Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Association Between Skin Acid Mantle, Natural Moisturizing Factors, and Antibacterial Activity Against S. aureus in the Stratum Corneum
Authors Li R , Rodrigues M , Li L, Winget J, Wang Y, Wang C, Smith E, Wei K
Received 21 February 2023
Accepted for publication 16 June 2023
Published 22 June 2023 Volume 2023:16 Pages 1595—1606
DOI https://doi.org/10.2147/CCID.S409534
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Rui Li,1 MyriamRubecca Rodrigues,1 Lijuan Li,2 Jason Winget,2 Yu Wang,1 Caroline Wang,1 Ed Smith,3 Karl Wei3
1Beauty Revealed, Procter & Gamble International Operations SA SG Branch, Singapore, Singapore; 2Corporate Functions Analytical, Procter & Gamble Mason Business Center, Cincinnati, OH, USA; 3Personal Care, Procter & Gamble Mason Business Center, Cincinnati, OH, USA
Correspondence: Rui Li, Procter & Gamble, SGIC, 70 Biopolis Street, Singapore, 138547, Singapore, Tel +65 85223528, Fax +65 67122211, Email [email protected] Karl Wei, 8700 Mason Montgomery Road, Mason, OH, 45040, USA, Tel +1 513-626-5792, Fax +1 513-622-1006, Email [email protected]
Purpose: The skin has evolved a system to prevent pathogenic microorganism colonization and infection. This study examined the role of natural moisturizing factors (NMFs) and skin pH on Staphylococcus aureus (S. aureus) growth and colonization on the human stratum corneum (SC).
Study Population and Methods: A survey study with 82 female participants was performed. Participants maintained their daily hygiene routine, except for refraining from using leave-on products on their forearms on the day of the test. Skin sampling was performed using adhesive tapes. An ex vivo method was developed to study the viability and growth of S. aureus on human SC sampled from normal skin. NMFs, including pyrrolidone carboxylic acid (PCA), urocanic acid (UCA), histidine, and proline in SC samples, were measured by liquid chromatography with tandem mass spectrometry. The impact of PCA and UCA on S. aureus growth and metabolic activity was measured by optical density and isothermal microcalorimetry, respectively.
Results: Heterogeneity of S. aureus viability on human SC samples was observed. Skin pH showed a significant negative association (p< 0.05) with SC antibacterial activity in the ex vivo assay. One unit of skin pH decrease corresponded to 68.1% of S. aureus cell death. The levels of PCA and histidine were significantly negatively associated (p< 0.05) with skin pH. The addition of 5 mM and 10 mM PCA significantly inhibited S. aureus growth by approximately 25% at 20 hours and reduced its metabolic activity in vitro.
Conclusion: The results indicate that PCA, one of the NMFs in human skin, plays an important role in regulating the human skin acid mantle in vivo and contributes to antibacterial activity against S. aureus.
Keywords: skin barrier function, skin moisturizer, skin pH, Staphylococcus aureus, skin acid mantle, 2-pyrrolidone-5-carboxylic acid
Introduction
At the end of the 19th century, researchers began measuring skin pH. The term “acid mantle” was first coined by Schade and Marchionini in 1928 to describe the acidic nature of the stratum corneum (SC).1
The universal method for measuring skin surface pH is using the glass planar electrode connected to a voltage meter.2 When using an electrode to measure pH on the surface of the skin, a standard volume of distilled water is applied to the skin. Protons in distilled water are released from intracellular water, amphiphilic free fatty acids (FFAs), and water-soluble compounds in corneocytes, and proton concentration is measured as the skin surface pH value by the pH meter.3 Acidification of the SC has been hypothesized to result from both exogenous (FFAs from sebaceous lipids, microbial metabolites, and lactic acid from eccrine glands) and endogenous epidermal (natural moisturizing factors [NMFs] from filaggrin catabolism, membrane sodium-hydrogen antiporter type 1, and FFAs from phospholipid hydrolysis) mechanisms.4,5
Among all the proposed mechanisms for SC acidification, NMFs have been mostly studied under pathological skin conditions and in vitro skin models. NMFs are hygroscopic molecules—in particular, amino acids and 2-pyrrolidone-5-carboxylic acid (PCA), a derivative of glutamine. These amino acids and derivatives mainly come from the total degradation of the histidine-rich protein, filaggrin, and the filaggrin-related protein, filaggrin-2.6 In the SC of the thenar eminence, levels of NMF in the SC of patients with atopic dermatitis (AD) were negatively correlated with skin surface pH.7 Additionally, an inverse relationship between FLG gene dose and forearm skin surface pH has been observed in FLG-deficient patients, with double-allele participants displaying the greatest elevation in skin surface pH.8 In another study, down regulation of the filaggrin-related protein encoded by FLG2 increased skin pH and reduced the amount of NMFs at the reconstructed human epidermis (RHE) surface.9 However, the correlation between levels of NMFs in the SC and skin pH has not been reported for people with generally good skin health.
