Back to Journals » Nature and Science of Sleep » Volume 15
Association Between Metabolic-Associated Fatty Liver Disease and Obstructive Sleep Apnea: A Cross-Sectional Study
Authors Huang J , Chen L , Li X, Chen M, Lin T, Chen G
Received 9 December 2022
Accepted for publication 20 February 2023
Published 28 February 2023 Volume 2023:15 Pages 49—57
DOI https://doi.org/10.2147/NSS.S398971
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Ahmed BaHammam
Jiefeng Huang,1– 3,* Lida Chen,4,* Xiuling Li,1– 3,* Menglan Chen,1– 3 Ting Lin,1– 3 Gongping Chen1– 3
1Department of Respiratory and Critical Care Medicine, the First Affiliated Hospital of Fujian Medical University, Fujian Medical University, Fuzhou, People’s Republic of China; 2Institute of Respiratory Disease, Fujian Medical University, Fujian Medical University, Fuzhou, People’s Republic of China; 3Department of Respiratory and Critical Care Medicine, National Regional Medical Center, Binhai Campus of the First Affiliated Hospital, Fujian Medical University, Fuzhou, People’s Republic of China; 4Department of Respiratory and Critical Care Medicine, Zhangzhou Affiliated Hospital of Fujian Medical University, Xiangcheng, Zhangzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Gongping Chen, Tel/Fax +86591-87981697, Email [email protected]
Aim: Emerging evidence has revealed that obstructive sleep apnea (OSA) is an independent risk factor for the development of a variety of adverse metabolic disease states. In this study, we evaluated the association between OSA severity and metabolic dysfunction-associated fatty liver disease (MAFLD) among Asian populations.
Materials and Methods: This was a cross-sectional, single-center study. The study cohort consisted of patients undergoing polysomnography and abdominal ultrasonography. Logistic regression analysis was used to evaluate the independent risk factors of MAFLD in patients with OSA.
Results: A total of 1065 patients (277 non-MAFLD and 788 MAFLD) were included in the study. The prevalence of MAFLD in non-OSA, mild-moderate OSA, and severe OSA patients was 58.16%, 72.41%, and 78.0%, respectively (p < 0.001). We identified significant differences in body mass index (BMI), apnea-hypopnea index (AHI), oxygen desaturation index (ODI), and lowest O2 saturation (LaSO2) between non-MAFLD and MAFLD patients (all p < 0.001). After adjusting for confounding variables, we used multivariate regression analysis to show that BMI, ODI, and triglyceride (TG) levels independently predicted the occurrence of MAFLD (odds ratio [OR] = 1.234, p < 0.001; OR = 1.022, p = 0.013; OR = 1.384, p = 0.001, respectively). Moreover, stratified analysis according to BMI indicated that TG levels were the predominant risk factor for MAFLD in a group of patients with a BMI < 23 kg/m2, while BMI, ODI, TG levels, and total cholesterol (TC) were the major risk factors for MAFLD in a group of patients with a BMI ≥ 23 kg/m2 (all p < 0.05).
Conclusion: OSA-associated chronic intermittent hypoxia was independently associated with the risk of MAFLD, especially in OSA patients with a BMI ≥ 23 kg/m2, suggesting that oxidative stress might play an important role in the pathogenesis of MAFLD in patients with OSA.
