Back to Journals » Clinical Epidemiology » Volume 15
Association Between Lipid Profile and Risk of Incident Systemic Sclerosis: A Nationwide Population-Based Study
Authors Kwon OC , Han K , Park MC
Received 19 July 2023
Accepted for publication 4 November 2023
Published 29 November 2023 Volume 2023:15 Pages 1095—1107
DOI https://doi.org/10.2147/CLEP.S427881
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Laura Horsfall
Oh Chan Kwon,1 Kyungdo Han,2 Min-Chan Park1
1Division of Rheumatology, Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea; 2Department of Statistics and Actuarial Science, Soongsil University, Seoul, Korea
Correspondence: Kyungdo Han, Department of Statistics and Actuarial Science, Soongsil University, 369 Sangdo-ro, Dongjak-gu, Seoul, 06978, Korea, Email [email protected] Min-Chan Park, Gangnam Severance Hospital, Yonsei University College of Medicine, 211 Eonjuro, Gangnam-Gu, Seoul, 06273, Korea, Email [email protected]
Background and Aims: Lipid metabolism is altered in systemic sclerosis (SSc), mediating activation of immune cells and fibroblasts. However, it is unclear whether altered lipid profile is associated with a risk of developing SSc. We aimed to assess the association between lipid profile and risk of incident SSc.
Methods: From a Korean nationwide database, individuals without SSc who underwent national health check-ups in 2009 were selected and followed-up through 2019. Serum levels of total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), and triglyceride were measured on the health check-up date in 2009. Individuals who developed SSc during follow-up were identified. Multivariable Cox models were performed to estimate the risk of incident SSc according to TC, HDL-C, LDL-C, and triglyceride levels, respectively.
Results: Of the 9,894,996 individuals selected, 1355 individuals developed SSc during a mean follow-up of 9.2 years (incidence rate=1.49 per 100,000 person-years). Levels of TC (adjusted hazard ratio [aHR] 0.959, 95% confidence interval [CI] 0.945– 0.974), HDL-C (aHR 0.968, 95% CI 0.950– 0.987), LDL-C (aHR 0.968, 95% CI 0.952– 0.983) were inversely associated with the risk of incident SSc, whereas no significant association was observed between levels of triglyceride (aHR 1.004, 95% CI 0.998– 1.011) and risk of incident SSc.
Conclusion: Serum levels of TC, HDL-C, and LDL-C were inversely associated with the risk of incident SSc. Our findings provide new insights that altered lipid profile could be considered a non-causal biomarker associated with incident SSc, which could help early diagnosis. The underlying mechanism for this association needs further studies.
Plain Language Summary: Lipid metabolism is altered in patients with systemic sclerosis (SSc), mediating activation of immune cells and fibroblasts. In this large population-based cohort study, we found that levels of TC, HDL-C, and LDL-C were inversely associated with the risk of incident SSc. Our findings suggest that levels of TC, HDL-C, and LDL-C could be considered in detecting individuals at high risk of incident SSc.
Keywords: systemic sclerosis, risk factor, total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol
Introduction
Systemic sclerosis (SSc) is a chronic autoimmune disease characterised by vasculopathy, inflammation, and tissue fibrosis.1 Patients with SSc could present with various manifestations. Raynaud phenomenon is the most common manifestation of SSc and blood perfusion of hands is lower in patients with SSc than controls.2 Interstitial lung disease is one of the leading causes of mortality in patients with SSc.3 Although survival rate has improved over time, SSc has the highest mortality among various rheumatic diseases.4,5 Given the high mortality rate of SSc, it is important to know risk factors of its development, to implement close monitoring of high risk individuals. Genetic factors and epigenetic modifications are suggested as factors that influence the development of SSc.1,6–8 In addition, middle age (45–64 years), female sex, family history of SSc, and exposure to silica have been reported as risk factors of SSc in a systemic review on 34 literatures.9
Diagnostic delay of SSc is an important unmet clinical need.10 Approximately a quarter of patients with SSc have a diagnostic delay of more than 10 years between the first appearance of raynaud phenomenon and the diagnosis of SSc.10,11 Importantly, earlier diagnosis leads to better outcomes via earlier treatment.12,13 Therefore, it is crucial to identify biomarkers for early diagnosis of SSc.
