Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 16
Association Between Sensitivity to Thyroid Hormone Indices and Diabetic Retinopathy in Euthyroid Patients with Type 2 Diabetes Mellitus
Authors Yang J , Ding W, Wang H, Shi Y
Received 8 December 2022
Accepted for publication 27 January 2023
Published 27 February 2023 Volume 2023:16 Pages 535—545
DOI https://doi.org/10.2147/DMSO.S399910
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Gian Paolo Fadini
Jie Yang, Wencui Ding, Haiying Wang, Yanan Shi
Department of Endocrinology, Cangzhou Central Hospital, Cangzhou, Hebei Province, 061000, People’s Republic of China
Correspondence: Jie Yang, Department of Endocrinology, Cangzhou Central Hospital, Cangzhou, Hebei Province, 061000, People’s Republic of China, Email [email protected]
Objective: Normal thyroid hormone (TH) levels and their relation to microvascular complications in patients with type 2 diabetes mellitus (T2DM) have been studied. However, the relationship between TH sensitivity and diabetic retinopathy (DR) remains unclear. Thus, this study aimed to investigate the relationship between TH sensitivity and the risk of DR in euthyroid T2DM patients.
Methods: This retrospective study analyzed 422 T2DM patients and calculated their sensitivity to TH indices. Multivariable logistic regression, generalized additive model, and subgroup analysis were performed to examine the association between sensitivity to TH indices and DR risk.
Results: After adjusting for covariates, the binary logistic regression model showed no statistically significant association between the sensitivity of TH indices and the risk of DR in euthyroid T2DM patients. However, a non-linear relationship was found between sensitivity to TH indices (thyroid-stimulating hormone index, thyroid feedback quantile index [TFQI]) and the risk of DR in the crude model; TFQI and DR in the adjusted model. The inflection point of the TFQI was 0.23. The effect size (odds ratio) on the left and right of the inflection point were 3.19 (95% confidence interval [CI]: 1.24 to 8.17 p=0.02) and 0.11 (95% CI: 0.01, 0.93 p=0.04), respectively. Moreover, this relationship was maintained by men stratified by sex. In euthyroid patients with T2DM, an approximate inverted U-shaped relationship and a threshold effect were demonstrated between TH index sensitivity and DR risk with sex differences. This study provided an in-depth understanding of the relationship between thyroid function and DR, which has important clinical implications for risk stratification and individual prediction.
Keywords: thyroid hormone sensitivity, diabetes retinopathy, non-linear relationship, sex differences
Introduction
Diabetic retinopathy (DR) is one of the most common microvascular complications of type 2 diabetes mellitus (T2DM), leading to an increased socioeconomic burden. In middle-aged and older adults, DR is the leading cause of vision impairment that often results in blindness.1 It affects 1 in 3 people with diabetes and is associated with an increased incidence of other severe vascular diseases, such as stroke and coronary artery disease (CAD). In 2015, 415 million cases of DR were diagnosed worldwide, which is expected to increase to 642 million by 2040.2 Therefore, it is essential to identify risk factors linked to the onset and progression of DR to reduce its prevalence.
Although the pathogenesis and etiology of DR have not yet been fully elucidated, an increased duration of diabetes, poor glycemic control, and hypertension are considered risk factors for DR.3 However, some people who have well-controlled blood sugar levels and no hypertension also experience severe DR, indicating the contribution of additional risk factors to the development of DR.4 Recently, the impact of thyroid hormones (THs) on diabetic microvascular illness has been highlighted. Several studies have demonstrated the critical role of TH metabolism in retinal development and increased retinal vascular density.5
The TH is required for retinal development and maturation via genomic and non-genomic pathways.6–8 TH deficiency may affect the adult retina and impair visual function, particularly color vision.9 Furthermore, a high level of thyroid-stimulating hormone (TSH) is an independent risk factor for DR,10 and subclinical hypothyroidism (SCH) is associated with a high incidence of DR.11 Although the correlation between TH levels and DR has been recently reported, there are relatively few studies on the relationship between various thyroid functions and DR in T2DM patients. Euthyroid thyroid function has been demonstrated to be associated with DR;12–14 however, their results are conflicting. Therefore, we attempted to investigate the relationship between DR and normal TH levels.
