Back to Journals » Journal of Pain Research » Volume 14
Association Between Sleep Quality and Pain Intensity in Mild Patients with COPD: A Community Study
Authors Xu Q, Wu K, Yang Y, Chang R, Qiu H , Wang Y, Lin T, Fu C , Chen Y, Wang N, Ruan X
Received 14 May 2021
Accepted for publication 22 July 2021
Published 25 August 2021 Volume 2021:14 Pages 2641—2649
DOI https://doi.org/10.2147/JPR.S310036
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jonathan Greenberg
Qian Xu,1 Kang Wu,2 Yi Yang,2 Rui Chang,1 Hua Qiu,2 Yingying Wang,1 Tao Lin,2 Chaowei Fu,1 Yue Chen,3 Na Wang,1 Xiaonan Ruan2
1School of Public Health, Fudan University, Pudong Preventive Medicine Research Institute of Fudan University, Shanghai, 200032, People’s Republic of China; 2Pudong New Area Center for Disease Control and Prevention, Pudong Preventive Medicine Research Institute of Fudan University, Shanghai, 200136, People’s Republic of China; 3School of Epidemiology and Public Health, Faculty of Medicine, University of Ottawa, Ottawa, ON, Canada
Correspondence: Na Wang
School of Public Health, Fudan University, Pudong Preventive Medicine Research Institute of Fudan University, No. 130, Dongan Road, Xuhui District, Shanghai, 200032, People’s Republic of China
Tel/Fax +86 21-54237455
Email [email protected]
Xiaonan Ruan
Pudong New Area Center for Disease Control and Prevention, Shanghai, 200136, People’s Republic of China
Tel/Fax +86 21-50342409
Email [email protected]
Purpose: Poor sleep quality and pain were common and had been proved as an important influenced factor of quality of life for patients with COPD. The association of sleep quality with pain has been observed in other population but remains unclear in mild patients with COPD from a community setting.
Methods: A cross-sectional study was conducted to include eligible mild patients with COPD in Pudong New District of Shanghai. A structured questionnaire was used to collect general and clinical information for the patients. The Chinese version of Pittsburgh Sleep Quality Index (PSQI) and the short form of McGill Pain Questionnaire (SF-MPQ) was used to assess sleep quality and intensity of pain. Logistic regression was performed to test the association between sleeping quality and pain intensity.
Results: Two hundred and sixty-four patients with COPD, with an average age of 64 years (SD 5.78 years), were enrolled, and of 52% were women. Seventy-one (26.9%) participants reported at least one exacerbation during the past year. About 28.2% of the patients were classified as having poor sleep quality. Sleep quality was significantly associated with PRI score (adjusted odds ratio (ORad)=2.16, 95% CI: 1.16– 4.00) and PPI rank (ORad=1.90, 95% CI: 1.08– 3.34). People with daytime disturbance were more likely to have pain (ORad =2.03, 95% CI: 1.18– 3.50).
Conclusion: Poor sleep quality was common in mild patients with COPD in community and was associated with higher pain intensity. Pain may involve an impairment of sleep quality.
Keywords: sleep, pain, chronic obstructive pulmonary disease, Pittsburgh sleep quality index, SF-MPQ
Introduction
Chronic obstructive pulmonary disease (COPD), characterized by persistent respiratory symptoms and airflow limitation, is a common disease and one of the leading causes of mortality in the world1 and patients with COPD are frequently complicated by sleep disorders.2,3 These sleep disorders and some nocturnal alterations in ventilation and symptoms, such as insomnia, cough and sputum4–6 may cause difficulty initiating and maintaining sleep, with possible results of daytime sleepiness,7 cognitive dysfunction,8 changes of immune function,9 even prone to acute exacerbations of COPD.10
Sleep quality, assessed by the Pittsburgh Sleep Quality Index (PSQI), has been commonly reported in patients with COPD and has been proved as an important predictor of quality of life in patients with COPD.11–14 As a likewise common problem in COPD, pain, with a pooled prevalence of 66% in moderate and severe patients with COPD,15 is associated with increased dyspnoea,16 fatigue,17 some specific comorbidities16 and has been proved to decrease the quality of life in patients with COPD.18 The impairment in pain and sleep can have a negative impact On health and well-being in patients with COPD. A pooled prevalence of 44% in co-occurrence of pain and sleep disturbance has been reported, in which 72% patients with chronic pain had insomnia.19 This association between pain and sleep quality might be bidirectional.20 The persistence and exacerbation of pain might cause the sleep disturbance.21 Whereas disturbed sleep could change the resistance to pain22 and non-disturbed sleep also might alleviate the pain as well.23 Sleep quality and pain have common influence factors, such as anxiety and depression. Previous studies suggested that poor sleep quality was a predictor of negative emotion,24,25 vice versa.26 Similar bidirectional association was also observed between pain and negative emotion.27
This association of poor sleep quality with body pain has been usually observed in general population28 as well as patients with lung cancer29 and COPD,30 but rarely in patients with mild COPD. According to a nationwide prevalence study in China, among adults with COPD, 56.4% had mild disease (GOLD 1) and only 7.4% had severe or very severe disease (GOLD 3 and GOLD 4).31 Therefore, in this study, we investigated the relationship between sleep quality and pain intensity in patient with mild COPD, with a community setting, to provide epidemiological evidence for prevention and management of patients with mild COPD in China.
