Back to Journals » Journal of Inflammation Research » Volume 16
Association Between Mean Platelet Volume and Benign Prostatic Hyperplasia: A Population Study from the TCLSIH Cohort Study
Authors Song Y, Gu Y, Guo H, Yang H, Wang X, Wu H, Wang A, Wang M, Wang H, Zhang Q, Liu L, Meng G, Liu B, Niu K
Received 10 April 2023
Accepted for publication 11 July 2023
Published 5 August 2023 Volume 2023:16 Pages 3259—3269
DOI https://doi.org/10.2147/JIR.S416404
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tara Strutt
Yanqi Song,1 Yeqing Gu,2 Honglei Guo,3 Honghao Yang,4– 6 Xuena Wang,4– 6 Hongmei Wu,4– 6 Aidi Wang,1 Mengxiao Wang,7 Haijin Wang,1 Qing Zhang,8 Li Liu,8 Ge Meng,6,8– 10 Baoshan Liu,1 Kaijun Niu4– 6
1Department of Traditional Chinese Medicine, Tianjin Medical University General Hospital, Tianjin, People’s Republic of China; 2Institute of Radiation Medicine, Chinese Academy of Medical Sciences & Peking Union Medical College, Tianjin, People’s Republic of China; 3Tianjin University of Traditional Chinese Medicine, Tianjin, People’s Republic of China; 4School of Public Health of Tianjin University of Traditional Chinese Medicine, Tianjin, People’s Republic of China; 5School of Integrative Medicine, Tianjin University of Traditional Chinese Medicine, Tianjin, People’s Republic of China; 6Nutritional Epidemiology Institute and School of Public Health, Tianjin Medical University, Tianjin, People’s Republic of China; 7Binzhou Hospital of Chinese Medicine, Shandong, People’s Republic of China; 8Health Management Centre, Tianjin Medical University General Hospital, Tianjin, People’s Republic of China; 9Tianjin Key Laboratory of Environment, Nutrition and Public Health, Tianjin, People’s Republic of China; 10Department of Toxicology and Sanitary Chemistry, School of Public Health, Tianjin Medical University, Tianjin, People’s Republic of China
Correspondence: Kaijun Niu; Baoshan Liu, Nutritional Epidemiology Institute and School of Public Health, Tianjin Medical University, 22 Qixiangtai Road, Heping District, Tianjin, 300070, People’s Republic of China, Tel +86-22-83336613, Email [email protected]; [email protected]; [email protected]
Purpose: This study aimed to prospectively investigate the association between mean platelet volume (MPV) levels and risk of benign prostatic hyperplasia (BPH) in a general Chinese adult male population, and assessed this association in metabolic syndrome (MetS) patients.
Patients and methods: This study included a total of 14,923 male participants free from BPH at baseline. MPV was measured by the method of laser-based flow cytometric impedance according to the complete blood sample. BPH was defined as total prostate volume (TPV) ≥ 30 mL, TPV was determined by transabdominal ultrasonography. Multivariable Cox proportional hazards models were fitted to calculate hazards ratios (HRs) and corresponding 95% confidence intervals (CIs) for BPH risk with NLR levels.
Results: During a median follow-up of 2.7 years, 4848 BPH cases were documented in total male participants, and 1787 BPH cases were documented in MetS participants. After adjusting for age, body mass index, smoking, alcohol and personal and family history of disease, the multivariable-adjusted HRs of BPH were 1.00 (reference), 1.03 (95% CIs 0.96, 1.11), 1.00 (95% CIs 0.92, 1.08) and 0.98 (95% CIs 0.90, 1.06), respectively, for participants with MPV in the 1st, 2nd, 3rd and 4th quartiles (P for trend = 0.47). In MetS patients, the multivariable-adjusted HRs of BPH were 1.00 (reference), 1.03 (95% CIs 0.90, 1.16), 0.99 (95% CIs 0.87, 1.14) and 1.01 (95% CIs 0.89, 1.15) (P for trend= 0.98), respectively.