The SC presents an antimicrobial barrier to pathogenic microbes that attempt to colonize from the environment. Indeed, Schiemann and Landau reported that normal hand skin could destroy Escherichia coli in 1919.10 Recently, a correlation between the skin acid mantle and antibacterial function was established in vivo. Namely, Hayashi et al demonstrated that skin pH negatively correlated with the antibacterial activity of the hands against E. coli.11 Colonization of the skin with the gram-positive bacteria Staphylococcus aureus (S. aureus) is a major pathogenic factor in AD.12 Notably, the skin pH is higher in patients with AD than in healthy controls, even on uninvolved sites without lesions.13–15 Moreover, a skin pH of 5.7–6.2 and low hydration are associated with high S. aureus abundance in patients with AD.16
The present study aimed to investigate the relationship between skin pH, levels of NMF in SC, and SC antibacterial activity against S. aureus.
Materials and Methods
Bacterial Strains and Culture Medium
The S. aureus ATCC 6538 strain was used. As a pre-culture, S. aureus was streaked on tryptone soya agar (Oxoid, Basingstoke, United Kingdom) and cultured overnight at 37°C. One single colony was inoculated into 20 mL tryptone soya broth and cultured overnight at 37°C and 180 revolutions per minute (rpm). Bacterial cells were then harvested by centrifugation, followed by resuspension and dilution to the desired level in test solutions or mediums.
Evaluation of Bacterial Growth Inhibition Through in vitro Experiments
In this study, S. aureus ATCC 6538 was grown in a 1% peptone and 0.5% yeast extract medium without added buffer, reflecting conditions present on skin. It has been estimated that UCA ranges between 6 and 12 mM in the SC.17 For bacterial growth inhibition assay, PCA and urocanic acid (UCA) were added to growth media at 2.5 mM, 5 mM, and 10 mM. Histidine and proline were added to the growth medium at 10 mM. The media pH was measured by a SevenCompact S220 PH meter (Mettler Toledo, Columbus, OH, USA). Bacterial growth in a 96-well plate (Nunclon 96 Flat Bottom Transparent, Nunclon, Thermo Fischer Scientific, Waltham, MA, USA) was measured by an Infinite 200Pro (Tecan, Mannedorf, Switzerland). S. aureus cell growth was assessed by measuring the optical density at 600 nm (OD600).
CalScreener Isothermal Microcalorimetry (IMC) Assay
IMC is a well-established method for measuring bacterial energy release, which is proportional to the metabolic reactions.18 The bacterial growth rate and inhibitory potential of PCA and UCA at various concentrations were determined using the CalScreener IMC assay (Symcel Sverige, Spanga, Sweden). The use of calorimetry to determine bacterial growth and the potency of antimicrobial compounds has been previously described.18 S. aureus ATCC 6538 was grown overnight and diluted to an inoculum of 106 colony-forming unit (CFU)/mL in fresh medium (1% peptone plus 0.5% yeast extract) containing different concentrations of PCA and UCA. For each treatment condition, 200 µL of culture was added to the presterilized plastic insert of the 48-well plate. The assay was run with continuous data collection for 24 hours. Data were analyzed using the CALview software (VieleTech, Woodland Park, CO, USA) for visualization of growth kinetics and total energy release.