Keywords: obstructive sleep apnea, chronic intermittent hypoxia, metabolic dysfunction-associated fatty liver disease, obesity
Introduction
Obstructive sleep apnea (OSA) is an increasingly common disorder that is characterized by periodic collapse of the upper airway, with consequent hypoxia or arousals during sleep. It has been reported that globally 936 million adults aged 30–69 may have mild to severe OSA, and 425 million may have moderate to severe OSA.1 Evidence has shown that OSA can contribute to the development of cardiovascular diseases, resulting in an increase in morbidity and mortality rates, and a decrease in quality-of-life scores.2–4 Metabolic abnormalities (such as obesity, hyperglycemia, dyslipidemia, and the metabolic syndrome) are associated with cardiovascular disease. OSA is strongly associated with obesity, insulin resistance, type two diabetes (T2DM), nonalcoholic fatty liver disease (NAFLD), and other related metabolic diseases;5 therefore, OSA may exacerbate the cardiometabolic risk associated with these metabolic abnormalities.6 Lipid peroxidation, induced by chronic intermittent hypoxia (CIH), results in the increased production of reactive oxygen species, which leads to hepatic inflammation and fibrosis, resulting in nonalcoholic steatohepatitis.7
During the 2000s, evidence emerged that linked OSA severity with the development and progression of NAFLD. For example, CIH (a hallmark of OSA) was identified as an independent risk factor in the onset and progression of NAFLD.7 The essential links between CIH and NAFLD involve insulin resistance, glucose dysregulation, dysfunction of key steps in hepatic lipid metabolism, oxidative stress, and hepatic steatosis and fibrosis, which together indicate that metabolic dysfunction plays a significant role in the pathogenesis of CIH-associated NAFLD.7 Recently, a consensus of international experts has proposed a change in nomenclature from NAFLD to metabolic dysfunction-associated fatty liver disease (MAFLD), which more accurately reflects the current knowledge the association between fatty liver diseases and metabolic dysfunction.8 Their consensus statement suggests that the diagnosis of MAFLD should be based on evidence of fat accumulation in the liver (hepatic steatosis), plus any one of the three following criteria: overweight/obesity, the presence of T2DM, or evidence of metabolic dysregulation.9
OSA is therefore closely related to metabolic disorders and NAFLD, leading us to speculate that OSA may contribute to the development of MAFLD. In this cross-sectional comparative study, we aimed to investigate the relationship between MAFLD and OSA severity, and to clarify the role of OSA as a risk factor for MAFLD and the influence of obesity in patients with these conditions.
Methods
Study Population
Patients who attended our sleep center from January 2013 to December 2020 that presented with a clinical suspicion of sleep-disordered breathing, including snoring, sleepiness and witnessed sleep apnea, were enrolled in the study. We selected subjects using complete abdominal imaging, then recorded their sleep symptoms, value on the Epworth sleepiness scale (ESS), history of alcohol consumption and smoking, medical history, and current medications. Patients who were previously diagnosed with or treated for OSA were excluded. Other exclusion criteria included: current use of hepatotoxic drugs (including some Chinese herbal medicines or chemotherapeutic drugs); severe cardiopulmonary chronic disease requiring hospitalization; acute inflammatory disease; or other sleep disorders such as restless leg syndrome or narcolepsy. This study complied with the Declaration of Helsinki. It was approved by the ethics committee of the First Affiliated Hospital of Fujian Medical University (Fuzhou, China), and all participants provided their written consent.
Anthropometric and Clinical Assessment
Body mass index (BMI) was calculated as: weight (kg; without heavy clothing) divided by the square of height (m). Waist circumference (WC; measured midway between the lower rib margin and the iliac crest) and neck circumference (NC; measured at mid-neck height between the mid-cervical spine to mid-anterior neck) were measured to within 1 mm. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured by an automated sphygmomanometer on the right arm in the sitting position after 5 minutes of rest. Blood samples were taken in the morning after a 12-hour fast and were analyzed to measure blood glucose, liver function (including alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), total bilirubin (T. bilirubin) and gamma glutamyl transferase (GGT)), and lipid profile (including levels of triglycerides (TG), total cholesterol (TC), high-density lipoprotein-cholesterol (HDL-C), and low-density lipoprotein- cholesterol (LDL-C)). All biochemical parameters were measured using the Modular P800 autoanalyzer (Roche, Tokyo, Japan). High-sensitivity C-reactive protein (hs-CRP) was analyzed using a BNII nephelometer (Dade Behring, Deerfield, IL, USA).