Clinically, thyroid function is assessed by measuring serum levels of free triiodothyronine (FT3), free thyroxine (FT4), and TSH. However, FT3 or TSH alone may not be adequate to reflect the regulation of TH homeostasis.15 Therefore, the sensitivity to TH indicators, including TSH-T4 resistance index (TT4RI), TSH index (TSHI), and thyroid feedback quantile index (TFQI), were proposed as quantitative markers of pituitary thyroid-stimulating function to interpret thyroid status comprehensively.16–18 Recently, the sensitivity of TH indicators has been demonstrated to be associated with metabolic disorders such as T2DM, obesity, metabolic syndrome, and non-alcoholic fatty liver disease (NAFLD).19,20 However, no study has investigated the relationship between TH sensitivity indices and the risk of DR. It is unclear whether changes in TH sensitivity influence the development of DR. Thus, this population-based study aimed to gain new insights into the association between TH sensitivity and the risk of DR in euthyroid patients with T2DM.
Materials and Methods
Study Population
This cross-hospital study included 422 T2DM patients admitted to the Endocrinology Department of Cangzhou Central Hospital between July 2017 and December 2019. The inclusion criteria were as follows: aged ≥ 18 years old with normal thyroid function and negative autoantibodies, including thyroid peroxidase antibody (TPOAb), thyroglobulin antibody, or thyrotropin receptor antibody, and diagnosed with T2DM according to the criteria of the American Diabetes Association.21 The exclusion criteria were as follows: those who had any acute complications of diabetes; a history of thyroid disease; any other endocrine disorder such as Addison’s disease, Cushing syndrome, pituitary adenoma, or hypopituitarism; retinopathy unrelated to diabetes; a severe infection; an inflammatory disease; malignant tumors; liver or renal dysfunction; and thyroid-function-altering medications or drugs. This study was approved by the Cangzhou Central Hospital Ethics Committee and was performed following the Declaration of Helsinki guidelines, including any relevant details. Due to the retrospective nature of the study, the requirement for informed consent was waived.
Definition of DR
All patients had their eyes examined and their fundi photographed using 45° four-field stereoscopic digital photography. The definition outlined in the American Academy of Ophthalmology classification was used to identify DR.
Assessment of Thyroid Function
Serum FT3, FT4, TSH, and TPOAb (reference range: 0–34.0 IU/mL) levels were measured using an electrochemiluminescence analyzer (Cobas e601, Roche, Basel, Switzerland). Patients with THs in this range were considered to be euthyroid patients: TSH (0.27–4.20 mIU/L, FT3 (3.08–6.78 pmol/L), and FT4 (11.97–21.88 pmol/L).
Indices of TH Sensitivity
The TSHI, TT4RI, and TFQI were used to assess central TH sensitivity. The calculation formulas of TSHI and TT4RI were derived from previous studies.22 The TFQI calculation method was developed by Laclaustra et al.19 Lower TT4RI, TSHI, and TFQI values indicate greater central TH sensitivity.
Clinical Examination and Laboratory Measurements
The covariates are demographic information, clinical data, and laboratory data. Demographic and clinical data were collected, including age, sex, height and weight, blood pressure, smoking and drinking status, history of hypertension, and diabetes duration. Body mass index (BMI) was calculated by dividing weight by height squared (kg/m2). Hypertension was defined as systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, or the use of antihypertensive drugs. The results obtained from measuring glycated hemoglobin (HbA1c), total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol (HDL-C) and triglyceride levels in euthyroid patients with T2DM were also obtained from the Hospital Information System.
Statistical Analyses
Categorical variables provide frequencies or percentages, while continuous variables show mean, standard deviation, or median (quartiles). To identify statistical differences in means and proportions between groups, one-way analysis of variance (normal distribution), the Kruskal–Wallis test (skewed distribution), and the Chi-square test (categorical variables) were used.