Quantitative Evaluation of S. Aureus Viable Cells
The Soleris Next Generation system (Neogen Corp, Lansing, MI, USA) is an automated and growth-based method for microbial detection and quantification. Carbon dioxide released by microbial growth triggers a color change in the pH indicator, which yields a positive detection time (DT). For S. aureus, a quantitative correlation between viable microbes and DTs has been observed using a Non-fermenting-Total Viable Count (NF-TVC) Vial (Neogen Corp, Lansing, MI, USA).19 Here, the linear regression curve for S. aureus was generated by plotting mean DT of replicates and mean Log (10) CFU/sample. For measuring the viable number of S. aureus in the liquid medium, 10 µL of broth was directly inoculated in the NF-TVC vial. For measuring the viable number of S. aureus on SC tape strips, the whole tape strip (D-squame, CuDerm, Dallas TX, USA) was inserted into the NF-TVC vial. Bacterial count in the liquid medium and on the SC tape strips was calculated using a linear regression curve (Log CFU/ sample= a*DT+b) (Supplementary Figure 1).
Ex vivo Antibacterial Activity Assay
It has been reported that S. aureus binds to skin corneocytes through the interaction between bacterial clumping factor B (ClfB) and skin ligands.20 Antibacterial activity of skin is normally studied by inoculating target microorganisms directly onto the skin.11,21 Here, an ex vivo method was developed to study S. aureus ATCC 6538 viability on human SC. S. aureus was directly inoculated on human SC collected by adhesive discs. Human SC tape strips were fixed on sterilized 2% agar. Three SC tape strip samples from the same participant were used for measuring skin antibacterial activity. Ten microliters of 0.1% polysorbate 80 was evenly spread on the SC of each sample with a 2 µL inoculation loop and dried in a biological safety cabinet. Then, 104–105 CFU of S. aureus cells in 10 µL of 1% peptone were inoculated on the SC and spread evenly. Samples were placed in an incubator at 37°C and 90% relative humidity for 4 hours. S. aureus cells in 10 µL of liquid inoculum and SC tape strips inoculated with S. aureus were measured by the Soleris system using the NF-TVC kit. Skin antibacterial activity for each participant was calculated using Log CFU changes (Log CFU [inoculum]- Log CFU [SC sample]). Positive values suggested bactericidal activity of human SC. Negative values suggested bacterial cell growth on human SC. Zero values suggested that S. aureus stayed static on the SC.
Scanning Electron Microscopy (SEM)
Human SC tape strips were treated following the ex vivo protocol above. After treatment, all samples were fixed with 4% paraformaldehyde and washed with deionized (DI) water three times. The freeze-drying method was used to preserve the biological structure of skin samples.22 A small piece (5 mm × 5 mm) was cut from the freeze-dried tape and mounted on a copper sample holder using conductive double-sided adhesive carbon tape, and then sputter-coated with a thin layer of platinum-palladium in a PP3010 preparation chamber (Quorum, Laughton, East Sussex, UK) at room temperature. The SEM analysis (Zeiss Crossbeam 540, Carl Zeiss AG, Jena, Germany) was conducted at an accelerating voltage of 2 kV using secondary electron detectors.
In vivo Study Design
To investigate skin pH and collect SC samples for NMF analysis and antibacterial activity assay, we conducted an observational study.
Participants
A total of 82 Singaporean female participants were recruited for this study. The study was conducted between December 2019 and January 2020. Participants were recruited by the Procter & Gamble (P&G) Singapore Innovation Centre consumer hub based on the following inclusion criteria: (1) healthy female aged between 18 and 60 years, (2) self-declared to be in general good health, and (3) read, signed, and received a copy of the Informed Consent form and product confidentiality form. Participants were excluded if: (1) they had self-declared skin symptoms (eg AD and rosacea-like dermatitis), (2) they were in receipt of any medication, and (3) the investigator believed any health and skin condition could jeopardize the safety of the participant, interfere with the evaluation, or confound the interpretation of the study results.