Definition of MAFLD
The diagnosis of MAFLD was based on the presence of hepatic steatosis (diagnosed by ultrasound) as well as the presence of one or more of the following symptoms: overweight/obesity (defined as having BMI ≥ 23 kg/m2); presence of T2DM; or evidence of metabolic dysregulation. Metabolic dysregulation was defined as the presence at least two of the following metabolic risk abnormalities: (A) a waist circumference ≥ 102 cm in men or 88 cm in women; (B) blood pressure ≥ 130/85 mmHg or a specific drug treatment; (C) TG ≥ 1.70 mmol/L or a specific drug treatment; (D) HDL-C < 1.0 mmol/L for men or < 1.3 mmol/L for women or a specific drug treatment; (E) pre-diabetes (defined by fasting glucose (Glu) levels from 5.6–6.9 mmol/L, 2-hour post-load Glu levels from 7.8–11.0 mmol/L, or glycated hemoglobin levels from 5.7–6.4%); (F) a HOMA-IR score ≥ 2.5; or (G) hs-CRP level > 2 mg/L. Patients who did not meet these criteria were defined as non-MAFLD.
Polysomnography Evaluation
All individuals underwent a full night (22:00 to 06:00) polysomnography testing (P Series Sleep System, Compumedics, Melbourne, Australia) to assess the following parameters: electroencephalogram, electrooculogram, submental and anterior tibialis electromyogram, electrocardiogram, arterial oxyhemoglobin saturation with pulse oximetry, nasal airflow, thoracoabdominal movement, snoring, and body position. We used the American Academy of Sleep Medicine (AASM) manual to score sleep and associated events,10 including the following indices: apnea-hypopnea index (AHI; representing the number of episodes of apnea and hypopnea per hour of sleep); oxygen desaturation index (ODI; 3% oxygen desaturation index per hour of sleep); LaSO2 (lowest arterial oxyhemoglobin saturation during sleep); mean SpO2 (mean arterial oxyhemoglobin saturation during sleep); and TS90% (% sleep time in which SpO2 < 90%). Apnea was defined as decrements in airflow ≥ 90% from baseline for ≥ 10s, measured using an oronasal thermal sensor. Hypopnea was defined as a ≥ 30% decrease in flow lasting ≥ 10s associated with ≥ 4% oxyhemoglobin desaturation, or as a ≥ 50% decrease in flow lasting ≥ 10s associated with ≥ 3% oxygen desaturation. Participants were classified according to their AHI levels as non-OSA (AHI < 5/h), mild-moderate OSA (AHI from 5–30/h), or severe OSA (AHI > 30/h).
Statistical Analysis
Data were analyzed using SPSS 26.0. The sample size was determined for this study using the Power Analysis and Sample Size (PASS) software v21.0.3. The cross-sectional study was planned to have 80% statistical power, with a two--sided α error of 5% and β error of 20%. The proportion of MAFLD was assumed to be 60% for OSA based on our previous research on the relationship between OSA and NAFLD.11 Based on these calculations, sample size calculation was performed and the required sample size was 95 for each group. Normally distributed continuous variables are presented as means with standard deviations (SD), and were analyzed using a Student’s t-test or an ANOVA test. Skewed variables are expressed as medians with interquartile ranges (IQR), and were analyzed using the Mann–Whitney U-test or the Kruskal–Wallis test. Categorical variables are expressed as frequencies and proportions, and differences were assessed using the chi-square test or Fisher’s exact test to determine significance. Multivariate logistic regression was used to identify independent risks of MAFLD. All p values were two-tailed, and the significance threshold was p < 0.05.
Results
A total of 1065 subjects, including 883 men and 182 women, were enrolled in the study, comprising 277 non-MAFLD and 788 MAFLD patients. The mean age for all study participants was 49.79 years, and the mean BMI was 27.41 kg/m2. When categorized into non-OSA, mild-moderate OSA, and severe OSA groups, the mean ages were 48.39 years, 51.16 years, and 49.00 years (p = 0.031) respectively, and the mean BMIs for the three groups were 25.63 kg/m2, 26.71 kg/m2, and 28.26 kg/m2 (p < 0.001).