Conclusion: A non-significant association was observed between MPV levels and risk of BPH, and no association in this association in MetS patients. Our findings support the notion that MPV levels may not be a target for BPH prevention and intervention.
Keywords: mean platelet volume, inflammation, benign prostatic hyerplasia, metabolic syndrome, prospective cohort study
Introduction
Prostate cancer (PC) is the fourth most frequent type of cancer in humans (7.3% of cases) and the second most lethal type of cancer in males.1 Benign prostatic hyperplasia (BPH) is a histological condition that causes in benign prostate gland enlargement and is a risk factor for developing PC.2,3 In BPH, the transition zone (TZ) and periurethral area exhibit uncontrollable proliferation of epithelial and fibromuscular tissue.4 Poor urine flow, frequent urination, difficulty starting the flow, post-void dribbling, and nocturia are some symptoms that may occur in men with BPH.4 Metabolic syndrome (MetS), a risk factor for cardiovascular disease (CVD), refers to a group of disorders including abdominal obesity, diabetes mellitus, hypertension, low high-density lipoprotein cholesterol (HDL-C), and hypertriglyceridemia, with insulin resistance as a potentially defining characteristic.5 MetS also play a significant role in the development and progression of BPH, according to recent studies.6,7
There is still much to learn about the pathophysiology and etiology of BPH. Adorini et al hypothesized that BPH was significantly influenced by inflammation.8 Widespread inflammatory infiltrates were present in BPH lesions, and the cytokines and growth factors secreted by these inflammatory cells may stimulate the proliferation of stromal and epithelial cells.2 BPH is believed to be an immune-mediated inflammatory illness, while the specific timing and causes of chronic inflammation are unknown.9
Mean platelet volume (MPV) is a fundamental metric of platelet size that has been found as a biomarker of platelet activity.10 Inflammation has reportedly been linked to some platelet indicators, such as MPV.10 The findings of many cross-sectional11 and case-control studies12 in the male population studying the association between MPV levels and BPH are equivocal. Due to the fact that MPV and BPH have the same pathophysiological mechanisms, increased MPV has largely been accepted as a proxy biomarker of low-grade systemic inflammation and endothelial dysfunction.13 Be that as it may, no epidemiologic data on the association between MPV levels and the risk of BPH are available.
As a result, we conducted a prospective cohort study in a large-scale Chinese adult male population to investigate how MPV levels are connected to the risk of BPH, and we tested this association in MetS patients.
Methods
Study Design and Participants
The Tianjin Chronic Low-grade Systemic Inflammation and Health (TCLSIH) Cohort Study, a prospective dynamic cohort that started in 2007 to investigate into the association between chronic low-grade inflammation and health status, is the foundation of this prospective study. Detailed discussions of the TCLSIH cohort study have already been provided.14,15 Participants in the study had to be 18 years of age or older and had lived in Tianjin, China, for at least five years. Between January 2010 and December 2017, participants responded to questionnaires about their smoking and alcohol consumption habits, as well as their disease histories. A comprehensive lifestyle questionnaire that includes personal details, nutritional intake, lifestyle characteristics, and health status has also been given to randomly selected participants from this population from May 2013. The Institutional Review Board of Tianjin Medical University approved the study protocol, and each participant gave written informed consent.
The TCLSIH study database from January 2010 to December 2020 was examined for the current study. A total of 35,238 Chinese adult male participants are included in this study. We excluded 4828 participants who were lost to follow-up (follow-up rate: 86.3%) from this group. We also excluded participants with other prostate diseases (n=1682) and those with CVD (n=1475), cancer (n=1362), or did not undergo MPV counts (n=2491), health examinations (n=3656), had BPH or a prior history of treatment for BPH (n= 4821) at baseline. 14,923 people made up the final study sample after these exclusions (mean [standard deviation] age: 44.0 [10.3] years). Figure 1 depicts the study population flow chart.