Materials and Methods
Study Population and Design
This was a cross-sectional study based on a community setting in Pudong New District of Shanghai, China, in the year of 2018. Ten communities were randomly selected from 46 communities in Pudong New District to recruit patients with mild patients in a register system established in 2014 including data of diagnosed COPD patients from local hospitals.32
The eligible subjects were included in this study only with the following criteria: (1) aged 40 years or older; (2) with mild (GOLD 1: forced expiratory volume in one second [FEV1]/forced vital capacity [FVC] <0.7 and FEV1 ≥80% predicted) COPD (verified by on-site spirometry during the survey); (3) local residents or lived in Pudong for more than two years; (4) willing and able to provide informed written consent, medical records and complete the survey (including questionnaire and spirometry) independently. Patients were excluded if they (1) were in severe or unstable medical conditions, such as cardiovascular, neurological, musculoskeletal diseases, and acute exacerbation (described by acute changes in respiratory symptoms) within one month; and/or (2) had cognitive impairment and mobility limitation. All included patients with COPD were previously diagnosed by physician and had a clam of COPD by Tenth Revision of International Classification of Disease codes related COPD: J43.x, and J44. x. All information was collected by using structured questionnaires in a face-to-face interview. Field investigators were trained together with a unified standard before the study was conducted and all questionnaires were double checked to ensure the accuracy and completeness of data. Finally, 264 out of 300 recruited mild patients with COPD fully completed the survey and submitted informed consents, with a response rate of 88.0%.
Measurements and Covariates
Spirometry
Spirometry was used to assess the severity of COPD among participants by measuring values of FEV1 and FVC as well as FEV1% predicted according to GOLD recommendation.1 The lung function was tested 10 minutes after the short-acting beta2-agonist (Salbutamol Sulfate Solution for inhalation) was given. The ratio of FEV1 and FVC (FEV1/FVC) was calculated and compared with values of FEV1% predicted to help select mild patients with COPD in this study.
Sleep Quality
Sleep quality was assessed by the Chinese version of Pittsburgh Sleep Quality Index (PSQI), which has been validated and widely used.33 In this questionnaire, information on 19 questions were collected to assess 7 domains of sleep quality: 1) perceived sleep quality, 2) sleep latency, 3) sleep duration, 4) sleep efficiency, 5) sleep disturbance, 6) use of sleep medication and 7) daytime dysfunction. Each domain of PSQI scored from 0 to 3, and then constituted a total PSQI score ranging from 0 to 21. A validated three-factor analysis was also used, which is favoured better than a single score in statistics34 Factor 1, Sleep Efficiency, includes sleep duration and efficiency (score, 0–6). Factor 2, Sleep Quality, includes the perceived sleep quality, sleep latency, and sleep medication use (score, 0–9). Factor 3, Daily Disturbances, includes sleep disturbances and daytime dysfunction (score, 0–6). A cut off of 7 was used to all participants categorized all patients into either the poor or good sleep quality group with a sensibility and specificity of 98.2% and 90.2%.35 Specific categories were used to describe some sleep characteristics: long sleep latency (>30 vs ≤30 minutes), short sleep duration (<7 vs ≥7 hours), sleep medication use (≥once a week vs <once a week).
Pain Assessment
Pain severity was assessed by the short-form McGill Pain Questionnaire (SF-MPQ)36 with satisfactory reliability and validity.37 The SF-MPQ had three sections. 1) The pain rating index (PRI) was comprised of sensory subscale with 11 items and affective subscale with 4 items. Each item was scored from 0 (none) to 3 (severe). The total PRI score was obtained by summing scores of 15 items (range 0–45). 2) A 100-mm visual analogue scale (VAS) scored from 0 to 100 was used to rate the intensity of average pain. 3) Present pain intensity (PPI) with 5 scales from 0 (no pain) to 5 (extreme pain) was used to assess intensity of current pain.