Table 1 displays the main clinical characteristics of subjects, according to the severity of OSA. Polysomnographic parameters, including AHI, ODI, and T90%, increased with OSA severity, while LaSO2 and average SpO2 decreased with OSA severity (all p < 0.001). BMI, neck circumference, and waist circumference increased with OSA severity (all p < 0.001). The prevalence of MAFLD in the non-OSA, mild-moderate OSA, and severe OSA groups was 57 out of 98 (58.16%), 302 out of 417 (72.41%), and 430 out of 550 (78.0%), respectively. These differences were found to be statistically significant (p < 0.001) (Figure 1).
 |
Table 1 Comparison of Main Clinical Characteristics of Subjects According to the Severity of OSA |
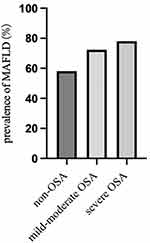 |
Figure 1 Shows the prevalence of MAFLD among patients, according to the severity of OSA. |
As shown in Table 2, patients with MAFLD were more likely to be young, male, and tended to be heavier compared with non-MAFLD patients (with a BMI of 25.17± 3.38 kg/m2 compared with 28.20 ± 4.06 kg/m2, p < 0.001). Patients with MAFLD also had a higher prevalence of metabolic comorbidities (diabetes, diastolic hypertension, hyperlipidemia), compared with those in the non-MAFLD group (all p < 0.001). In terms of polysomnographic parameters, the MAFLD group had a higher AHI (35.10/h compared with 24.00/h, p < 0.001) and ODI (26.45/h compared with 15.90/h, p < 0.001), and a lower LaSO2 (77% compared with 81%, p < 0.001).
 |
Table 2 Comparison of Basic Characteristics and Sleep Parameters in Patients with Non-MAFLD and MAFLD |
Table 3 shows the metabolic and liver function parameters of non-MAFLD and MAFLD patients. MAFLD patients typically had multiple metabolic disorders, including increased TC, TG, LDL, fasting glucose, glycated hemoglobin, and decreased HDL-C compared with non-MAFLD patients (all p < 0.001). Additionally, the liver enzymes ALT, AST, and GGT were present at significantly higher levels in patients in the MAFLD group (all p < 0.001). There were no statistically significant differences in terms of levels of creatinine, blood urea nitrogen, total bilirubin, and alkaline phosphatase between the two groups.
 |
Table 3 Comparison of Biochemical Characteristics in Patients with Non-MAFLD and MAFLD |
Finally, multivariate regression analyses suggested that BMI, ODI and TG levels were independent predictors for MAFLD, after adjusting for confounding variables (the odds ratios [OR] were 1.234, p < 0.001; 1.022, p = 0.013; and 1.384, p = 0.001, respectively). To further understand the association between MAFLD and obesity in patients with OSA we also performed multivariable logistic regression analyses, stratified by BMI. In the group that had a BMI < 23 kg/m2, TG levels were an independent risk factor for MAFLD (OR = 3.808, p = 0.013), while in the group with a BMI ≥ 23 kg/m2, BMI, ODI, TG levels, and TC were independent risk factors for MAFLD (OR = 1.191, p < 0.001; OR = 1.020, p = 0.043; OR = 1.281, p = 0.008, respectively) (Table 4).
 |
Table 4 Multivariate Logistic Regression Analysis of OSA and MAFLD |
Discussion
The results of this cross-sectional analysis suggest that patients with a higher degree of OSA severity showed a higher prevalence of MAFLD, and that BMI, ODI, and TG levels could be used as independent predictors for MAFLD. Furthermore, stratified analysis according to BMI indicated that ODI was only significantly correlated with MAFLD in patients with a BMI ≥ 23 kg/m2, after adjusting for other confounding parameters. Therefore, this study provides key information about the clinical significance of MAFLD in patients with established OSA.