Questionnaire, Skin pH Measurement, and SC Sample Collection
All participants refrained from using any leave-on products on volar forearms on the day of the test. The participant’s age, ethnicity, and personal cleansing information were collected by questionnaire. Two locations in the center of right and left forearms were selected for skin pH measurement. The pH meter (Horiba Scientific D-72G LAQUAact with glass probe, Horiba, Kyoto, Japan) was firstly equilibrated in the test room for 15 min and calibrated using a pH buffer 2, 4, and 7 (METTLER TOLEDO 51350002, 51302068, Columbus, OH, USA). Twenty µL of DI water was added between the skin surface and the probe for pH measurement. The acclimation time was calculated by using the latest wash time and the time of skin pH measurement. Adhesive tape discs (D-Squame 100, CuDerm, Dallas, TX, USA) were sanitized by 70% ethanol, followed by ultraviolet exposure for 10 min before placement on the volar forearm. SC was collected by three rounds of repeated sticking and peeling with a pressurizer (D-Squame Pressure instrument, CuDerm, Dallas, TX, USA) to saturate the adhesive tape disc.23 Four SC samples were collected from each forearm. A total of eight samples were collected from each participant.
NMF Measurement
SC specimens were collected from each participant and analyzed for NMFs (L-Proline, PCA, cis- and trans-UCA, and L-Histidine) using ion-pairing reverse-phase liquid chromatography coupled with tandem mass spectrometry as described previously.24 Briefly, a 25 µL aliquot of the internal standard solution (Histidine-D3; L-Proline-D3; 2-Pyrrolidone-5-carboxylic-D5 Acid; cis-Urocanic-13C3 Acid) was added to each tube containing the tape strip followed by 1.0 mL of water containing 0.1% formic acid and 0.1% heptafluorobutyric acid. The tubes were capped, vortexed for 10 seconds, and then placed on a sonicator for 10 minutes. An aliquot of the extraction solution was removed for gradient chromatographic separation on an Atlantis T3 column (2.1 × 50 mm, 3-µm particles; Waters, Milford, MA, USA). Detection and quantitation were performed by tandem mass spectrometry (AB-5000, Sciex, Framingham, MA, USA) operating under multiple reaction monitoring conditions. The concentration of a given NMF in the study specimens was determined from its corresponding peak area ratio by interpolation from the regression curve of calibration standards. The nominal range of quantitation was 20 ng/mL to 20,000 ng/mL for each NMF. The concentration of each NMF determined in the acid extract was multiplied by the extraction volume to obtain a mass NMF/strip value. The found mass of each NMF was then normalized by the protein amount in the acid extract determined by bicinchoninic acid (BCA) assay (Pierce Biotechnology/Thermo Scientific, Rockford, IL, USA) using bovine serum albumin as a standard.
Statistical Analysis
JMP 16 software (SAS Institute, Cary, NC, USA) was used for the statistical analysis. The skin pH data were averaged across all four sites for each participant. Antibacterial activity data, represented by relative logarithmic reduction in viable bacteria compared with initial inoculum, were averaged across the three SC samples from the participant. The NMF measures were normalized to their associated protein measures (as determined by BCA assay) and Log base 10 was taken. Linear regressions of skin pH on NMFs and Log CFU/skin sample on pH were performed with a significance level of p<0.05.
Results
Skin Surface pH Results
Overall, 82 female participants were included, and their primary ethnicity was Chinese (Table 1). The distribution of pH was centered around 5.16, within the range of 4.33 to 6.22. An outlier (pH 7.22) was removed from the correlation analysis to avoid a leveraging effect, though no abnormal record was identified for this participant. Participants were divided into four age groups (Table 1). No significant difference in skin pH was observed among the age groups (p=0.49). Acclimation time after wash varied widely among the cohort (Supplementary Figure 2). No significant correlation was observed between acclimation time and skin pH (p=0.49) (Supplementary Figure 3).
 |
Table 1 Baseline Characteristics |
Regression of SC Antibacterial Activity on Skin pH
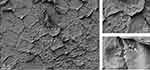
SEM analysis demonstrated that S. aureus binds to the corneocyte surface in the ex vivo model. Moreover, dividing cells were visualized, suggesting growth of bacterial cells on corneocytes (Figure 1).