Anxiety/Depression
Anxiety and depression were assessed by Chinese version of Hospital Anxiety and Depression Scale (HADS). HADS consists of 14 items, seven reflecting anxiety subscale (HADS Anxiety) and seven reflecting depression subscale (HADS depression).38 Each item had a four-point (0–3) response. Hence, the total scores ranged from 0 to 21 for anxiety subscale and 0 to 21 for depression subscale. The cut-off value was recommended by 8–10 for doubtful cases and ≥11 for definite case.39 A cut-off score of 8/9 for HADS was used with a sensibility and specificity of 93.7% and 72.6% for anxiety, and 84.6% and 90.3% for depression.40
Variables for Demographic and Clinical Information
During the interview, height and weight were measured and the body mass index (BMI) was calculated. Demographic information, including age, gender, retirement (retired vs unretired), education level (>9 vs ≤9 years), diagnosis date of COPD, self-reported exacerbation based on “symptom description” (during the past 12 months, no vs yes), history of common chronic diseases (physician diagnosed hypertension, cardiovascular diseases, type 2 diabetes, or chronic kidney disease), regular medicine use (self-reported and matched with recent medical records, yes vs no), were collected by using a structured questionnaire. Living habits such as ex/current smoker (≥1 cigarette a day for more than 6 months, yes vs no), self-reported second-hand smoke (exposure of more than 15 minutes a day, yes vs no), and exercise (any physical activities for more than 30 minutes, <once per week vs ≥once per week) were also collected in the questionnaire.
Statistical Analysis
All data were entered twice with Epidata 3.1 for double check. Considering the distribution of data, age was categorized by median (≤65 vs >65), while BMI was classified by WHO criteria (≤25 vs >25). Duration of COPD (<10 vs ≥10 years), numbers of self-reported comorbidity (0 vs 1 vs ≥2) were calculated and categorized. The scores of PSQI and pain intensity were presented by medium and interquartile ranges due to skewed distributions. However, to compare with previous research, the means of Skewed data were also mentioned in the text. The independent-samples t-test and χ2 test were used to compare variables between mild COPD patients with good and poor sleep.
To assess the association between sleep quality and pain intensity, logistic regression analysis was performed with PSQI category (>7 vs ≤7) as the primary outcome variable. Three confirmed factors were also performed by logistic regression as the secondary outcome, which were categorized by quartile (≤ low quartile vs >low quartile) Considering that the distributions of PPI, VAS, and total PRI scores were positively skewed, VAS and total PRI scores were regrouped into ≤median or >median and PPI rank into pain or no pain groups. The variables of Demographic information (eg, age, sex and BMI, retirement), clinical information (eg, exacerbation, numbers of comorbidities) and living habits (eg, smoking, exercise) were adjusted in the logistic regression analysis. We also performed the sensitivity analysis by excluding patients with anxiety or depression, to test the robustness of results. All the analysis were performed by SPSS (Version 22.0, IBM Corporation, New York, New York) as well. Two-sided P values less than 0.05 were considered statistically.
Ethics Approval and Informed Consent
All procedures performed in studies involving human participants were in accordance with the ethical standards of institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The Ethics Committee of Fudan University School of Public Health Institutional Review Board approved this study (IRB#2016-07-0597). All participants provided written informed consent for participation. Informed consent was obtained from all individual participants included in the study. They were informed that all information collected in this study have been completely anonymized so that their identity cannot be identified via the paper.
Results
Of 300 eligible patients, 264 patients with mild COPD completed the survey, with a response rate of 88.0%. The average age of the 264 participants was 64 years (SD 5.78 years) and about 51.8% participants were female. Majority of patients (155/264, 58.7%) reported more than one comorbidity. The most common self-reported comorbidity was hypertension (43.2%,114/264), followed by cardiovascular diseases (14.0%, 37/264), type 2 diabetes (9.8%, 26/264) and others (5.7%,15/264). Of patients, 26.9% (71/264) reported having exacerbation in the past 12 months. Only 21% patients (58/263) were current smokers. The prevalence of anxiety and depression were 6.6% and 9.9%, respectively (Table 1).
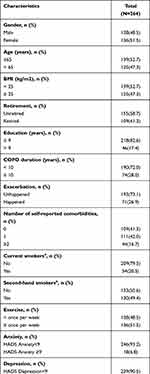 |
Table 1 Characteristics of Study Population (N=264) |
Of patients with mild COPD, only 13.6% participants (36/264) reported problem to initiate sleep (sleep latency >30 minutes), 5.3% (14/264) used sleep medication and over 60% patients (166/264) had a sleep duration of no less than 7 hours. As shown in Table 1, The median (IQR) PSQI score was 5.0 (3.0, 8.0) and the prevalence of poor sleepers (PSQI>7) was 28.2%. The median (IQR) of factor 1 (Sleep efficiency), factor 2 (Sleep quality) and factor 3 (daytime function) were 1.0 (0.0, 3.0), 2.0 (1.0, 3.0) and 2.0 (1.0, 3.0), respectively. The median scores of PRI and VAS were 1.0 (0.0, 3.0) and 0.0 (0.0, 10.0). Of all participants, 38.1% (104/264) reported pain by PPI rank. As shown in Table 2, of patients with poor sleep, 74.3% reported higher pain intensity by PRI and 48.6% had mild and above pain within 24 hours of the interview (PPI). Both PRI and PPI showed significant difference between patients with different sleep conditions and prompt that patients with poor sleep suffered from higher pain intensity.