 |
Figure 1 Study population flow chart. Abbreviations: BPH, benign prostatic hyperplasia; MPV, mean platelet volume. Note: BPH was defined as TPV ≥ 30 mL. |
Definition of Newly Diagnosed BPH
The gold standard for prostate assessment is prostate pathological cytology; be that as it may, it is not suggested for large-scale population studies due to its invasiveness, the risk of complications, and significant expense.16 Previous studies have investigated the relative adequacy of transabdominal and transrectal ultrasonographies for prostatic assessment, supplanting the transrectal studies with less expensive, simpler and less invasive transabdominal studies in large-scale population screening.17 BPH was defined as total prostate volume (TPV) ≥ 30 mL as indicated by a previous study.18 TPV was determined by transabdominal ultrasonography (7–12 MHz, Royal Philips) using the formula for an elliptical volume [height (cm) ×width (cm) × length (cm) × π/6].19 Transabdominal ultrasonographies with TPV measurements were performed by a single high-volume radiologist, who performs roughly 4800 such assessments every year.
Assessment of MPV
The following morning, venous blood samples from the participants were taken after a minimum 12-hour overnight fast. For the purpose of measuring MPV, blood was drawn and placed into tripotassium ethylenediaminetetraacetic acid (EDTA) (7.2 mg) tubes. Lance et al demonstrated that 120 minutes after venipuncture is the ideal time to estimate MPV.20 An automatic hematology analyzer was used in this study to examine blood samples 120 minutes after venipuncture. A flow cytometric impedance method based on lasers was used to measure MPV in participants. We evaluated these hematological parameters as both quartiles and continuous variables to determine the specific relationship between them and the presence of BPH, allowing us to more effectively and flexibly use the available data.
Assessment of Other Variables
A series of physical measurements and physiological tests were performed on all participants during standardized physical examinations.21 The participants were asked to stand without shoes while their height (to the nearest 0.1 cm) and weight (to the nearest 0.1 kg) were recorded. BMI was calculated by dividing weight (kg) by height squared (m2). Waist circumference was also measured according to a recognized methodology (to the nearest 0.1 cm).
Trained nurses drew fasting blood samples from the participants’ antecubital veins. Fasting blood samples were analyzed for fasting blood glucose (FBG), blood lipids (including triglycerides [TG], total cholesterol [TC], low-density lipoprotein cholesterol [LDL-C], and HDL-C), alanine aminotransferase (ALT), and high-sensitivity C-reactive protein (hsCRP). Diabetes mellitus was characterized as FBG ≥ 7.0 mmol/L or having a self-reported history of diabetes mellitus.22 Hyperlipidemia was characterized as TC ≥ 5.17 mmol/L or TG ≥ 1.7 mmol/L or LDL-C ≥ 3.37 mmol/L or taking antilipemic medications.23 The TM-2655 oscillometric equipment (A&D Company, Ltd., Tokyo, Japan) was used by trained nurses to measure blood pressure at least twice.24 Participants rested for at least 5 minutes in a quiet environment before taking their blood pressure readings, and then kept their upper right arms at heart level, feet on the ground, and backs supported.25 At least one minute passed between the measurements. Each participant’s blood pressure was determined by averaging the two closest readings. Systolic blood pressure ≥ 140 mmHg, diastolic blood pressure ≥ 90 mmHg, or a history of hypertension were all considered to be characteristics of hypertension.26
Social and demographic variables, including age, smoking and alcohol consumption habits, educational level, occupation, monthly household income, and individual and familial history of disease, were gathered using a health status questionnaire. A shortened version of the International Physical Activity Questionnaire (IPAQ), which collects data on the number of minutes spent in vigorous-intensity activities, moderate-intensity activities, strolling, and sitting over the previous seven days, was used to quantify physical activity (PA) (h/week).27 The following method was used to calculate the number of metabolic equivalent (MET) hours per week (MET hours/week): MET coefficient of activity × duration (hours) × frequency (days), the corresponding MET coefficients were 8.0, 4.0, and 3.3, respectively.27 The sum of the scores for each activity was used to calculate the total PA levels. A validated extended self-administered food frequency questionnaire (FFQ) with 100 food items was used to ascertain the usual dietary intake. By combining data from the FFQ and the Chinese food composition table, we were capable of calculating the mean total energy intake for each participant.28 MetS was defined using criteria from the American Heart Association scientific statements of 2009 criteria.29 All covariates used in the current analysis were collected at baseline and follow-up periods.