To understand the correlation between skin pH and SC antibacterial activity, the SC samples obtained in the study were tested using an ex vivo method. In brief, 104–105 CFU of S. aureus cells were inoculated on the SC samples. After incubating the SC samples at 37°C and 90% relative humidity for 4 hours, the number of viable S. aureus cells (Log CFU/sample) were measured by the Soleris system using the NF-TVC kit. Skin antibacterial activity for each sample was presented using Log CFU changes between initial inoculum and after incubation. Overall, 48% (39/81) of participants showed a positive Log CFU reduction value (Supplementary Figure 4). One outlier (Log CFU reduction = 4.93) was removed with three sigma rules. The distribution of Log CFU reduction was centered at 0.26 ± 0.91 for 80 participants. Skin pH was significantly associated with S. aureus Log CFU reduction on SC (Figure 2). The regression coefficient was −0.496 (p=0.0078). Therefore, as skin pH decreased by one unit, the average Log CFU reduction increased by 0.496, which corresponds to 68.1% of S. aureus cell death.
 |
Figure 2 Regression analysis between skin pH and S. aureus Log CFU changes measured by ex vivo antibacterial activity assay. Abbreviations: CFU, colony-forming unit; S. aureus, Staphylococcus aureus. |
Regression of Skin pH on NMF
Four different NMFs in SC were measured by liquid chromatography with tandem mass spectrometry. Of these, PCA and histidine were significantly negatively associated with skin pH (p<0.05) (Figure 3).
Verification of the Effects of PCA and UCA on S. Aureus Growth and Metabolic Activity
PCA and UCA (2.5 mM to 10 mM) were added to the bacterial growth medium, mimicking reported concentrations found on human skin. The addition of PCA and UCA reduced the medium pH (Table 2).
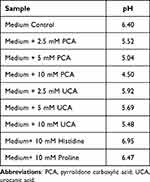 |
Table 2 PCA and UCA Impact on Medium pH |
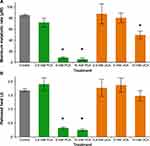
The cell density of the cultures was monitored by recording the OD600 values (Figure 4A). S. aureus growth was affected by 5 mM and 10 mM of PCA and the final cell density was reduced at 20 hours by approximately 25% (from OD600 of 0.14 to 0.10) compared with the control. PCA (2.5 mM) and UCA (10 mM) only reduced the bacterial cell growth rate. Minor impacts on bacterial growth rate were shown by 2.5 mM and 5 mM UCA.
S. aureus growth was also evaluated in the presence of 10 mM histidine and 10 mM proline. The addition of histidine increased the medium pH from 6.40 to 6.95 (Table 2). 10 mM proline had no impacts on S. aureus growth compared to the control. 10 mM histidine increased bacterial growth rate in the Log phase (Supplementary Figure 5).
The thermograms obtained by exposing S. aureus ATCC 6538 to different concentrations of PCA and UCA are displayed in Figure 4B and C. Treating the cells with UCA and PCA led to a shift in the lag phase duration and a lower maximum heat flow (Figure 4B). The cumulative released heat was plotted against time (Figure 4C). PCA treatment with 5 mM and 10 mM showed a significantly lower maximum metabolic rate and released heat compared with the control sample (p<0.0001) (Figure 5). UCA treated with 10 mM showed a significantly lower maximum metabolic rate compared with the control sample (p=0.0024).
Discussion
The aim of the present study was to determine the association between the skin acid mantle, NMFs, and antibacterial activity of the SC using S. aureus as a model microorganism.