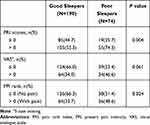 |
Table 2 Sleep Quality and Pain Intensity in Patients with Mild COPD |
Table 3 illustrated the results from multiple logistic regression analysis. COPD patients with higher pain intensity were more likely to have poor sleep quality. Compared with COPD patients without pain according to PPI, those with pain had a poorer sleep quality, with adjusted odds ratio (OR) of 1.90 (95% CI: 1.08–3.34). Similar results were observed when using PRI (ORad=2.16, 95% CI: 1.16–4.00).
 |
Table 3 Association Between Sleep Quality (PSQI>7 Vs PSQI≤7) and Pain in Patients with Mild COPD |
The association of sleep factors with higher pain intensity according to PRI scores was only observed in daytime disturbance, with the adjusted odds ratio (OR) of 2.03 (95% CI:1.18–3.50) (Table 4). The association of sleep with pain intensity remained when participants with anxiety/depression were excluded, with the corresponding ORs for PRI, VAS, and PPI rank of 2.14 (95% CI:1.14 −4.02), 2.01 (95% CI:1.06–3.82), and 2.18 (95% CI:1.15–4.27) respectively.
 |
Table 4 Association Between Different Sleep Factor with Pain in Patients with Mild COPD |
Discussion
Although previous studies have investigated the relationship between sleep and pain in general population,41,42 to our knowledge, this is the first study to assess the association of sleep quality with pain intensity in patients with mild COPD based on a community population. In our study, poor sleep quality was significantly associated with higher pain intensity in patients with mild COPD.
About 28% of our study patients had poor sleep (PSQI>7). Previous studies reported a higher prevalence of poor sleep quality of more than 50% in patients with COPD, especially in severe/very severe patients.11,13,14 However, recent research in Asian patients with mild to moderate COPD by Lee, S. H. showed that 33.1% patients reported having a poor night sleep assessed by COPD and Asthma Sleep Impact Scale (CASIS),43 which was slightly higher than that in our study. This difference in the severity of COPD patients might be the underlying cause. There were about 66% patients with moderate COPD in the research of Lee, S. H,43 but all participants were mild patients with COPD in our research. The prevalence of pain assessed by PPI (PPI>0) was 38.1% and the intensity of pain assessed by SF-MPQ was the main influencing factor to sleep in our research. A systematic review in patients with COPD revealed that the prevalence of pain ranged from 32 to 60%.44 For those with mild COPD the prevalence of 37.9%, reported from the research of Tian Xiao, was close to our results.45
Our research found that different measures of pain including PRI score, VAS and PPI were associated with a higher risk for poor sleep quality in mild patients with COPD, which suggested that higher pain intensity in patients with COPD reduced their sleep quality. This result was similar to the finding from the previous researches. Among patients with COPD,46 insomnia was independently associated with pain (β=0.71, p<0.05). Among patients with Parkinson’s disease,47 poor sleep quality was associated with different types of pain, like akathisic pain (OR: 4.69, p<0.001) and radiating pain (OR: 3.98, p<0.05). Among adolescents and young adults, higher levels of pain intensity were associated with higher PSQI scores (β = 0.23, p<0.001 and β=0.14, p< 0.01).48 Similar association between poor sleep quality and increased pain was also observed among patients with stable heart failure.49
In addition to overall sleep quality assessed by total PSQI score, we also investigated the impact of pain on sleep efficiency, sleep quality and daytime disturbance respectively. No statistically significant association was observed between sleep efficiency/ quality and pain, which was consistent with some other studies. However, the relationship between shorter total sleep time and pain has been reported previously,50,51 and their association might be bidirectional.52 Use of sleep medication might contribute to reduce the interference from pain with sleep duration.49 However, due to the low proportion of use of sleep medication, we failed to examine their association. Patients in our study had low scores of SF-MPQ and low intensity of pain, which might be inadequate to interrupt the sleep and interfere with sleep latency. Like previous studies in elder adults and patients in other chronic conditions, we found that pain was associated with daytime dysfunction, such as daytime sleepiness,49 fatigue,53 and increased napping.54 However, PSQI merely contained two questions about daytime dysfunction. More detailed instruments and information about daytime dysfunction were necessary.