The following statistical analyses were conducted and studied: (1) Was there any relationship between TH sensitivity indices and DR risk, and if yes, was it linear or non-linear? To provide preliminary insight into their relationship, we used a generalized additive model (GAM). If there were a linear relationship between the two, univariate and multivariate linear regression models would be used to assess their associations. According to the recommendation from the Strengthening the Reporting of Observational Studies in Epidemiology statement, three models would be built: an unadjusted model, a model adjusted for demographics, and a fully-adjusted model. The relevant covariates that affected the matched odds ratio (OR) by at least 10% when included in this model are considered the adjusted variables for the fully-adjusted model.23 If the non-linear association were identified, a two-piecewise linear regression model would be used to determine the threshold effect of the TH sensitivity indices and DR risk in terms of the smoothing plot. When the ratio of TH sensitivity and DR appears in a smooth curve, the recursive method automatically calculates the inflection point at which the maximum model likelihood will be used. (2) Which factors interfered with or altered their relationship? The stratified binary logistic regression models were employed for the subgroup analyses. The likelihood ratio test investigated subgroup modification and interaction. (3) What was the independent effect on TH sensitivity and DR risk when the potential confounders or modifiers were removed? Further stratified binary logistic regression models were utilized for the subgroup analyses. We also used hierarchical interaction analysis to investigate the robustness of the results in various subgroups.
The statistical software packages R (http://www.R-project.org, The R Foundation) and EmpowerStats (http://www.empowerstats.com, X&Y Solutions, Inc., Boston, MA) were applied to all the analyses. P values less than 0.05 (two-sided) were deemed statistically significant.
Results
Baseline Characteristics of the Participants with or Without DR
This study included 422 participants. Table 1 displays the demographic characteristics of the research population. Participants in the DR group were more likely to be older, be male, have a lower BMI, and have diabetes for longer than those in the non-DR group. Significant differences were not observed between the two groups for FT3, TSH, TSHI, TT4RI, and TFQI (P > 0.05). However, the FT4 level in patients with DR was significantly low (P < 0.001).
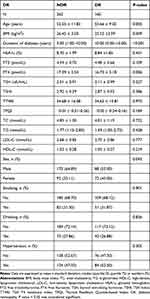 |
Table 1 Clinical and Laboratory Characteristics of the Study Participants |
Associations Between Sensitivity to TH Indices and DR
The multivariate regression analysis between sensitivity to TH indices and DR is presented in Table S1. In the unadjusted, minimally adjusted, and fully adjusted models, TSHI, TT4RI, and TFQI were not associated with the risk of DR (P > 0.05). For sensitivity analysis, TSHI, TT4RI, and TFQI were converted from continuous variables to categorical variables (based on tertile). The categorical-transformed variables were then reintroduced into the model; however, the trend remained nonsignificant (P > 0.05).
The results of multivariate models based on logistic regression were not statistically significant, which may indicate a non-linear relationship between sensitivity to TH indices and DR. Through the GAM and smooth curve fitting, an approximate inverted U-shaped relationship was observed between the sensitivity to TH indices, except for TT4RI, and unfavorable DR risk in T2DM patients. This relationship was evident in both unadjusted and adjusted models (adjusted sex, age, BMI, duration of diabetes, HbA1c, HDL-C, FT3, and FT4) (Figure 1).
These data were modeled into a piecewise binary logistic regression model to fit two different slopes, and the best-fit model was selected through the log-likelihood ratio test. The inflection point of TSHI, TT4RI, and TFQI was obtained through a recursive algorithm, and thereafter, the effect sizes and confidence interval were calculated on the left and right sides of the inflection point (Table 2). Notably, the results illustrated a threshold effect for TSHI, and TFQI, which were fitted according to the smoothing curve (Figure 1). However, these results differed slightly between the unadjusted and adjusted models. In the unadjusted model, the effect sizes (OR) for TSHI on the left and right sides of the inflection point at 3.28 were 1.22 (95% confidence interval [CI]: 0.77 to 1.92, p=0.40) and 0.17 (95% CI: 0.04 to 0.73, p=0.02), respectively; in the adjusted model, the effect sizes (OR) for TSHI on the left and right sides of the inflection point at 3.54 were 1.45 (95% CI: 0.91 to 2.31, p=0.12) and 0.06 (95% CI: 0.00 to 1.64 p=0.10), respectively. After adjusting for covariates, the inflection points remained the same; however, the results of both binary logistic regression models became nonsignificant. In the unadjusted model, the effect sizes (OR) for TFQI on the left and right sides of the inflection point at 0.18 were 1.34 (95% CI: 0.62 to 2.90, p=0.45) and 0.13 (95% CI: 0.02 to 0.66 p=0.01), respectively; in the adjusted model, the effect size (OR) of TFQI on the left and right sides of the inflection point at 0.23 were 3.19 (95% CI: 1.24 to 8.17 p=0.02) and 0.11 (95% CI: 0.01, 0.93 p=0.04), respectively. Contrary to the results for the TSHI, the results of both binary logistic regression models demonstrated an association between TFQI and DR occurrence after adjustment.