The acid mantle of the skin has been known for decades and the maintenance of acidic SC pH is regulated by several pathways: the filaggrin degradation pathway; hydrolysis of FFAs from phospholipids; and proton pumps.25 The current study firstly observed that the level of NMFs negatively correlated with skin pH for female participants with generally good skin health. UCA and PCA, as part of NMFs from filaggrin degradation, are believed to be key proton donors.26 PCA is the major NMF that is produced within the SC.27 PCA showed better proton donation ability than UCA in vitro. For example, 10 mM UCA reduced the medium pH from 6.40 to 5.48, while 10 mM PCA reduced the medium pH to 4.50. Thus, PCA may play a stronger role than UCA in maintaining SC acidity. Several functions have been proposed for the acid mantle defense against S. aureus colonization on human skin: (1) direct growth inhibition by the acidic pH condition, (2) reduced S. aureus expression of proteins for skin adhesion, and (3) reduced expression of bacterial adhesion clumping factor B ligands on corneocytes.20,28–30 Our study suggests that an acidic skin pH directly regulates S. aureus growth and viability on human SC. In the in vitro experiments, both bacterial cell growth and metabolic activity were inhibited below pH 5.0 in the presence of 5 mM and 10 mM PCA. PCA (10 mM) reduced S. aureus maximum metabolic rate from 85.5 µW to 5.72 µW and total released heat from 1.68 J to 0.25 J. IMC directly measures energy emitted from biological processes in microorganisms.19 Reduced energy production could be associated with cellular damage on enzyme activity and electron transport chain during respiration.31
One limitation of our study is that other antibacterial factors in SC were not studied. Skin contains different amounts of antimicrobial peptides and lipids that limit colonization of commensal and opportunistic microorganisms.32 Moreover, their antimicrobial activity could be regulated by pH. Dermcidin showed improved bactericidal activity against E. coli under an acidic pH.33 Latendorf et al identified a new class of antimicrobial peptide-Cationic Intrinsically Disordered Antimicrobial Peptides (CIDAMPs) with activity under acidic conditions.34 Fatty acids and sphingosines are two major antimicrobial lipids in SC. Both showed improved bactericidal activity at acidic pH.35,36 To fully understand the germ protection mechanism of human SC, it would be important to assess other targets in future studies. Another limitation is that participant hygiene habits and the practice of using personal cleansing products was not strictly controlled. This could be one confounding factor for measuring skin pH, NMFs, and skin antibacterial activity ex vivo. Lastly, our work exclusively focused on S. aureus. Future studies should explore the role of the acid mantle in regulating other opportunistic microorganisms and natural commensal microorganisms on the skin surface.
Conclusion
In this study, the proposed mechanism could lead to the development of both leave-on and rinse-off products to maintain the skin acid mantle. PCA is known as a major NMF for skin hydration and could be explored as a new agent for the skin acid mantle. Thus, by maintaining and increasing the PCA amount in SC, there is potential to provide improved skin health and a germ protection barrier for individuals.
Abbreviations
AD, atopic dermatitis; BCA, bicinchoninic acid; CFU, colony-forming unit; CIDAMP, Cationic Intrinsically Disordered Antimicrobial Peptide; DI, deionized; DT, detection time; FFA, free fatty acid; IMC, isothermal microcalorimetry; NF-TVC, Non-fermenting-Total Viable Count; MRM, multiple reaction monitoring; NMFs, natural moisturizing factors; P&G, Procter & Gamble; PCA, pyrrolidone carboxylic acid; RHE, reconstructed human epidermis; rpm, revolutions per minute; SC, stratum corneum; SEM, scanning electron microscopy; UCA, urocanic acid.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
Ethics Approval and Informed Consent
The study protocol was approved by the Human Research Committee of the P&G Singapore Innovation Center (SGIC PRT ID: 965). Informed written consent was obtained from all participants after the procedures were explained with documentation. All experiments were conducted in accordance with the principles of the Declaration of Helsinki.
Acknowledgments
We thank Feng Yue, Miranda Farage, Jamie Fitzgerald, Kunal Gujraty, Angelica Caranza, Pius Parakattil, HiuFung Lau, and Pradipta Sarkar for their useful comments and contributions regarding this research. We also thank Ganna Oliynyk (Symcel AB) for her technical support and guidance on IMC experiments. The clinical study was performed in collaboration with Sara Holloway (P & G International Operations SA SG Branch).