The biobehavioural mechanisms underlying the association between sleep and pain are not entirely clear. One hypothesis was related to inflammation. Previous studies found that both pain55 and insomnia symptoms56 were significantly associated with higher levels of inflammation biomarkers. Lower sleep duration was proved to increase the level in blood of C-reactive protein (CRP),57 IL-658 and TNF-α.59 These cytokines might result in histamine-induced vasodilation, causing the redness, swelling, pain, and warmth, which were characteristics of general pain.60,61 Moreover, it has been found that negative mood plays an possible medium adjusting role in the association between sleep and pain in non-clinically depressed people.62 Depression and anxiety are common comorbidities in patients with COPD as well,63 which may partially explain the association between sleep quality and pain in this population. However, in the present research, only 12.9% patients had anxiety/depression problems. After excluding those patients with anxiety/depression, the association between sleep and pain remained significant.
The strength of our study mainly includes the use of reliable and validated scales for assessment of sleep quality and pain intensity, as well as recruitment of patients with COPD from community settings. There are also several limitations. First, as a cross-section study, we have to preclude the causal inference between sleep and pain. Secondly, SF-MPQ provides the assessment for the intensity of pain, but not for a comprehensive understanding of pain. Further study should collect more clinical and non-clinical information on pain, such as pain extents and the impact of pain. Thirdly, although we assumed that daytime dysfunction was related with increased pain, more detailed and comprehensive instrument to evaluate this relationship in patients with mild COPD was needed. Moreover, the current analysis examined the association only in mild COPD patients, patients with other severity of COPD were not included.
Conclusion
Our study in community mild COPD patients found that poor sleep quality was associated with higher pain intensity and daytime disturbance might play a major role in this association. Further studies are needed to explore this association in patients with moderate/severe COPD.
Data Sharing Statement
The raw data required to reproduce these findings cannot be shared at this time as the data also forms part of an ongoing study.
Ethics Approval and Informed Consent
All procedures performed in studies involving human participants were in accordance with the ethical standards of institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The Ethics Committee of Fudan University School of Public Health Institutional Review Board approved this study (IRB#2016-07-0597). Informed consent was obtained from all individual participants included in the study. They were informed that all information collected in this study have been completely anonymized so that their identity cannot be identified via the paper.
Author Contributions
Q.X.: Data collection, Analysis and Writing; K.W, Y.Y, R.C, H.Q, Y.W and T.L: Data collection and Investigation; C.F, X.R and N.W: Conception and Design; Y.C and N.W: Revising. All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave approval of the final version to be published; and agree to be accountable for all aspects of the work.
Funding
This study was funded by National Natural Science Foundation of China under Grant: 82073634, Pudong New District Health Commission under Grant: No. PW2017A-9 and the National Key Research and Development Program of China under Grant: No. 2018YFC1313600. The sponsor had no role in the design or conduct of this research.
Disclosure
The authors declare that they have no conflicts of interest for this work.
References
1. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease; 2020 Nov 4. Available from: http://www.goldcopd.org/.
2. Budhiraja R, Siddiqi TA, Quan SF. Sleep disorders in chronic obstructive pulmonary disease: etiology, impact, and management. J Clin Sleep Med. 2015;11(3):259–270. doi:10.5664/jcsm.4540