 |
Table 2 Threshold Effect Analysis of Sensitivity to Thyroid Hormone Indices on DR Using the Two-Piecewise Linear Regression Model |
Associations Between Sensitivity to TH Indices and DR Interaction with Sex
Furthermore, to determine the trend of effect sizes, a hierarchical analysis and interactive analyses with sex as the stratified variable were conducted to study the stability of the non-linear relationship between sensitivity to TH indices and DR risk in different subgroups. The results of smooth curve-fitting and threshold effect analysis showed that sex played an interactive role (P-interaction: 0.017 for TSHI and sex; P-interaction: 0.012 for TFQI and sex) in a non-linear relationship between sensitivity to TH indices and the incidence of DR (Table 3; Figure 2). A similar non-linear relationship and threshold effect to the general population between TSHI and TFQI and incident DR was detected in males with adjustments for the above-mentioned covariates. Inflection points were also found in females; however, the relationship between the sensitivity to TH indices and DR risk in females did not conform to the curve relationship. Additionally, TT4RI and DR showed a nonsignificant linear relationship between males and females.
 |
Table 3 Threshold Effect Analysis of Sensitivity to Thyroid Hormone Indices on DR Using the Two-Piecewise Linear Regression Model with Sex Differences |
Discussion
Based on the hospital data, this study investigated the relationship between TH sensitivity and DR incidence. In euthyroid patients with T2DM, an approximate inverted U-shaped relationship was found between sensitivity to the TH indices TSHI and TFQI and the risk of DR. Males had a similar relationship between TSHI and TT4RI and the prevalence of DR in subgroup analyses that were stratified by sex. This study is the first to use the TH sensitivity index instead of FT3, FT4, and TSH as a predictor of DR. A direct connection between TH resistance and DR occurrence and progression was demonstrated, providing a novel hypothesis for the early detection and treatment of DR in euthyroid patients with T2DM.
Traditionally, a long durations of diabetes, hypertension, and poor glycemic control have been the main risk factors for DR. However, recent research has revealed that, in addition to the established risk factors, thyroid function is tightly linked to the development of DR.24 T2DM patients with subclinical hypothyroidism have been found to have an increased risk of developing DR.11 Similarly, a meta-analysis observed that subclinical hypothyroidism increased DR risk in diabetic patients by 2.13-fold.25 However, the correlation between normal levels of TH and DR in patients with T2DM has yet to be extensively researched. Several research studies have attempted to cast light on the subject. Zou et al13 demonstrate that FT3 levels in euthyroid patients with T2DM were negatively correlated with the risk of DR within the normal range. Lower than normal FT4 levels were reported to be associated with DR prevalence in T2DM patients after controlling for traditional DR risk factors in a study by Kong et al.12 Despite most previous studies proving otherwise, our results with a linear multivariate logistic regression revealed no relationship between FT3, FT4, TSH, and the risk of DR. Remarkably, using smoothed curve fitting and threshold saturation effect analysis, a non-linear relationship was observed between FT4 and DR, and the results are presented in Tables S2 and S3 and Figure S1A–C. The study hypothesizes that the disparities in the results may be due to the different criteria used to assess the thyroid function and composition of participants. Furthermore, the interaction of TSH, FT4, and FT3 is strong, and complicated relationships occur spontaneously along the hypothalamus–pituitary–thyroid (HPT) axis.26 Thus, measuring just one parameter might not sufficiently explain the connection between the thyroid system and the risk of DR. Consequently, comprehensive indicators were utilized for further evaluation.