Editorial support for this manuscript was provided by Cactus Life Sciences (part of Cactus Communications), funded by P&G International Operations.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The funding for this study was provided by P&G International Operations.
Disclosure
All authors are employees of the study sponsor, P&G International Operations. The authors report no other conflicts of interest in this work.
References
1. Schade H, Marchionini A. Der Säuremantel der Haut (Nach Gaskettenmessungen). Klin Wochenschr. 1928;7(1):12–14. doi:10.1007/BF01711684
2. Stefaniak AB, Plessis J, John SM, et al. International guidelines for the in vivo assessment of skin properties in non-clinical settings: part 1. pH. Skin Res Technol. 2013;19(2):59–68.
3. Parra JL, Paye M, Group EEMCO. EEMCO guidance for the in vivo assessment of skin surface pH. Skin Pharmacol Appl Skin Physiol. 2003;16(3):188–202.
4. Fluhr JW, Elias PM. Stratum corneum pH: formation and Function of the ‘Acid Mantle’. Exog Dermatol. 2002;1:163–175.
5. Elias PM. The how, why and clinical importance of stratum corneum acidification. Exp Dermatol. 2017;26(11):999–1003.
6. Le Lamer M, Pellerin L, Reynier M, et al. Defects of corneocyte structural proteins and epidermal barrier in atopic dermatitis. Biol Chem. 2015;396(11):1163–1179.
7. Kezic S, O’Regan GM, Lutter R, et al. Filaggrin loss-of-function mutations are associated with enhanced expression of IL-1 cytokines in the stratum corneum of patients with atopic dermatitis and in a murine model of filaggrin deficiency. J Allergy Clin Immunol. 2012;129(4):1031–1039 e1.
8. Gruber R, Elias PM, Crumrine D, et al. Filaggrin genotype in ichthyosis vulgaris predicts abnormalities in epidermal structure and function. Am J Pathol. 2011;178(5):2252–2263.
9. Pendaries V, Le Lamer M, Cau L, et al. In a three-dimensional reconstructed human epidermis filaggrin-2 is essential for proper cornification. Cell Death Dis. 2015;6:e1656.
10. Schiemann O, Landau H. Über Händedesinfektion und Händereinigung in ihrer Bedeutung zur Verhütung von Krankheitsübertragungen. Zeitschr f Hygiene. 1919;88:129–184.
11. Hayashi K, Mori I, Takeda K, et al. Analysis of hand environment factors contributing to the hand surface infection barrier imparted by lactic acid. Skin Res Technol. 2021;27(6):1135–1144.
12. Ogonowska P, Gilaberte Y, Barańska-Rybak W, Nakonieczna J. Colonization With Staphylococcus aureus in Atopic Dermatitis Patients: attempts to Reveal the Unknown. Front Microbiol. 2020;11:567090.
13. Seidenari S, Giusti G. Objective assessment of the skin of children affected by atopic dermatitis: a study of pH, capacitance and TEWL in eczematous and clinically uninvolved skin. Acta Derm Venereol. 1995;75(6):429–433.
14. Eberlein-König B, Schäfer T, Huss-Marp J, et al. Skin surface pH, stratum corneum hydration, trans-epidermal water loss and skin roughness related to atopic eczema and skin dryness in a population of primary school children. Acta Derm Venereol. 2000;80(3):188–191.
15. Knor T, Meholjić-Fetahović A, Mehmedagić A. Stratum corneum hydration and skin surface pH in patients with atopic dermatitis. Acta Dermatovenerol Croat. 2011;19(4):242–247.
16. Hülpüsch C, Tremmel K, Hammel G, et al. Skin pH-dependent Staphylococcus aureus abundance as predictor for increasing atopic dermatitis severity. Allergy. 2020;75(11):2888–2898.
17. Safer D, Brenes M, Dunipace S, Schad G. Urocanic acid is a major chemoattractant for the skin-penetrating parasitic nematode Strongyloides stercoralis. Proc Natl Acad Sci U S A. 2007;104(5):1627–1630.