3. Tsai SC. Chronic obstructive pulmonary disease and sleep related disorders. Curr Opin Pulm Med. 2017;23(2):124–128. doi:10.1097/MCP.0000000000000351
4. Agusti A, Hedner J, Marin JM, Barbe F, Cazzola M, Rennard S. Night-time symptoms: a forgotten dimension of COPD. Eur Respir Rev. 2011;20(121):183–194. doi:10.1183/09059180.00004311
5. Hartman JE, Prinzen J, van Lummel RC, Ten Hacken NH. Frequent sputum production is associated with disturbed night’s rest and impaired sleep quality in patients with COPD. Sleep Breath. 2015;19(4):1125–1133. doi:10.1007/s11325-014-1111-9
6. Basile M, Baiamonte P, Mazzuca E, et al. Sleep disturbances in COPD are associated with heterogeneity of airway obstruction. COPD. 2018;15(4):350–354. doi:10.1080/15412555.2018.1504015
7. Budhiraja R, Parthasarathy S, Budhiraja P, Habib MP, Wendel C, Quan SF. Insomnia in patients with COPD. Sleep. 2012;35(3):369–375. doi:10.5665/sleep.1698
8. Fernandez-Mendoza J, Calhoun S, Bixler EO, et al. Insomnia with objective short sleep duration is associated with deficits in neuropsychological performance: a general population study. Sleep. 2010;33(4):459–465. doi:10.1093/sleep/33.4.459
9. Ganz FD. Sleep and immune function. Crit Care Nurse. 2012;32(2):e19–25. doi:10.4037/ccn2012689
10. Omachi TA, Blanc PD, Claman DM, et al. Disturbed sleep among COPD patients is longitudinally associated with mortality and adverse COPD outcomes. Sleep Med. 2012;13(5):476–483. doi:10.1016/j.sleep.2011.12.007
11. Akinci B, Aslan GK, Kiyan E. Sleep quality and quality of life in patients with moderate to very severe chronic obstructive pulmonary disease. Clin Respir J. 2018;12(4):1739–1746. doi:10.1111/crj.12738
12. Dignani L, Toccaceli A, Lucertini C, Petrucci C, Lancia L. Sleep and quality of life in people with COPD: a descriptive-correlational study. Clin Nurs Res. 2016;25(4):432–447. doi:10.1177/1054773815588515
13. Nunes DM, Mota RM, de Pontes Neto OL, Pereira ED, de Bruin VM, de Bruin PF. Impaired sleep reduces quality of life in chronic obstructive pulmonary disease. Lung. 2009;187(3):159–163. doi:10.1007/s00408-009-9147-5
14. Scharf SM, Maimon N, Simon-Tuval T, Bernhard-Scharf BJ, Reuveni H, Tarasiuk A. Sleep quality predicts quality of life in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2010;6:1–12. doi:10.2147/COPD.S15666
15. Lee AL, Harrison SL, Goldstein RS, Brooks D. Pain and its clinical associations in individuals with COPD: a systematic review. Chest. 2015;147(5):1246–1258. doi:10.1378/chest.14-2690
16. Bentsen SB, Rustoen T, Miaskowski C. Differences in subjective and objective respiratory parameters in patients with chronic obstructive pulmonary disease with and without pain. Int J Chron Obstruct Pulmon Dis. 2012;7:137–143. doi:10.2147/COPD.S28994
17. Bentsen SB, Gundersen D, Assmus J, Bringsvor H, Berland A. Multiple symptoms in patients with chronic obstructive pulmonary disease in Norway. Nurs Health Sci. 2013;15(3):292–299. doi:10.1111/nhs.12031
18. Merino M, Villoro R, Hidalgo-Vega A, Carmona C; Collaborative Working Group E-E. Health-related quality of life of patients diagnosed with COPD in Extremadura, Spain: results from an observational study. Health Qual Life Outcomes. 2019;17(1):189. doi:10.1186/s12955-019-1244-4
19. Mathias JL, Cant ML, Burke ALJ. Sleep disturbances and sleep disorders in adults living with chronic pain: a meta-analysis. Sleep Med. 2018;52:198–210. doi:10.1016/j.sleep.2018.05.023
20. Cheatle MD, Foster S, Pinkett A, Lesneski M, Qu D, Dhingra L. Assessing and managing sleep disturbance in patients with chronic pain. Sleep Med Clin. 2016;11(4):531–541. doi:10.1016/j.jsmc.2016.08.004
21. Bonvanie IJ, Oldehinkel AJ, Rosmalen JG, Janssens KA. Sleep problems and pain: a longitudinal cohort study in emerging adults. Pain. 2016;157(4):957–963. doi:10.1097/j.pain.0000000000000466
22. Lavigne G, Smith MT, Denis R, Zucconi M. Pain and Sleep. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. W.B. Saunders; 2011:1442–1451.