Statistical Analysis
The normal distributions of continuous variables was evaluated using the Kolmogorov–Smirnov test (n > 2000). For continuous variables, the baseline characteristics of the participants were reported as medians (25th and 75th percentiles), and for categorical variables, as percentages.
The follow-up time for each participant was determined from the completion of the initial survey to the last time of follow-up, the incident BPH event date, or the date of loss to follow-up, whichever came earlier. To investigate the association between MPV levels and the risk of BPH in the general Chinese adult male population and MetS patients, Cox proportional hazards models were used. The significance level of interaction terms between the quartile categories of MPV and follow-up time was evaluated in order to examine the proportional hazard assumption. Three progressively multivariable Cox regression models were fitted. In model 1, 95% confidence intervals (CIs) for crude hazard ratios (HRs) were calculated. In model 2, potential confounding variables including age and BMI were further adjusted for. In model 3, additionally adjusted for smoking status, alcohol drinking status, hypertension, hyperlipidemia, diabetes mellitus, family history of disease (including CVD, hypertension, hyperlipidemia and diabetes mellitus). To investigate the effect (mediating role) of inflammation on the associations with the risk of BPH, inflammatory markers were adjusted. Linear trend tests were performed across MPV quartile categories by modeling these quartiles as ordinal variables.
All statistical tests were two-sided and a P value < 0.05 was considered statistically significant. The analyses were conducted using SAS version 9.4 for Windows (SAS Institute Inc, Cary, NC, USA).
Results
Characteristics of Study Subjects
The population as a whole has a mean age (standard deviation) of 44.0 (10.3) years. During this time, a total of 4848 participants were diagnosed with BPH for the first time. Participant baseline characteristics are displayed in Table 1 in relation to incident BPH. Males with BPH had a higher average age than men without BPH (P< 0.0001). Participants with BPH tended to have a lower level of education (P< 0.0001), ALT, “Animal foods” dietary pattern score (P< 0.001), and household income (P= 0.01), but had a higher level of BMI, waist, TG, LDL, FBG, SBP, DBP, “Sweets” dietary pattern score (P< 0.0001) and TC (P< 0.001). They tend to have a history of hypertension and hyperlipidemia and a family history of CVD, hypertension and diabetes mellitus (P< 0.0001). There was a spread between smoking, drinking, and employment status among men with BPH. Other than that, there were no notable variations between the groups.
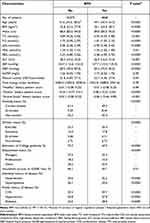 |
Table 1 Participant Baseline Characteristics by BPH Status (n = 14,923) |
Association Between MPV Levels and Risk of BPH
During a median follow-up of 2.7 y (range: 1.0–9.0 y), we identified 4848 incident BPH cases. The association between MPV levels and the risk of BPH is demonstrated in Table 2. After adjusting for demographic factors and lifestyle factors, the HRs of BPH were 1.00 (reference), 1.03 (95% CIs 0.96, 1.11), 1.00 (95% CIs0.92, 1.08) and 0.98 (95% CIs0.90, 1.06), respectively, for participants with MPV in the 1st, 2nd, 3rd and 4th quartiles (P for trend = 0.47). The risk of BPH and MPV levels were shown to be non-significantly associated.
 |
Table 2 Adjusted Relationships of MPV to BPH (n = 14,923) |
Association between MPV Levels and risk of BPH in MetS Patients
At a baseline median age of 46.9 years, the study sample included 4283 patients with MetS. During this period, a total of 1787 MetS patients received a new diagnosis of BPH. As shown in Table 3, the association between MPV levels and the risk of BPH in MetS patients are demonstrated, with the highest quartile of MPV levels had a multivariable HRs of 1.01 (95% CIs 0.89, 1.15; P for trend= 0.98), compared with those in the lowest quartile. Based on this result, in the MetS population, there was a non-significant association between MPV levels and the risk of BPH.
 |
Table 3 Adjusted Relationships of MPV to BPH in Population with Metabolic Syndrome (n = 4283) |
Discussion
In this large-scale prospective study of a Chinese adult male population, we demonstrated that no association between MPV levels and the risk of BPH. To the extent that we are aware, this is the first large-scale general population study that examined the topic of MPV levels and the risk of BPH conducted in Asia, and evaluated it in MetS patients.