OSA is a factor that may contribute to the development of nonalcoholic fatty liver disease (NAFLD). Studies in both rodents and humans have suggested that OSA and CIH are involved in the pathogenesis and exacerbation of NAFLD.12–14 OSA and NAFLD appear to share common intermediary mechanisms including insulin resistance, inflammation, and oxidative stress, and successful treatment of OSA was shown to slow the progression of NAFLD.15 It has therefore been proposed to screen patients with OSA for NAFLD, and NAFLD patients for OSA.16 In 2020, a panel of international experts proposed to rename NAFLD to MAFLD,9 which was considered to better emphasize the role of metabolic dysfunction in the disease. The disease pathophysiology is closely related to metabolic syndrome, T2DM, and obesity, and so this term may lead to a more precise recognition and comprehension of fatty liver disease, which would be beneficial to basic research, clinical practice, and public health.8 Some researchers think that OSA may in fact be a manifestation of metabolic syndrome, because of its independent associations with each of the components of metabolic syndrome, particularly visceral obesity, hypertension, insulin resistance and dyslipidemia.17,18 Therefore, we considered whether OSA was associated with MAFLD. In this study, we found that the prevalence of MAFLD tended to increase in correlation with the severity of OSA, reaching 78% in patients with severe OSA. This indicates that OSA may promote the development of MAFLD. Recently, a study involving a cohort of patients with chronic liver disease who underwent screening polysomnographies found that patients with MAFLD have a higher prevalence of OSA, a higher likelihood of severe OSA, and more severe nocturnal desaturation compared with non-MAFLD patients.19 In agreement with findings by Tomar et al,19 our study found that patients with MAFLD had higher levels of AHI and ODI, as well as worse nocturnal desaturation parameters. We further found that ODI was an independent risk factor for MAFLD.
Various factors contribute to the development and progression of MAFLD, including genetic background, alterations of the gut microbiota, and increased insulin resistance in the adipose tissue and skeletal muscle, which affects hepatic lipid metabolism and contributes to further hepatic fat accumulation and inflammation.20 OSA can induce dysfunction of multiple organs, predominantly due to the repetitive occurrence of hypoxia-reoxygenation, which results in constant low-grade inflammation, insulin resistance, sympathetic overactivity and oxidative stress.21 In this study, we identified ODI, which is regarded as a marker of CIH, to be independently associated with MAFLD. This suggests that CIH-dependent deleterious effects (including increased oxidative stress, inflammation, insulin resistance, and lipid metabolism disorder) may be responsible for some aspects of the pathophysiology of MAFLD in patients with OSA. However, it is possible that other underlying factors, such as obesity or dyslipidemia, may be either mediators or confounders of MAFLD; therefore, we further examined the independent risk factors for MAFLD among two patient subgroups with different BMIs. We found that neither ODI nor BMI were associated with MAFLD in patients with a BMI < 23 kg/m2 (after stratification by BMI). This might be because obese patients may tend to have more severe OSA. A better understanding of the interaction between OSA and MAFLD is required to fully understand the links between the two disease states, and to determine the efficacy of OSA interventions, such as CPAP treatment and weight control, on MAFLD.