23. Aili K, Nyman T, Svartengren M, Hillert L. Sleep as a predictive factor for the onset and resolution of multi-site pain: a 5-year prospective study. Eur J Pain. 2015;19(3):341–349. doi:10.1002/ejp.552
24. Baglioni C, Battagliese G, Feige B, et al. Insomnia as a predictor of depression: a meta-analytic evaluation of longitudinal epidemiological studies. J Affect Disord. 2011;135(1):10–19. doi:10.1016/j.jad.2011.01.011
25. Soehner AM, Kaplan KA, Harvey AG. Insomnia comorbid to severe psychiatric illness. Sleep Med Clin. 2013;8(3):361–371. doi:10.1016/j.jsmc.2013.04.007
26. Scott AJ, Webb TL, Rowse G. Does improving sleep lead to better mental health? A protocol for a meta-analytic review of randomised controlled trials. BMJ Open. 2017;7(9):e016873. doi:10.1136/bmjopen-2017-016873
27. Tunks ER, Crook J, Weir R. Epidemiology of chronic pain with psychological comorbidity: prevalence, risk, course, and prognosis. Can J Psychiatry. 2008;53(4):224–234. doi:10.1177/070674370805300403
28. Blay SL, Andreoli SB, Gastal FL. Chronic painful physical conditions, disturbed sleep and psychiatric morbidity: results from an elderly survey. Ann Clin Psychiatry. 2007;19(3):169–174. doi:10.1080/10401230701468099
29. Nishiura M, Tamura A, Nagai H, Matsushima E. Assessment of sleep disturbance in lung cancer patients: relationship between sleep disturbance and pain, fatigue, quality of life, and psychological distress. Palliat Support Care. 2015;13(3):575–581. doi:10.1017/S1478951513001119
30. Hansen J, Molsted S, Ekholm O, Hansen HH. Pain prevalence, localization, and intensity in adults with and without COPD: results from the Danish health and morbidity survey (a self-reported survey). Int J Chron Obstruct Pulmon Dis. 2020;15:3303–3311. doi:10.2147/copd.S275234
31. Fang L, Gao P, Bao H, et al. Chronic obstructive pulmonary disease in China: a nationwide prevalence study. Lancet Respir Med. 2018;6(6):421–430. doi:10.1016/s2213-2600(18)30103-6
32. Xiao T, Qiu H, Chen Y, et al. Prevalence of anxiety and depression symptoms and their associated factors in mild COPD patients from community settings, Shanghai, China: a cross-sectional study. BMC Psychiatry. 2018;18(1):89. doi:10.1186/s12888-018-1671-5
33. Tsai PS, Wang SY, Wang MY, et al. Psychometric evaluation of the Chinese version of the Pittsburgh Sleep Quality Index (CPSQI) in primary insomnia and control subjects. Qual Life Res. 2005;14(8):1943–1952. doi:10.1007/s11136-005-4346-x
34. Cole JC, Motivala SJ, Buysse DJ, Oxman MN, Levin MJ, Irwin MR. Validation of a 3-factor scoring model for the Pittsburgh sleep quality index in older adults. Sleep. 2006;29(1):112–116. doi:10.1093/sleep/29.1.112
35. Liu XC, Tang MQ, Hu L. 匹兹堡睡眠质量指数的信度和效度研究 [Reliability and validity of the Pittsburgh sleep quality index]. Chin J Psychiatry. 1996;29(2):103–107.
36. Melzack R. The short-form McGill pain questionnaire. Pain. 1987;30(2):191–197. doi:10.1016/0304-3959(87)91074-8
37. Strand LI, Ljunggren AE, Bogen B, Ask T, Johnsen TB. The short-form McGill pain questionnaire as an outcome measure: test-retest reliability and responsiveness to change. Eur J Pain. 2008;12(7):917–925. doi:10.1016/j.ejpain.2007.12.013
38. Snaith RP. The hospital anxiety and depression scale. Health Qual Life Outcomes. 2003;1(1):29. doi:10.1186/1477-7525-1-29
39. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–370. doi:10.1111/j.1600-0447.1983.tb09716.x
40. Botega NJ, Bio MR, Zomignani MA, Garcia JC, Pereira WA. Mood disorders among inpatients in ambulatory and validation of the anxiety and depression scale HAD. Rev Saude Publica. 1995;29(5):355–363.
41. Mork PJ, Nilsen TI. Sleep problems and risk of fibromyalgia: longitudinal data on an adult female population in Norway. Arthritis Rheum. 2012;64(1):281–284. doi:10.1002/art.33346
42. Odegard SS, Sand T, Engstrom M, Stovner LJ, Zwart JA, Hagen K. The long-term effect of insomnia on primary headaches: a prospective population-based cohort study (HUNT-2 and HUNT-3). Headache. 2011;51(4):570–580. doi:10.1111/j.1526-4610.2011.01859.x
43. Lee SH, Kim KU, Lee H, Park HK, Kim YS, Lee MK. Sleep disturbance in patients with mild-moderate chronic obstructive pulmonary disease. Clin Respir J. 2019;13(12):751–757. doi:10.1111/crj.13085