Considering the discrepancies found by using a single parameter in previous studies, comprehensive indicators TSHI, TT4RI, and TFQI were recommended as new central TH resistance indicators. Among them, TFQI is supported by empirical co-distribution of FT4 and TSH, which have the advantage of not producing extreme values during thyroid dysfunction.19 These new comprehensive indicators can systematically reflect the regulation of TH homeostasis. They may be less biased than single indicators with a better explanation for the various associations between TH changes and the risk of DR. TSHI and TT4RI are significantly linked to the prevalence of coronary heart disease (CAD),27 NAFLD,28 and chronic kidney disease.29 A strong link was discovered between TFQI and obesity, metabolic syndrome, and diabetes-related mortality.30 However, to our knowledge, no study has focused on the relationship between sensitivity to THs and the risk of DR.
The HPT axis regulates circulating THs through a negative feedback mechanism. All these TH indices measure central sensitivity/resistance, ie, the grade of pituitary gland inhibition by FT4 levels. Thus, they evaluate the set point of the central regulation of TH concentration.19 The HPT axis is essential in retinal development, photoreceptor differentiation, cone opsin expression, and regulating photoreceptor survival.31 Among them, TH receptor-β (TRβ) is an essential regulator of sensory development in the cochlea and retina.32 In contrast, in the hypothalamus and pituitary glands, TRβ mediates inhibitory actions of THs on the expression of thyrotropin-releasing hormone and TSH to control the HPT axis,33 which may be the physiopathological basis for the association between TH sensitivity indices and retinopathy. Dysregulation of the HPT axis often coexists with obesity, metabolic syndrome, diabetes, and consequent biological alterations related to the impact of THs on the metabolic functions of the body, including glucose metabolism, insulin secretion, homocysteine metabolism, lipid metabolism, chronic inflammation, and vascular endothelial function damage.24 Thus, a strong link was established to prove the association between the TH sensitivity index and DR risk.
In the unadjusted, minimally adjusted, and fully adjusted multivariate linear logistic regression of this study, there was no significant relationship between TSHI, TT4RI, and TFQI and the risk of DR. These data were further investigated by using logistic regression with cubic spline functions and smooth curve fitting (cubic spline smoothing). Eventually, the relationship between the TSHI and TFQI and undesirable DR risk was non-linear. This non-linear association was still significant for TFQI after adjusting for multiple covariates, which indicated that on the left side of the inflection point of TFQI (0.23), patients were at a 2.19-fold increased risk of DR with an increase in TH resistance and on the right side of the inflection point, patients were at an 89% decreased risk of DR with decreasing TH resistance. However, the possible explanations for the non-linear relationship between sensitivity to TH metrics and the risk of DR in euthyroid patients with T2DM remain unknown. A variety of factors may cause this. First, when a change in one variable does not conform to a continuous change in the other variable, it may be defined as a non-linear relationship between two variables, which could imply that the correlation between the two variables is erratic or does not exist. Non-linear objects, on the contrary, can be connected in a reasonably predictable manner, albeit in a more complex manner than that between linear connections. As a result, the non-linear relationship appears to be similar to the actual relationship between TH sensitivity and DR risk. In biological mechanisms, alterations in the TH sensitivity index imply changes in the HPT axis tuning point and the accompanying TH levels, leading to altered metabolic and energy balances, which may trigger an increased or reduced risk of DR. TH sensitivity indices may reconcile the conflicting results of the relationship between a single TH and the risk of DR, which may require further laboratory research. Second, this non-linear relationship between TFQI and DR may persist after controlling for multiple covariates, which may be supported by a previous study that found TFQI to be more stable than TSH in assessing TH sensitivity.19 Moreover, the similar non-linear relationship between TFQI and DR suggested that FT4 may contribute more to the relationship between thyroid function and DR in the euthyroid range than that of TSH or FT3 in patients with diabetes. Thus, FT4 may be the ideal parameter for assessing thyroid status in euthyroid patients with T2DM compared to TSH and FT3, with age and metformin application having little effect.12 Third, numerous studies have demonstrated that changes in TH levels within the normal range were frequently accompanied by hypertension,34 glucose metabolism dysfunction,35 abnormal insulin secretion,36 vascular endothelial impairment,37 and progression of vascular neurodegeneration,38 which are the main pathological risk factors for developing DR. Overall, sensitivity to THs may play a non-linear role in the development of DR in euthyroid patients with T2DM.