MPV is a frequently used laboratory biomarker of platelet activity in inflammatory conditions.30 Measuring MPV made it easy to measure platelet activity and aggregation capacity. Lower MPV levels may indicate increased large platelets consumption in inflammatory conditions.13 The association between MPV levels and the risk of BPH has been investigated in a variety of studies. According to a cross-sectional study, MPV values significantly decreased in all grades of histological prostatitis after BPH surgery, although there was no association between MPV levels and the degree of the inflammation.11 In addition, a case-control study also found that MPV might be a predictor of the development of BPH.12 Despite the fact that it is a simple and inexpensive biomarker of inflammation and is associated to a number of disorders,31–33 the association between MPV levels and the risk of BPH has not been examined in previous studies. According to the current study’s findings, there is no association between MPV levels and the risk of BPH.
BPH is the most common urologic disease that impacts elderly men; it affects about 25% of men in their 50s, 1/3 of men in their 60s, and 50% of men in their 80s.34 Recent studies clearly show that BPH is an immunological inflammatory disease, even though the pathogenesis is still not fully grasped and a variety of processes appear to be involved in its development and progression.35,36 Scientists are intrigued by the association between BPH and chronic inflammation. Studies have shown that there is a significant concentration of inflammatory cells around the prostate gland, which raises the possibility that this is where the prostate immune response begins.37 As a result, chronic inflammation may have a role in the development and progression of BPH, which may be an immunological inflammatory disease.36,38
Recently, Zhao et al showed that MPV might be used as a predictor of inflammation caused by MetS in the development of BPH.12 Patients with MetS have been reported to be at increased risk of BPH, indicating that MetS has a wide range of clinical repercussions.6,7 The combination of numerous cardiovascular risk factors, such as insulin resistance, obesity, dyslipidemia, and high blood pressure, characterizes the clinical condition known as MetS.29 It can be broadly defined as a systemic inflammatory state, and chronic inflammation-driven tissue remodeling, and prostate enlargement as a result of remodeling of prostate tissue is the first stage in the development of BPH.39,40 The theory that MetS may encourage a chronically inflammatory-driven prostate enlargement and that it may possibly be a predictor or driver of the progression of BPH is backed up by a number of studies.12,41
Even if the biological pathways between elevated MPV and MetS are yet unknown, in a previous study, it was found that elevated MPV was independently associated with the number of positive MetS components and that MPV was higher when MetS was present.42 MPV may function as a predictor of the presence or absence of MetS-induced inflammation as BPH progresses. As a result, we investigated the association between MPV levels and the risk of BPH in MetS patients in this study but were unable to detect any association (HR = 1.01; 95% CI 0.89, 1.15; P for trend =0.98). Combined, the inflammatory response brought on by MetS may not have an impact on the association between MPV levels and the risk of BPH in MetS patients.
In comparison to earlier investigations, the current study included several significant strengths. This study’s participants were drawn from a large population-based sample, making it more widely applicable than studies conducted in specific clinical populations. Furthermore, we also made adjustments for a number of significant potential confounding variables that might have affected the association between MPV levels and the risk of BPH. Naturally, there are certain limitations on this study. First, the etiology of BPH development in patients was not well investigated. It is obvious that there are other causes of BPH besides inflammation, and many other variables are probably also involved in this morphological change. On this issue, further research on males who have BPH at first presentation is required to determine whether MPV affects how BPH progresses over time. Nevertheless, we believe the present study provides satisfactory preliminary data regarding the association between MPV levels and the risk of BPH. Second, although we have adjusted a few potential confounding factors, there are still many obscure factors that may influence the association between MPV levels and the risk of BPH. At last, our study has a wide age range (18–90 years). Thus, youthful participants may not have the time to develop BPH in a generally short follow-up period (median: 2.7 years), which may underestimate the association of MPV levels and the risk of BPH. Nonetheless, although the follow-up period is short, the sample size of the study is large enough that it reinforces the statistical reliability of our results. It will take the long-term studies to confirm our findings.