18. Cirnski K, Coetzee J, Herrmann J, Müller R. Metabolic Profiling to Determine Bactericidal or Bacteriostatic Effects of New Natural Products using Isothermal Microcalorimetry. J Vis Exp. 2020;164.
19. Limberg BJ, Johnstone K, Filloon T, Catrenich C. Performance Equivalence and Validation of the Soleris Automated System for Quantitative Microbial Content Testing Using Pure Suspension Cultures. J AOAC Int. 2016;99(5):1331–1337.
20. Feuillie C, Vitry P, McAleer MA, et al. Adhesion of Staphylococcus aureus to Corneocytes from Atopic Dermatitis Patients Is Controlled by Natural Moisturizing Factor Levels. mBio. 2018;9(4):e01184–18.
21. Billhimer WL, Berge CA, Englehart JS, Rains GY, Keswick BH. A modified cup scrub method for assessing the antibacterial substantivity of personal cleansing products. J Cosmet Sci. 2001;52(6):369–375.
22. Williams RC. Electron microscopy of sodium desoxyribonucleate by use of a new freeze-drying method. Biochim Biophys Acta. 1952;9(3):237–239.
23. Chng KR, Tay AS, Li C, et al. Whole metagenome profiling reveals skin microbiome-dependent susceptibility to atopic dermatitis flare. Nat Microbiol. 2016;1(9):16106.
24. Wei KS, Stella C, Wehmeyer KR, et al. Effects of season stratum corneum barrier function and skin biomarkers. J Cosmet Sci. 2016;67(3):185–203.
25. Proksch E. pH in nature, humans and skin. J Dermatol. 2018;45(9):1044–1052.
26. Rippke F, Schreiner V, Schwanitz HJ. The acidic milieu of the horny layer: new findings on the physiology and pathophysiology of skin pH. Am J Clin Dermatol. 2002;3(4):261–272.
27. Rawlings AV, Harding CR. Moisturization and skin barrier function. Dermatol Ther. 2004;17 Suppl 1:43–48.
28. Rippke F, Schreiner V, Doering T, Maibach HI. Stratum corneum pH in atopic dermatitis: impact on skin barrier function and colonization with Staphylococcus Aureus. Am J Clin Dermatol. 2004;5(4):217–223.
29. Miajlovic H, Fallon PG, Irvine AD, Foster TJ. Effect of filaggrin breakdown products on growth of and protein expression by Staphylococcus aureus. J Allergy Clin Immunol. 2010;126(6):1184–1190.e3.
30. Lambers H, Piessens S, Bloem A, Pronk H, Finkel P. Natural skin surface pH is on average below 5, which is beneficial for its resident flora. Int J Cosmet Sci. 2006;28(5):359–370.
31. Zhou C, Fey PD. The acid response network of Staphylococcus aureus. Curr Opin Microbiol. 2020;55:67–73.
32. Brogden NK, Mehalick L, Fischer CL, Wertz PW, Brogden KA. The emerging role of peptides and lipids as antimicrobial epidermal barriers and modulators of local inflammation. Skin Pharmacol Physiol. 2012;25(4):167–181.
33. Schittek B, Hipfel R, Sauer B, et al. Dermcidin: a novel human antibiotic peptide secreted by sweat glands. Nat Immunol. 2001;2(12):1133–1137.
34. Latendorf T, Gerstel U, Wu Z, et al. Cationic Intrinsically Disordered Antimicrobial Peptides (CIDAMPs) Represent a New Paradigm of Innate Defense with a Potential for Novel Anti-Infectives. Sci Rep. 2019;9(1):3331.
35. Wang LL, Johnson EA. Inhibition of Listeria monocytogenes by fatty acids and monoglycerides. Appl Environ Microbiol. 1992;58(2):624–629.
36. Bibel DJ, Aly R, Shinefield HR. Antimicrobial activity of sphingosines. J Invest Dermatol. 1992;98(3):269–273.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.