One important aspect of MAFLD progression is the pathological accumulation of triglycerides and other lipids in hepatocytes.22 Emerging clinical research suggests that most patients with MAFLD exhibit atherogenic dyslipidemia, characterized by high plasma triglyceride concentrations.23,24 Consistent with the these data, our study also found that TG levels were associated with MAFLD in patients with OSA, regardless of patient BMI. This suggests that lean MAFLD patients may also undergo severe visceral fat accumulation. A study from Tang et al showed that triglycerides may also play a causal role in the pathogenesis of OSA, due to the hydrophobic structure of the TG resulting in its aggregation and deposition in extracellular fluids, increasing the risk of OSA even without the confounding effects of obesity. Therefore, intervention to prevent changes in triglyceride levels may also help to reduce the risk of MAFLD and OSA.25
There are several limitations to this study. First, this study was a cross-sectional and single-center study on an Asian population, which could not demonstrate a direct causal relationship between OSA and MAFLD. Further well-designed, prospective, and multi-center studies investigating this possible association are therefore required. Second, NAFLD was diagnosed using ultrasonography rather than through biopsy. However, according to new guidelines, ultrasonography is recommended for the diagnosis of MAFLD, and has been determined to have acceptable accuracy and to correlate with histological findings.9 Third, this study did not assess the influence of treatment of OSA on MAFLD, which would be valuable to further understand the relationship between CIH and MAFLD. Finally, sleep duration has also been shown to be independently associated with an increased risk of NAFLD;26 however, this study did not analyze the correlation between sleep fragmentation and liver injury.
Conclusions
In summary, our research demonstrated that the prevalence of MAFLD increased with OSA severity, and identified BMI, ODI and TG levels as factors that may promote MAFLD in patients with OSA. Therefore, interventions such as treatment of OSA, weight control, and the regulation of lipid metabolism could be key to reduce the risk of MAFLD in patients with OSA, especially in those with a BMI ≥ 23 kg/m2. Future prospective cohort studies are needed to closely examine the risk of patients with OSA developing MAFLD.
Data Sharing Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the First Affiliated Hospital of Fujian Medical University.
Acknowledgments
We thank Alison Inglis, PhD, from Liwen Bianji (Edanz) for editing the English text of a draft of this manuscript.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The work was supported by the National Natural Science Foundation of China (No. 82070088) and Natural Science Foundation of Fujian Province (No. 2020J01985).
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Benjafield AV, Ayas NT, Eastwood PR, et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir Med. 2019;7(8):687–698. doi:10.1016/S2213-2600(19)30198-5
2. Querejeta Roca G, Redline S, Punjabi N, et al. Sleep apnea is associated with subclinical myocardial injury in the community. The ARIC-SHHS study. Am J Respir Crit Care Med. 2013;188(12):1460–1465. doi:10.1164/rccm.201309-1572OC
3. Dredla BK, Castillo PR. Cardiovascular consequences of obstructive sleep apnea. Curr Cardiol Rep. 2019;21(11):137. doi:10.1007/s11886-019-1228-3
4. Povitz M, Bolo CE, Heitman SJ, Tsai WH, Wang J, James MT. Effect of treatment of obstructive sleep apnea on depressive symptoms: systematic review and meta-analysis. PLoS Med. 2014;11(11):e1001762.
5. Li M, Li X, Lu Y. Obstructive sleep apnea syndrome and metabolic diseases. Endocrinology. 2018;159(7):2670–2675. doi:10.1210/en.2018-00248
6. Drager LF, Togeiro SM, Polotsky VY, Lorenzi-Filho G. Obstructive sleep apnea: a cardiometabolic risk in obesity and the metabolic syndrome. J Am Coll Cardiol. 2013;62(7):569–576. doi:10.1016/j.jacc.2013.05.045