We further evaluated the sex-specific association between TH sensitivity and DR risk in addressing the sex differences found in previous studies.39 With adjusted covariates, an inverted U-shaped relationship and threshold effect were similar between TSHI and TFQI and incident DR in males and females. Sex hormones (such as estrogen and testosterone) regulate thyroid function,40 and estrogen levels may influence diabetes development, particularly in patients aged 60. Sex hormone differences may explain some sex differences in the association between TH sensitivity and DR risk discovered in this study. However, since this study did not examine sex hormone levels, further study is required to investigate this idea.
The strengths of this study included specific populations, innovative TH indices, and non-linear relationship-fitting results with sex differences. However, the current study had several limitations that must be considered. First, given that this was a cross-sectional study, causality could not be inferred. Therefore, further studies are needed to determine the relative risk between TH sensitivity and DR risk in T2DM patients. Second, due to the short duration of the hospital stay, a single thyroid function was only tested once, which may have presented statistical errors. Third, diabetes increases the risk of low T3 syndrome and hypothyroidism.41 As this study did not assess the trans-T3 levels of the participants, the relationship between sensitivity to TH indices and DR risk may have been distorted. Fourth, this was a single-institute study where all participants were Chinese. As a result, our findings may not apply to all Chinese patients with T2DM or patients of other ethnicities.
Conclusions
The study findings supported a non-linear relationship between TH sensitivity and the prevalence of DR in T2DM patients, particularly males, who had normal thyroid function. These findings aid in understanding the interaction between TH sensitivity and DR risk, which could have important clinical implications for risk stratification and personalized forecasting. These findings may serve as the foundation for future large-scale cohort studies. Prospective cohort studies are also needed to investigate the link between TH sensitivity and DR risk in euthyroid patients with T2DM.
Data Sharing Statement
The datasets generated and analyzed during this study are available from the corresponding author on reasonable request.
Declarations Ethics Approval and Consent to Participate
The study was approved by the Institutional Ethics Committee of Cangzhou Central Hospital for retrospective analysis. Due to the retrospective nature of this study the ethics committee waived the need for written informed consent of the included patients. However, we de-identified and anonymized patient records/information prior to analysis to protect the patients’ privacy.
Acknowledgments
The authors thank the participants of this study for their contribution to this research.
Funding
This work was supported by Cangzhou Key R&D Program (No. 204106137).
Disclosure
The authors report no conflicts of interest in this work.
References
1. White NH, Pan Q, Knowler WC, et al. Risk factors for the development of retinopathy in prediabetes and type 2 diabetes: the diabetes prevention program experience. Diabetes Care. 2022;45(11):2653–2661. doi:10.2337/dc22-0860
2. Solomon SD, Chew E, Duh EJ, et al. Diabetic retinopathy: a position statement by the American Diabetes Association. Diabetes Care. 2017;40(3):412–418. doi:10.2337/dc16-2641
3. Lin KY, Hsih WH, Lin YB, Wen CY, Chang TJ. Update in the epidemiology, risk factors, screening, and treatment of diabetic retinopathy. J Diabetes Investig. 2021;12(8):1322–1325. doi:10.1111/jdi.13480
4. Zhang L, Krzentowski G, Albert A, Lefebvre PJ. Risk of developing retinopathy in Diabetes Control and Complications Trial type 1 diabetic patients with good or poor metabolic control. Diabetes Care. 2001;24(7):1275–1279. doi:10.2337/diacare.24.7.1275
5. Mendoza A, Hollenberg AN. New insights into thyroid hormone action. Pharmacol Ther. 2017;173:135–145. doi:10.1016/j.pharmthera.2017.02.012
6. Roberts MR, Srinivas M, Forrest D, Morreale de Escobar G, Reh TA. Making the gradient: thyroid hormone regulates cone opsin expression in the developing mouse retina. Proc Natl Acad Sci U S A. 2006;103(16):6218–6223. doi:10.1073/pnas.0509981103
7. Hiroi Y, Kim HH, Ying H, et al. Rapid nongenomic actions of thyroid hormone. Proc Natl Acad Sci U S A. 2006;103(38):14104–14109. doi:10.1073/pnas.0601600103
8. Cheng SY, Leonard JL, Davis PJ. Molecular aspects of thyroid hormone actions. Endocr Rev. 2010;31(2):139–170. doi:10.1210/er.2009-0007
9. Glaschke A, Weiland J, Del Turco D, Steiner M, Peichl L, Glosmann M. Thyroid hormone controls cone opsin expression in the retina of adult rodents. J Neurosci. 2011;31(13):4844–4851. doi:10.1523/JNEUROSCI.6181-10.2011
10. Cao X, Lu M, Xie RR, et al. A high TSH level is associated with diabetic macular edema: a cross-sectional study of patients with type 2 diabetes mellitus. Endocrine Connections. 2022;11(7):5.