Accruing studies suggest that lipid metabolism affects immune responses and could play an important role in the pathogenesis of autoimmune rheumatic diseases.14–17 For SSc in particular, altered lipid metabolism is suggested as one of the key mediators for activating immune cells and fibroblasts.14,15 Indeed, studies have reported altered lipid profile in patients with SSc compared with controls.14 Although the pattern of changes in individual lipid parameters (total cholesterol [TC], high-density lipoprotein cholesterol [HDL-C], low-density lipoprotein cholesterol [LDL-C], and triglyceride) varied among studies according to the disease duration of SSc and the medications used for the treatment of SSc, altered lipid profile has been consistently observed throughout studies.18–22
However, there have been no clinical data assessing whether the altered lipid profile in individuals without SSc is associated with the risk of future development of SSc. None of the previous studies assessing risk factors of SSc have examined lipid profile as a possible risk factor.9 Given that lipid metabolism plays a crucial role in immune responses,16 and that altered lipid profile is observed in patients with SSc, we hypothesize that lipid profile could be associated with the risk of incident SSc. This study aimed to investigate lipid profile as a non-causal biomarker independently associated with incident SSc, which has not been studied previously. For this purpose, we used a large nationwide population-based cohort and assessed the association between lipid profile and the risk of future development of SSc.
Materials and Methods
Data Source
Data were extracted from the Korean National Health Insurance Service (NHIS) claims database, which covers approximately 97% of the whole population of South Korea. Comprehensive data, including demographics, medical treatments and procedures, and disease diagnoses according to the International Classification of Diseases-10th Revision (ICD-10) code and rare intractable disease (RID) code,23 are available in the NHIS database. The RID code is provided for a specific rare disease based on diagnostic criteria provided by the NHI. The RID code is determined through rigorous review by the NHIS and the corresponding health institution for the fulfilment of the diagnostic criteria. Among individuals registered in the NHIS, those aged ≥ 40 years or employees of any age are recommended to undergo national health examinations every two years. The health check-up data include anthropometric measurements, laboratory data (fasting plasma glucose levels and lipid profiles), and lifestyle behaviours (smoking status, alcohol consumption, and physical activity) based on standardized self-reporting questionnaires.24 This study was approved by the Institutional Review Board (IRB) of Gangnam Severance Hospital (IRB No: 3–2022-0338). Owing to the retrospective nature of this study and the anonymized patient data, the requirement for informed consent was waived and approved by the IRB of Gangnam Severance Hospital. This study complied with the ethical standards in the 1964 Declaration of Helsinki. All data accessed complied with relevant data protection and privacy regulations. Data are presented according to the STROBE checklist (Supplementary Material).
Study Cohort
Individuals who underwent national health check-ups in 2009 were initially extracted from the NHIS database. A total of 10,601,274 individuals were identified. The following patients were excluded: (i) age < 20 years (n = 15,431); (ii) diagnosed with SSc before 2009 (n = 890); (iii) those with missing data (n = 663,828); (iv) expired within a year from baseline (n = 26,012); and (v) incident SSc within a year from baseline (n = 117). Finally, 9,894,996 individuals were included for analysis (Figure 1). All individuals were followed up from the date of health check-up in 2009 (referred to as baseline) to the date of SSc diagnosis, or December 2019, whichever came first. The person time of observation duration was calculated excluding the first year of follow-up.
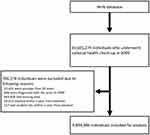 |
Figure 1 Flow diagram of the study population. Abbreviations: NHIS, National Health Insurance Service; SSc, systemic sclerosis. |
Lipid Profile
The serum levels of TC, HDL-C, LDL-C, and triglyceride were measured on the date of national health check-up in 2009. The levels were analyzed as continuous variables.