7. Mesarwi OA, Loomba R, Malhotra A. Obstructive sleep apnea, hypoxia, and nonalcoholic fatty liver disease. Am J Respir Crit Care Med. 2019;199(7):830–841. doi:10.1164/rccm.201806-1109TR
8. Eslam M, Sanyal AJ, George J. MAFLD: a consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology. 2020;158(7):1999–2014 e1991. doi:10.1053/j.gastro.2019.11.312
9. Eslam M, Newsome PN, Sarin SK, et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol. 2020;73(1):202–209. doi:10.1016/j.jhep.2020.03.039
10. Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM manual for the scoring of sleep and associated events. deliberations of the sleep apnea definitions task force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8(5):597–619. doi:10.5664/jcsm.2172
11. Qi JC, Huang JC, Lin QC, et al. Relationship between obstructive sleep apnea and nonalcoholic fatty liver disease in nonobese adults. Sleep Breath. 2016;20(2):529–535. doi:10.1007/s11325-015-1232-9
12. Savransky V, Bevans S, Nanayakkara A, et al. Chronic intermittent hypoxia causes hepatitis in a mouse model of diet-induced fatty liver. Am J Physiol Gastrointest Liver Physiol. 2007;293(4):G871–877. doi:10.1152/ajpgi.00145.2007
13. Agrawal S, Duseja A, Aggarwal A, et al. Obstructive sleep apnea is an important predictor of hepatic fibrosis in patients with nonalcoholic fatty liver disease in a tertiary care center. Hepatol Int. 2015;9(2):283–291. doi:10.1007/s12072-015-9615-3
14. Chen LD, Chen MX, Chen GP, et al. Association between obstructive sleep apnea and non-alcoholic fatty liver disease in pediatric patients: a meta-analysis. Pediatr Obes. 2021;16(3):e12718. doi:10.1111/ijpo.12718
15. Kim D, Ahmed A, Kushida C. Continuous positive airway pressure therapy on nonalcoholic fatty liver disease in patients with obstructive sleep apnea. J Clin Sleep Med. 2018;14(8):1315–1322. doi:10.5664/jcsm.7262
16. Aron-Wisnewsky J, Clement K, Pepin JL. Nonalcoholic fatty liver disease and obstructive sleep apnea. Metabolism. 2016;65(8):1124–1135. doi:10.1016/j.metabol.2016.05.004
17. Vgontzas AN, Bixler EO, Chrousos GP. Sleep apnea is a manifestation of the metabolic syndrome. Sleep Med Rev. 2005;9(3):211–224. doi:10.1016/j.smrv.2005.01.006
18. Tasali E, Ip MS. Obstructive sleep apnea and metabolic syndrome: alterations in glucose metabolism and inflammation. Proc Am Thorac Soc. 2008;5(2):207–217. doi:10.1513/pats.200708-139MG
19. Tomar A, Bhardwaj A, Choudhary A, Bhattacharyya D. Association of obstructive sleep apnea with nocturnal hypoxemia in metabolic-associated fatty liver disease patients: a cross-sectional analysis of record-based data. J Family Med Prim Care. 2021;10(8):3105–3110. doi:10.4103/jfmpc.jfmpc_412_21
20. Sakurai Y, Kubota N, Yamauchi T, Kadowaki T. Role of Insulin Resistance in MAFLD. Int J Mol Sci. 2021;22(8):4156. doi:10.3390/ijms22084156
21. Arnaud C, Dematteis M, Pepin JL, Baguet JP, Levy P. Obstructive sleep apnea, immuno-inflammation, and atherosclerosis. Semin Immunopathol. 2009;31(1):113–125. doi:10.1007/s00281-009-0148-5
22. Heeren J, Scheja L. Metabolic-associated fatty liver disease and lipoprotein metabolism. Mol Metab. 2021;50:101238.
23. Cheng YM, Kao JH, Wang CC. The metabolic profiles and body composition of lean metabolic associated fatty liver disease. Hepatol Int. 2021;15(2):405–412. doi:10.1007/s12072-021-10147-0
24. Chen YL, Li H, Li S, et al. Prevalence of and risk factors for metabolic associated fatty liver disease in an urban population in China: a cross-sectional comparative study. BMC Gastroenterol. 2021;21(1):212.
25. Tang H, Zhou Q, Zheng F, Wu T, Tang YD, Jiang J. The Causal Effects of Lipid Profiles on Sleep Apnea. Front Nutr. 2022;9:910690. doi:10.3389/fnut.2022.910690
26. Um YJ, Chang Y, Jung HS, et al. Sleep duration, sleep quality, and the development of nonalcoholic fatty liver disease: a cohort study. Clin Transl Gastroenterol. 2021;12(10):e00417.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
