcription Polymerase Chain Reaction Assay (RT-PCR). We excluded studies that focused on children and did not reported the clinical diagnostic criteria along with duplicate publications, single case reports, reviews, editorials, letters or (c) studies provide insufficient information on the relevant topic.
Data Extraction and Variables
Two authors (AT and SRR), who involved in the literature screening, also extracted the data independently from the selected studies. Differences were settled by conversation or a third analyst (SMSI). We obtained the following variables: first author, year of publication, number of patients, age, sex, number of severe and non-severe patients, and the prevalence of several symptoms including fever, cough, myalgia or fatigue, dyspnea and headache. “Patients with any of the following features at the time of, or after, admission was classified as severe cases: (1) respiratory distress (≥30 breaths per min); (2) oxygen saturation at rest ≤93%; (3) ratio of the partial pressure of arterial oxygen to the fractional concentration of oxygen inspired air ≤300 mm Hg; or (4) severe disease complications (eg, respiratory failure, the requirement of mechanical ventilation, septic shock, or non-respiratory organ failure).11
Data Analyses
All analyses were performed by R software (version 3.6.1). The odds ratios (OR) was considered to describe the severity of clinical symptoms in severe patients compared to non-severe patients. Due to the presence of heterogeneity in studies, Mantel-Haenszel random-effect models were utilised to estimate the average effect along with its precision, which can provide a more reliable estimate of the 95% confidence intervals (CI). To assess heterogeneity, we used the I2 statistic and Cochran’s Q test.
Study Quality and Publication Bias
We used The Newcastle-Ottawa Scale (NOS) for assessing the quality of studies in meta-analysis.20 The NOS summarised 8 aspects of each study: case definition adequacy, representativeness of the cases, selection of controls, the definition of controls, comparability of cases and controls on the basis of the design or analysis, ascertainment of exposure, the same method of ascertainment for cases and controls, and comparison of nonresponse rate between cases and controls. Studies with a score 6 or more out of 9 total points were considered as high-quality studies. We used funnel plot and Egger’s test to assess the publication bias. Furthermore, we utilized the Quality In Prognosis Studies (QUIPS) tool to assess the risk of bias which includes six domains such as (1) study participation, (2) study attrition, (3) prognostic factor measurement, (4) outcome measurement, (5) study confounding, and (6) statistical analysis and reporting.
Ethical Approval
This study needs no ethical approval since our study used published article for data collection.
Results
The search produced 255 articles. Out of these, we excluded 160 duplicate articles and considered the remaining 95. After a screening of the title and abstract, 52 articles were excluded. Finally, we reviewed the full text and removed 23 articles since they were reviews, case reports, editorials and had lack of information. Twenty articles6,8,21–38 including 5390 COVID-19 patients eventually met the inclusion criteria and were included. All the included studies were hospital-based cross-sectional. The flow diagram of the literature search is shown in Figure 1.
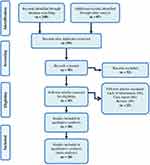 |
Figure 1 Flow diagram of literature search for including studies in meta-analysis. |
Among the participants, 2997 (55.60%) were males, and 974 (18.07%) reported severe conditions (Table 1). Fever (88.47%, 95% CI: 80.74–93.35%) was the most prevalent symptoms observed in COVID-19 patients, followed by cough (64.65%, 95% CI: 58.42–70.43%), fatigue (38.45%, 95% CI: 32.52–44.74%), dyspnea (23.59%, 95% CI: 16.48–32.58%) and myalgia (22.46%, 95% CI: 16.36–30.02%). The prevalence was estimated from random effect model and significant (p<0.05) heterogeneities were observed for the estimates with I2 ranging from 91% to 98% (see Figure 2).
 |
Table 1 Basic Characteristics of Included Studies in the Meta-Analysis and Estimating Prevalence of Several Symptoms Related to Novel Coronavirus (COVID-19) Patients |
 |
Figure 2 Meta-Analysis of symptoms of COVID-19. A–E represent the prevalence of fever, cough, fatigue, dyspnoea and myalgia, respectively. |
We found that all studies reported the presence of pneumonia and abnormal chest imaging in most of the patients on admission. Four studies reported that ground-glass opacity was the most common feature on chest computed tomography (CT), which was followed by bilateral patchy shadowing.6,8,21,23 The findings from the CT were almost similar to the CXR as reported by the studies. The most prevalent comorbidities were hypertension and diabetes,22 which are followed by acute respiratory distress syndrome (ARDS)6,8,21 and among them, a large proportion of patients worsened in a shorter period of time and developed multi-organ disorder.