11. Heidari Z, Asadzadeh R. Subclinical hypothyroidism is a risk factor for diabetic retinopathy in patients with type 2 diabetes mellitus. Med J Islam Repub Iran. 2021;35:186. doi:10.47176/mjiri.35.186
12. Kong X, Wang J, Gao G, et al. Association between free thyroxine levels and diabetic retinopathy in euthyroid patients with type 2 diabetes mellitus. Endocr Res. 2020;45(2):111–118. doi:10.1080/07435800.2019.1690504
13. Zou J, Li Z, Tian F, et al. Association between normal thyroid hormones and diabetic retinopathy in patients with type 2 diabetes. Biomed Res Int. 2020;2020:8161797. doi:10.1155/2020/8161797
14. Hu Y, Hu Z, Tang W, Liu W, Wu X, Pan C. Association of thyroid hormone levels with microvascular complications in euthyroid type 2 diabetes mellitus patients. Diabetes Metab Syndr Obes. 2022;15:2467–2477. doi:10.2147/DMSO.S354872
15. Yang S, Lai S, Wang Z, Liu A, Wang W, Guan H. Thyroid Feedback Quantile-based Index correlates strongly to renal function in euthyroid individuals. Ann Med. 2021;53(1):1945–1955. doi:10.1080/07853890.2021.1993324
16. van der Spek AH, Fliers E, Boelen A. The classic pathways of thyroid hormone metabolism. Mol Cell Endocrinol. 2017;458:29–38. doi:10.1016/j.mce.2017.01.025
17. Jostel A, Ryder WD, Shalet SM. The use of thyroid function tests in the diagnosis of hypopituitarism: definition and evaluation of the TSH Index. Clin Endocrinol. 2009;71(4):529–534. doi:10.1111/j.1365-2265.2009.03534.x
18. Hoermann R, Midgley JEM, Larisch R, Dietrich JW. The role of functional thyroid capacity in pituitary thyroid feedback regulation. Eur J Clin Invest. 2018;48(10):e13003. doi:10.1111/eci.13003
19. Laclaustra M, Moreno-Franco B, Lou-Bonafonte JM, et al. Impaired sensitivity to thyroid hormones is associated with diabetes and metabolic syndrome. Diabetes Care. 2019;42(2):303–310. doi:10.2337/dc18-1410
20. Lai S, Li J, Wang Z, Wang W, Guan H. Sensitivity to thyroid hormone indices are closely associated with NAFLD. Front Endocrinol. 2021;12:766419. doi:10.3389/fendo.2021.766419
21. American Diabetes A. Standards of medical care in diabetes--2014. Diabetes Care. 2014;37(Suppl 1):S14–S80. doi:10.2337/dc14-S014
22. Mehran L, Delbari N, Amouzegar A, Hasheminia M, Tohidi M, Azizi F. Reduced sensitivity to thyroid hormone is associated with diabetes and hypertension. J Clin Endocrinol Metab. 2022;107(1):167–176. doi:10.1210/clinem/dgab646
23. Kernan WN, Viscoli CM, Brass LM, et al. Phenylpropanolamine and the risk of hemorrhagic stroke. N Engl J Med. 2000;343(25):1826–1832. doi:10.1056/NEJM200012213432501
24. Stefanowicz-Rutkowska MM, Baranowska-Jurkun A, Matuszewski W, Bandurska-Stankiewicz EM. Thyroid dysfunction in patients with diabetic retinopathy. Endokrynol Pol. 2020;71(2):176–183. doi:10.5603/EP.a2020.0013
25. Wu J, Yue S, Geng J, et al. Relationship between diabetic retinopathy and subclinical hypothyroidism: a meta-analysis. Sci Rep. 2015;5:12212. doi:10.1038/srep12212
26. Botello A, Herran M, Salcedo V, Rodriguez Y, Anaya JM, Rojas M. Prevalence of latent and overt polyautoimmunity in autoimmune thyroid disease: a systematic review and meta-analysis. Clin Endocrinol. 2020;93(4):375–389. doi:10.1111/cen.14304