44. van Dam van Isselt EF, Groenewegen-Sipkema KH, Spruit-van EM, et al. Pain in patients with COPD: a systematic review and meta-analysis. BMJ Open. 2014;4(9):e005898. doi:10.1136/bmjopen-2014-005898
45. Xiao T, Zhou X, He Y, et al. Pain problems for patients with mild and moderate chronic obstructive pulmonary disease - a community-based study in Shanghai. J Pain Res. 2017;10:2247–2252. doi:10.2147/JPR.S141940
46. Hynninen MJ, Pallesen S, Hardie J, et al. Insomnia symptoms, objectively measured sleep, and disease severity in chronic obstructive pulmonary disease outpatients. Sleep Med. 2013;14(12):1328–1333. doi:10.1016/j.sleep.2013.08.785
47. Rana AQ, Qureshi ARM, Shamli Oghli Y, et al. Decreased sleep quality in Parkinson’s patients is associated with higher anxiety and depression prevalence and severity, and correlates with pain intensity and quality. Neurol Res. 2018;40(8):696–701. doi:10.1080/01616412.2018.1462880
48. de la Vega R, Racine M, Sanchez-Rodriguez E, et al. Pain extent, pain intensity, and sleep quality in adolescents and young adults. Pain Med. 2016;17(11):1971–1977. doi:10.1093/pm/pnw118
49. Conley S, Feder SL, Jeon S, Redeker NS. Daytime and nighttime sleep characteristics and pain among adults with stable heart failure. J Cardiovasc Nurs. 2019;34(5):390–398. doi:10.1097/JCN.0000000000000593
50. Weingarten JA, Dubrovsky B, Basner RC, Redline S, George L, Lederer DJ. Polysomnographic measurement of sleep duration and bodily pain perception in the sleep heart health study. Sleep. 2016;39(8):1583–1589. doi:10.5665/sleep.6026
51. Irwin MR, Olmstead R, Carrillo C, et al. Sleep loss exacerbates fatigue, depression, and pain in rheumatoid arthritis. Sleep. 2012;35(4):537–543. doi:10.5665/sleep.1742
52. Finan PH, Goodin BR, Smith MT. The association of sleep and pain: an update and a path forward. J Pain. 2013;14(12):1539–1552. doi:10.1016/j.jpain.2013.08.007
53. Chen YW, Camp PG, Coxson HO, et al. A comparison of pain, fatigue, dyspnea and their impact on quality of life in pulmonary rehabilitation participants with chronic obstructive pulmonary disease. Copd. 2018;15(1):65–72. doi:10.1080/15412555.2017.1401990
54. Foley DJ, Vitiello MV, Bliwise DL, Ancoli-Israel S, Monjan AA, Walsh JK. Frequent napping is associated with excessive daytime sleepiness, depression, pain, and nocturia in older adults: findings from the national sleep foundation ‘2003 Sleep in America’ poll. Am J Geriatr Psychiatry. 2007;15(4):344–350. doi:10.1097/01.JGP.0000249385.50101.67
55. DeVon HA, Piano MR, Rosenfeld AG, Hoppensteadt DA. The association of pain with protein inflammatory biomarkers: a review of the literature. Nurs Res. 2014;63(1):51–62. doi:10.1097/nnr.0000000000000013
56. Fernandez-Mendoza J, Baker JH, Vgontzas AN, Gaines J, Liao D, Bixler EO. Insomnia symptoms with objective short sleep duration are associated with systemic inflammation in adolescents. Brain Behav Immun. 2017;61:110–116. doi:10.1016/j.bbi.2016.12.026
57. Grandner MA, Buxton OM, Jackson N, Sands-Lincoln M, Pandey A, Jean-Louis G. Extreme sleep durations and increased C-reactive protein: effects of sex and ethnoracial group. Sleep. 2013;36(5):769–779e. doi:10.5665/sleep.2646
58. Rohleder N, Aringer M, Boentert M. Role of interleukin-6 in stress, sleep, and fatigue. Ann N Y Acad Sci. 2012;1261(1):88–96. doi:10.1111/j.1749-6632.2012.06634.x
59. Chennaoui M, Sauvet F, Drogou C, et al. Effect of one night of sleep loss on changes in tumor necrosis factor alpha (TNF-α) levels in healthy men. Cytokine. 2011;56(2):318–324. doi:10.1016/j.cyto.2011.06.002
60. Dirckx M, Stronks DL, van Bodegraven-hof EA, Wesseldijk F, Groeneweg JG, Huygen FJ. Inflammation in cold complex regional pain syndrome. Acta Anaesthesiol Scand. 2015;59(6):733–739. doi:10.1111/aas.12465
61. Sommer C, Leinders M, Üçeyler N. Inflammation in the pathophysiology of neuropathic pain. Pain. 2018;159(3):595–602. doi:10.1097/j.pain.0000000000001122
62. O’Brien EM, Waxenberg LB, Atchison JW, et al. Negative mood mediates the effect of poor sleep on pain among chronic pain patients. Clin J Pain. 2010;26(4):310–319. doi:10.1097/AJP.0b013e3181c328e9
63. Bordoni B, Marelli F, Morabito B, Sacconi B. Depression, anxiety and chronic pain in patients with chronic obstructive pulmonary disease: the influence of breath. Monaldi Arch Chest Dis. 2017;87(1):811. doi:10.4081/monaldi.2017.811
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