Figure 3 shows the meta-analysis of the association between symptoms with severe and non-severe patients. Higher odds of dyspnea (OR 2.43 95% CI: 1.52–3.89) was observed in the severe group. The odds of fever (OR 1.04, 95% CI: 0.85–1.28), cough (OR 1.12, 95% CI: 0.91–1.38), fatigue (OR 1.14, 95% CI: 0.96–1.36), and myalgia (OR 0.78, 95% CI: 0.54–1.13) were also found higher in the severe COVID-19 patients. However, except dyspnea, our meta-analysis did not find any statistically significant association with the severity of the disease.
Study Quality and Publication Bias
All studies included for the meta-analysis were ranked as high-quality studies (Supplementary Tables 1 and 2). The publication bias was examined for the following symptoms: fever, cough, fatigue, dyspnea and myalgia. Figure 4 shows the results of possible publication bias, which were assessed by funnel plots and Egger’s test. The results of the Egger’s test (all p<0.05) suggest that there exist no significant publication bias.
 |
Figure 4 Funnel plot with Egger’s test for assessing publication bias. (A) Fever; (B) cough; (C) fatigue; (D) dyspnoea; (E) myalgia. |
Discussion
This systematic review and meta-analysis provide a recent summary of findings of the association between COVID-19 symptoms and its association with disease severity. Our findings suggest patients with dyspnea had more than twice the odds of having a severe COVID-19 illness at the time of hospital admission. This indicates that COVID-19 patients with dyspnea having more than double chance of becoming a severe patient. However, we did not find a significant association between fever, cough, myalgia or fatigue and headache with the severity of symptoms, which is contradictory to the previous systematic review and meta-analysis.39,40 The main reason for this difference could be the stricter inclusion criteria in our study that limits the inclusion of some low-powered case-series studies. Furthermore, previous studies only analysed the prevalence of symptoms, while we meta-analysed and presented the Mantel-Haenszel (MH) odds ratio of the severity of symptoms, which is the first time since the outbreak occurred.
Our meta-analysis found that fever is the predominantly reported clinical manifestation in COVID-19 confirmed cases. This was followed by cough, myalgia or fatigue and less proportionally dyspnea and headache. Furthermore, we identified that most of the included studies were limited to middle-aged to older adults, and commonly included male. Our finding is consistent with previous systematic review and meta-analysis that reported significantly higher prevalence of fever and less commonly cough, fatigue and headache (all p < 0.05).39,40 The prevalence of fever was 92% (95% CI: 89.4–96.2)27 and 83.3% (95 CI: 78.4–87.7),39 while we found fever in 87.89% (95% CI: 83.22–81.39%). However, we only included adults, while the previous studies also included children. The frequency of fever in COVID-19 patients on admission is similar in SARS and MERS outbreak, but the prevalence of cough is higher in SARS and COVID-19, compared to MERS.41,42
Despite a comprehensive analysis, several limitations should be taken into consideration in our study. Firstly, we only included studies from the Wuhan, which limits the number of studies, as well as clinical and demographic characteristics of patients, may be different from other parts of the world, in particular, we were unable to include clinical studies from Europe and America. This also limits the geographical and ethnic differences that were not excluded. Secondly, in the pooled analysis, the varying treatment pattern from the hospitals could not be identified, which could vary greatly. Finally, substantial heterogeneities were predominantly observed in several parameters in the meta-analysis given the variation of patient recruitment, intervention technique and treatment provided.