27. Yu N, Wang L, Zeng Y, et al. The association of thyroid hormones with coronary atherosclerotic severity in euthyroid patients. Horm Metab Res. 2022;54(1):12–19. doi:10.1055/a-1718-6283
28. Turker F, Oral A, Sahin T, et al. Does the FT3-to-FT4 ratio easily predict the progression of NAFLD and NASH cirrhosis? J Int Med Res. 2021;49(11):3000605211056841. doi:10.1177/03000605211056841
29. Chen Y, Zhang W, Wang N, et al. Thyroid parameters and kidney disorder in type 2 diabetes: results from the METAL study. J Diabetes Res. 2020;2020:4798947. doi:10.1155/2020/4798947
30. Sun Y, Teng D, Zhao L, et al. Impaired sensitivity to thyroid hormones is associated with hyperuricemia, obesity, and cardiovascular disease risk in subjects with subclinical hypothyroidism. Thyroid. 2022;32(4):376–384. doi:10.1089/thy.2021.0500
31. Yang F, Ma H, Ding XQ. Thyroid hormone signaling in retinal development, survival, and disease. Vitam Horm. 2018;106:333–349. doi:10.1016/bs.vh.2017.05.001
32. El-Eshmawy MM, Shahin M. Thyroid and eye: where they meet in clinical practice. Endocr Metab Immune Disord Drug Targets. 2020;20(1):39–49. doi:10.2174/1871530319666190618120107
33. Abel ED, Ahima RS, Boers ME, Elmquist JK, Wondisford FE. Critical role for thyroid hormone receptor beta2 in the regulation of paraventricular thyrotropin-releasing hormone neurons. J Clin Invest. 2001;107(8):1017–1023. doi:10.1172/JCI10858
34. Gumieniak O, Perlstein TS, Hopkins PN, et al. Thyroid function and blood pressure homeostasis in euthyroid subjects. J Clin Endocrinol Metab. 2004;89(7):3455–3461. doi:10.1210/jc.2003-032143
35. Jun JE, Jin SM, Jee JH, et al. TSH increment and the risk of incident type 2 diabetes mellitus in euthyroid subjects. Endocrine. 2017;55(3):944–953. doi:10.1007/s12020-016-1221-1
36. Ortega E, Koska J, Pannacciulli N, Bunt JC, Krakoff J. Free triiodothyronine plasma concentrations are positively associated with insulin secretion in euthyroid individuals. Eur J Endocrinol. 2008;158(2):217–221. doi:10.1530/EJE-07-0592
37. Lekakis J, Papamichael C, Alevizaki M, et al. Flow-mediated, endothelium-dependent vasodilation is impaired in subjects with hypothyroidism, borderline hypothyroidism, and high-normal serum thyrotropin (TSH) values. Thyroid. 1997;7(3):411–414. doi:10.1089/thy.1997.7.411
38. Villanueva I, Alva-Sanchez C, Pacheco-Rosado J. The role of thyroid hormones as inductors of oxidative stress and neurodegeneration. Oxid Med Cell Longev. 2013;2013:218145. doi:10.1155/2013/218145
39. Porcu E, Medici M, Pistis G, et al. A meta-analysis of thyroid-related traits reveals novel loci and gender-specific differences in the regulation of thyroid function. PLoS Genet. 2013;9(2):e1003266. doi:10.1371/journal.pgen.1003266
40. Mauvais-Jarvis F, Clegg DJ, Hevener AL. The role of estrogens in control of energy balance and glucose homeostasis. Endocr Rev. 2013;34(3):309–338. doi:10.1210/er.2012-1055
41. Moura Neto A, Parisi MC, Alegre SM, Pavin EJ, Tambascia MA, Zantut-Wittmann DE. Relation of thyroid hormone abnormalities with subclinical inflammatory activity in patients with type 1 and type 2 diabetes mellitus. Endocrine. 2016;51(1):63–71. doi:10.1007/s12020-015-0651-5
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