Conclusion
In summary, this study showed no association between MPV levels and the risk of BPH in Chinese adult male population, participants with MetS were not found to have such an association either, which persisted even after adjusting for potential confounders.
Despite the fact that BPH is not a deadly disease, its symptoms may make a patient’s quality of life miserable. To further comprehend the biological mechanisms of BPH and evaluate whether or not an elevated level of MPV is a result of BPH, additional experimental and longitudinal investigations are required.
Abbreviations
MPV, mean platelet volume; BPH, benign prostatic hyperplasia; PC, prostate cancer; TZ, transition zone; MetS, metabolic syndrome; CVD, cardiovasculardisease; TCLSIH, Tianjin ChronicLow-grade Systemic Inflammation and Health; TPV, total prostate volume; EDTA, ethylenediaminetetraacetic acid; FBG, fasting blood glucose; TG, triglycerides; TC, total cholesterol; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; hsCRP, high-sensitivity C-reactive protein; ALT, alanine aminotransferase; PA, Physical activity; IPAQ, International Physical Activity Questionnaire; MET, Metabolic equivalent; FFQ, food frequency questionnaire.
Data Sharing Statement
The raw/processed data required to reproduce these findings cannot be shared at this time as the data also forms part of an ongoing study.
Ethics Approval and Informed Consent
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors. Informed consent was obtained from all individual participants included in the study.
Acknowledgments
The authors thank Health Management Centre of Tianjin Medical University General Hospital for their strong support and help in the field investigation. The authors appreciate the cooperation and participation of teacher, nurses, students, and participants.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by grants from the Study of Diet and Nutrition Assessment and Intervention Technology (No. 2020YFC2006300) from Active Health and Aging Technologic Solutions Major Project of National Key R&D Program——Study on Intervention Strategies of Main Nutrition Problems in China (No. 2020YFC2006305), National Natural Science Foundation of China (Nos. 81941024, 81872611, 82103837, 81903315 and 8197141228), Tianjin Major Public Health Science and Technology Project (No. 21ZXGWSY00090), National Health Commission of China (No. SPSYYC 2020015), Food Science and Technology Foundation of Chinese Institute of Food Science and Technology (No. 2019-12), 2014 and 2016 Chinese Nutrition Society (CNS) Nutrition Research Foundation—DSM Research Fund (Nos. 2016-046, 2014-071 and 2016-023), China.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. De Nunzio C, Kramer G, Marberger M, et al. The controversial relationship between benign prostatic hyperplasia and prostate cancer: the role of inflammation. Eur Urol. 2011;60(1):106–117. doi:10.1016/j.eururo.2011.03.055
3. Zhang L, Wang Y, Qin Z, et al. Correlation between prostatitis, Benign prostatic hyperplasia and prostate cancer: a systematic review and meta-analysis. J Cancer. 2020;11(1):177–189. doi:10.7150/jca.37235
4. Devlin CM, Simms MS, Maitland NJ. Benign prostatic hyperplasia - what do we know? BJU Int. 2021;127(4):389–399. doi:10.1111/bju.15229
5. Post JM, Beebe-Dimmer JL, Morgenstern H, et al. The metabolic syndrome and biochemical recurrence following radical prostatectomy. Prostate Cancer. 2011;2011:245642. doi:10.1155/2011/245642
6. Park YW, Kim SB, Kwon H, et al. The relationship between lower urinary tract symptoms/benign prostatic hyperplasia and the number of components of metabolic syndrome. Urology. 2013;82(3):674–679. doi:10.1016/j.urology.2013.03.047
7. Pan JG, Jiang C, Luo R, Zhou X. Association of metabolic syndrome and benign prostatic hyperplasia in Chinese patients of different age decades. Urol Int. 2014;93(1):10–16. doi:10.1159/000354026