Further Study
This is a study conducted at the early stage of COVID 19 outbreak. Therefore, we were not able to get enough information regarding the disease. However, in the future, we will be able to conduct the meta-analysis with more information. We believe that meta-analysis with more information can help us to better identify the association between symptoms and severity of disease in hospitalized novel coronavirus (COVID-19) patients.
Conclusion
Our review suggests that dyspnoea is associated with the severity of COVID-19. Therefore, besides the new onset of such symptom, people with existing respiratory illnesses, such as chronic obstructive pulmonary diseases, need to be careful about the onset of such symptom and should seek medical attention early.
Acknowledgments
SMSI is funded by a senior research fellowship from the Institute for Physical Activity and Nutrition (IPAN), Deakin University and a post-doctorate fellowship from the National Heart Foundation of Australia and received a career transition grant from the High Blood Pressure Research Council of Australia.
Disclosure
The authors declare that they have no known competing interests.
References
1. Cascella M, Rajnik M, Cuomo A, et al. Features, evaluation, and treatment of coronavirus (COVID-19). In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2020.
2. Wu JT, Leung K, Leung GM. Nowcasting and forecasting the potential domestic and international spread of the 2019-nCoV outbreak originating in Wuhan, China: a modelling study. Lancet. 2020;395(10225):689–697. doi:10.1016/S0140-6736(20)30260-9
3. Clark A, Jit M, Warren-Gash C, et al. Global, regional, and national estimates of the population at increased risk of severe COVID-19 due to underlying health conditions in 2020: a modelling study. Lancet Glob Health. 2020;8(8):e1003–e1017. doi:10.1016/S2214-109X(20)30264-3
4. Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382(13):1199–1207. doi:10.1056/NEJMoa2001316
5. Abd El-Aziz TM, Stockand JD. Recent progress and challenges in drug development against COVID-19 coronavirus (SARS-CoV-2) - an update on the status. Infect Genet Evol. 2020;83:104327. doi:10.1016/j.meegid.2020.104327
6. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi:10.1016/S0140-6736(20)30183-5
7. Singhal T. A review of coronavirus disease-2019 (COVID-19). Indian J Pediatr. 2020;87(4):281–286. doi:10.1007/s12098-020-03263-6
8. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi:10.1001/jama.2020.1585
9. Yang Y, Lu QB, Liu MJ, et al. Epidemiological and clinical features of the 2019 novel coronavirus outbreak in China. MedRxiv. 2020. doi:10.1101/2020.02.10.20021675
10. Xiao Y, Torok ME. Taking the right measures to control COVID-19. Lancet Infect Dis. 2020;20(5):523–524. doi:10.1016/S1473-3099(20)30152-3
11. Liu Y, Yan LM, Wan L, et al. Viral dynamics in mild and severe cases of COVID-19. Lancet Infect Dis. 2020;20(6):656–657. doi:10.1016/S1473-3099(20)30232-2
12. Bialek S, Boundy E, Bowen V; COVID, C. and R. Team. Severe outcomes among patients with coronavirus disease 2019 (COVID-19)—United States, February 12–March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(12):343–346. doi:10.15585/mmwr.mm6912e2
13. Brosnahan SB, Jonkman AH, Kugler MC, Munger JS, Kaufman DA. COVID-19 and respiratory system disorders: current knowledge, future clinical and translational research questions. Arterioscler Thromb Vasc Biol. 2020;40(11):2586–2597. doi:10.1161/ATVBAHA.120.314515
14. Shi S, Qin M, Shen B, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5(7):802–810. doi:10.1001/jamacardio.2020.0950
15. Sobhani S, Aryan R, Kalantari E, et al. Association between clinical characteristics and laboratory findings with outcome of hospitalized COVID-19 patients: a report from Northeast Iran. Interdiscip Perspect Infect Dis. 2021;2021:1–5. doi:10.1155/2021/5552138
16. Wang T, Du Z, Zhu F, et al. Comorbidities and multi-organ injuries in the treatment of COVID-19. Lancet. 2020;395(10228):e52. doi:10.1016/S0140-6736(20)30558-4
17. Yek C, Warner S, Wiltz JL, et al. Risk factors for severe COVID-19 outcomes among persons aged ≥18 years who completed a primary COVID-19 vaccination series - 465 health care facilities, United States, December 2020-October 2021. MMWR. 2022;71(1):19–25. doi:10.15585/mmwr.mm7101a4
18. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):1–6. doi:10.1371/journal.pmed.1000097
19. Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–2012. doi:10.1001/jama.283.15.2008
20. Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa. 2011;2(1):1–2.
21. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi:10.1016/S0140-6736(20)30211-7
22. Guan WJ, Liang WH, Zhao Y, et al. Comorbidity and its impact on 1590 patients with Covid-19 in China: a nationwide analysis. Eur Respir J. 2020;55(5):
23. Guan W, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi:10.1056/NEJMoa2002032
24. Liu J, Liu Y, Xiang P, et al. Neutrophil-to-lymphocyte ratio predicts severe illness patients with 2019 novel coronavirus in the early stage. MedRxiv. 2020. doi:10.1101/2020.02.10.20021584
25. Zhang JJ, Dong X, Cao YY, et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020;75(7):1730–1741. doi:10.1111/all.14238
26. Xu X, Wu X, Jiang X, et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ;2020. m606. doi:10.1136/bmj.m606
27. Guo T, Fan Y, Chen M, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020;5(7):811. doi:10.1001/jamacardio.2020.1017
28. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi:10.1016/S0140-6736(20)30566-3
29. Liu K, Fang Y, Deng Y, et al. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J. 2020;133(9):1025–1031. doi:10.1097/CM9.0000000000000744
30. Chen T, Dai Z, Mo P, et al. Clinical characteristics and outcomes of older patients with coronavirus disease 2019 (COVID-19) in Wuhan, China: a single-centered, retrospective study. J Gerontol. 2020;75(9):1788–1795. doi:10.1093/gerona/glaa089
31. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–481. doi:10.1016/S2213-2600(20)30079-5
32. Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934. doi:10.1001/jamainternmed.2020.0994
33. Jie L, Li S, Cai Y, et al. Epidemiological and clinical characteristics of 17 hospitalized patients with 2019 novel coronavirus infections outside Wuhan, China. medRxiv. 2020. doi:10.1101/2020.02.11.20022053
34. Mo P, Xing Y, Xiao Y, et al. Clinical characteristics of refractory COVID-19 pneumonia in Wuhan, China. Clin Infect Dis. 2020. doi:10.1093/cid/ciaa270
35. Rong-Hui D, Liang L, Yang C, et al. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: a prospective cohort study. Eur Respir J. 2020;55:2000524. doi:10.1183/13993003.00524-2020
36. Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ;2020. m1091. doi:10.1136/bmj.m1091
37. Deng Y, Liu W, Liu K, et al. Clinical characteristics of fatal and recovered cases of coronavirus disease 2019 (COVID-19) in Wuhan, China. Chin Med J. 2020;133(11):1261–1267. doi:10.1097/cm9.0000000000000824
38. Wang L, He W, Yu X, et al. Coronavirus disease 2019 in elderly patients: characteristics and prognostic factors based on 4-week follow-up. J Infect. 2020;80(6):639–645. doi:10.1016/j.jinf.2020.03.019
39. Fu L, Wang B, Yuan T, et al. Clinical characteristics of coronavirus disease 2019 (COVID-19) in China: a systematic review and meta-analysis. J Infect. 2020;80(6):656–665. doi:10.1016/j.jinf.2020.03.041
40. Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, et al. Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis. Travel Med Infect Dis. 2020;34:101623. doi:10.1016/j.tmaid.2020.101623
41. de Groot RJ, Baker SC, Baric RS, et al. Middle East respiratory syndrome coronavirus (MERS-CoV): announcement of the Coronavirus Study Group. J Virol. 2013;87(14):7790–7792. doi:10.1128/JVI.01244-13
42. Yin Y, Wunderink RG. MERS, SARS and other coronaviruses as causes of pneumonia. Respirology. 2018;23(2):130–137. doi:10.1111/resp.13196
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