8. Adorini L, Penna G, Fibbi B, Maggi M. Vitamin D receptor agonists target static, dynamic, and inflammatory components of benign prostatic hyperplasia. Ann N Y Acad Sci. 2010;1193(1):146–152. doi:10.1111/j.1749-6632.2009.05299.x
9. Kramer G, Steiner GE, Handisurya A, et al. Increased expression of lymphocyte-derived cytokines in benign hyperplastic prostate tissue, identification of the producing cell types, and effect of differentially expressed cytokines on stromal cell proliferation. Prostate. 2002;52(1):43–58. doi:10.1002/pros.10084
10. Rifaioglu MM, Demirbas O, Gokce H, Davarci M. Mean platelet volume-a predictive factor for the diagnosis of nonsymptomatic prostatitis: results of univariate and multivariate models. Am J Mens Health. 2017;11(1):35–40. doi:10.1177/1557988315621144
11. Karaman H, Karakukcu C, Kocer D. Can mean platelet volume serve as a marker for prostatitis? Int J Med Sci. 2013;10(10):1387–1391. doi:10.7150/ijms.6126
12. Zhao S, Tang J, Shao S, Yan Y. The relationship between benign prostatic hyperplasia/lower urinary tract symptoms and mean platelet volume: the role of metabolic syndrome. Urol Int. 2016;96(4):449–458. doi:10.1159/000443313
13. Gasparyan AY, Ayvazyan L, Mikhailidis DP, Kitas GD. Mean platelet volume: a link between thrombosis and inflammation? Curr Pharm Des. 2011;17(1):47–58. doi:10.2174/138161211795049804
14. Yu B, He H, Zhang Q, et al. Soft drink consumption is associated with depressive symptoms among adults in China. J Affect Disord. 2015;172:422–427. doi:10.1016/j.jad.2014.10.026
15. Song K, Du H, Zhang Q, et al. Serum immunoglobulin M concentration is positively related to metabolic syndrome in an adult population: tianjin Chronic Low-Grade Systemic Inflammation and Health (TCLSIH) Cohort Study. PLoS One. 2014;9(2):e88701. doi:10.1371/journal.pone.0088701
16. Zhu C, Wu J, Wu Y, et al. Triglyceride to high-density lipoprotein cholesterol ratio and total cholesterol to high-density lipoprotein cholesterol ratio and risk of benign prostatic hyperplasia in Chinese male subjects. Front Nutr. 2022;9:999995. doi:10.3389/fnut.2022.999995
17. Blanc M, Sacrini A, Avogadro A, et al. Prostatic volume: suprapubic versus transrectal ultrasonography in the control of benign prostatic hyperplasia. Radiol Med. 1998;95(3):182–187.
18. Huh JS, Kim YJ, Kim SD. Prevalence of Benign prostatic hyperplasia on Jeju Island: analysis from a cross-sectional community-based survey. World J Mens Health. 2012;30(2):131–137. doi:10.5534/wjmh.2012.30.2.131
19. Zhao SC, Xia M, Tang JC, Yan Y. Associations between metabolic syndrome and clinical benign prostatic hyperplasia in a northern urban Han Chinese population: a prospective cohort study. Sci Rep. 2016;6(1):33933. doi:10.1038/srep33933
20. Lance MD, van Oerle R, Henskens YM, Marcus MA. Do we need time adjusted mean platelet volume measurements? Lab Hematol. 2010;16(3):28–31. doi:10.1532/LH96.10011
21. Su Q, Yu B, He H, et al. Nut consumption is associated with depressive symptoms among chinese adults. Depress Anxiety. 2016;33(11):1065–1072. doi:10.1002/da.22516
22. Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15(7):539–553. doi:10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S
23. Expert Panel on Detection E, Treatment of High Blood Cholesterol in A. Executive summary of the third report of The National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA. 2001;285(19):2486–2497. doi:10.1001/jama.285.19.2486
24. Kobalava ZD, Kotovskaya YV, Babaeva LA, Moiseev VS. Validation of TM-2655 oscillometric device for blood pressure measurement. Blood Press Monit. 2006;11(2):87–90. doi:10.1097/01.mbp.0000200484.49540.12
25. Mancia G, De Backer G, Dominiczak A, et al. 2007 guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2007;25(6):1105–1187. doi:10.1097/HJH.0b013e3281fc975a
26. Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA. 2003;289(19):2560–2572. doi:10.1001/jama.289.19.2560
27. Craig CL, Marshall AL, Sjostrom M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381–1395. doi:10.1249/01.MSS.0000078924.61453.FB
28. Yang Y, Wang GY, Pan X. China Food Composition. Beijing: Peking University Medical Press; 2002.
29. Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640–1645. doi:10.1161/CIRCULATIONAHA.109.192644
30. Balbaloglu O, Korkmaz M, Yolcu S, Karaaslan F, Beceren NG. Evaluation of mean platelet volume (MPV) levels in patients with synovitis associated with knee osteoarthritis. Platelets. 2014;25(2):81–85. doi:10.3109/09537104.2013.776162
31. Velez-Paez JL, Tercero-Martinez W, Jimenez-Alulima G, et al. Neutrophil-to-lymphocyte ratio and mean platelet volume in the diagnosis of bacterial infections in COVID-19 patients. A preliminary analysis from Ecuador. Infez Med. 2021;29(4):530–537. doi:10.53854/liim-2904-5
32. Cai N, Chen ZQ, Tao M, Fan WT, Liao W. Mean platelet volume and red blood cell distribution width is associated with prognosis in premature neonates with sepsis. Open Med. 2021;16(1):1175–1181. doi:10.1515/med-2021-0323
33. Chen L, Zhang Q. Predictive value of mean platelet volume for aneurysm recurrence in patients with aneurysmal subarachnoid hemorrhage after endovascular treatment. World Neurosurg. 2021;145:e32–e37. doi:10.1016/j.wneu.2020.09.003
34. Kramer G, Mitteregger D, Marberger M. Is benign prostatic hyperplasia (BPH) an immune inflammatory disease? Eur Urol. 2007;51(5):1202–1216. doi:10.1016/j.eururo.2006.12.011
35. Krieger JN, Nyberg L, Nickel JC. NIH consensus definition and classification of prostatitis. JAMA. 1999;282(3):236–237. doi:10.1001/jama.282.3.236
36. Bostanci Y, Kazzazi A, Momtahen S, Laze J, Djavan B. Correlation between benign prostatic hyperplasia and inflammation. Curr Opin Urol. 2013;23(1):5–10. doi:10.1097/MOU.0b013e32835abd4a
37. Bostwick DG, de la Roza G, Dundore P, Corica FA, Iczkowski KA. Intraepithelial and stromal lymphocytes in the normal human prostate. Prostate. 2003;55(3):187–193. doi:10.1002/pros.10224
38. Li J, Li Y, Cao D, et al. The association between histological prostatitis and benign prostatic hyperplasia: a single-center retrospective study. Aging Male. 2022;25(1):88–93. doi:10.1080/13685538.2022.2050360
39. Gustafson B, Hammarstedt A, Andersson CX, Smith U. Inflamed adipose tissue: a culprit underlying the metabolic syndrome and atherosclerosis. Arterioscler Thromb Vasc Biol. 2007;27(11):2276–2283. doi:10.1161/ATVBAHA.107.147835
40. Lepor H. Pathophysiology, epidemiology, and natural history of benign prostatic hyperplasia. Rev Urol. 2004;6(Suppl 9):S3–S10.
41. Gacci M, Vignozzi L, Sebastianelli A, et al. Metabolic syndrome and lower urinary tract symptoms: the role of inflammation. Prostate Cancer Prostatic Dis. 2013;16(1):101–106. doi:10.1038/pcan.2012.44
42. Tavil Y, Sen N, Yazici HU, Hizal F, Abaci A, Cengel A. Mean platelet volume in patients with metabolic syndrome and its relationship with coronary artery disease. Thromb Res. 2007;120(2):245–250. doi:10.1016/j.thromres.2006.10.005
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