Definition of SSc and Covariates
SSc was defined as the RID code V138.25 Covariates were defined as follows: hypertension, ICD-10 codes I10‒I13 and I15 with prescriptions for anti-hypertensive agents or systolic blood pressure (BP) ≥ 140 mmHg or diastolic BP ≥ 90 mmHg; type 2 diabetes, ICD-10 codes E11–14 with prescriptions for anti-diabetic agents or fasting plasma glucose level ≥ 126 mg/dL; dyslipidemia, ICD-10 code E78 with prescriptions for lipid-lowering agents or serum TC ≥ 240 mg/dL; and chronic kidney disease (CKD), estimated glomerular filtration rate (GFR) < 60 mL/min/1.73m2 calculated by Modification of Diet in Renal Disease equation.26
Statistical Analysis
Baseline characteristics of the participants were summarized using descriptive analyses. Continuous variables were summarized as mean ± standard deviation. For continuous variables, values of the extreme 5% were identified as outliers and were treated by trimming.27 Categorical variables were summarized as numbers (%). Baseline characteristics were compared using independent samples t-test for continuous variables, and chi-square test for categorical variables. The incidence rate of SSc was expressed as the number of events per 100,000 person-years. Cox proportional hazard models were performed to evaluate the association between lipid profile and the risk of incident SSc. The lipid profile (ie, exposure) was analyzed as a continuous variable. Hazard ratios (HRs) and 95% confidence intervals (CIs) according to the levels of TC, HDL-C-, LDL-C, and triglyceride, respectively, were estimated. Model 1 was a crude model. Model 2 was adjusted for age and sex, which are known confounders (ie, covariates that affect both independent and dependent variables) of lipid profile and SSc. Model 3 was additionally adjusted for potential explanatory variables (age, sex, body mass index [BMI], residence, income, smoking status, alcohol consumption, physical activity, hypertension, type 2 diabetes, and CKD). Model 4 was adjusted for potential mediators (the use of lipid-lowering agents) in addition to the covariates adjusted in model 3. Age and BMI were not categorized and were adjusted as continuous variables. Restricted cubic spline analysis was used to assess the potential non-linear relationships between lipid profile and risk of incident SSc. Five knots were selected at 20th, 40th, 60th, 80th, and 100th percentiles. Subgroup analysis was performed to evaluate whether there is a subset of individuals in whom the association between lipid profile and risk of incident SSc is more prominent. All p-values were two-sided, and a p-value < 0.05 was considered statistically significant. Statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA).
Results
Baseline Characteristics
During a mean follow-up duration of 9.2 ± 1.1 years, 1355 of 9,894,996 individuals developed SSc. The comparison of baseline characteristics between those who did not develop SSc and those who developed SSc during follow-up are shown in Table 1. Given the much higher incidence of SSc in females, we stratified the participants by sex. In males, levels of TC (194.2 ± 36.1 mg/dL vs 187.7 ± 35.2 mg/dL, p = 0.005) and LDL-C (112.0 ± 38.8 mg/dL vs 104.7 ± 34.9 mg/dL, p = 0.004) were lower in individuals who developed SSc during follow-up, while the levels of HDL-C (53.1 ± 25.3 mg/dL vs 51.7 ± 17.5 mg/dL, p = 0.381) and triglyceride (128.6 [128.6–128.7] mg/dL vs 133.8 [124.3–144.1] mg/dL, p = 0.286) did not differ between the two groups. In females, levels of HDL-C (59.7 ± 30.5 mg/dL vs 56.4 ± 30.6 mg/dL, p < 0.001) were lower and levels of triglyceride (95.9 [95.9–96.0] mg/dL vs 102.4 [99.2–105.6] mg/dL, p = 0.002) were higher in those who developed SSc during follow-up, whereas the levels of TC (195.9 ± 37.6 mg/dL vs 193.7 ± 37.9 mg/dL, p = 0.051) and LDL-C (115.5 ± 38.3 mg/dL vs 114.8 ± 34.3 mg/dL, p = 0.550) did not differ between the two groups.
 |
Table 1 Comparison of Baseline Characteristics According to the Development of Systemic Sclerosis During Follow Up Stratified by Sex |
Risk of Incident SSc According to the Lipid Profile
The incidence rate of SSc in the total study population was 1.49 per 100,000 person-years. The cumulative incidence of SSc over time by sex is shown in Figure 2. The incidence was consistently higher in female than in male over time. In the Cox proportional hazard models (Table 2), levels of TC (model 4; adjusted HR 0.959, 95% CI 0.945–0.974), HDL-C (model 4; adjusted HR 0.936, 95% CI 0.900–0.974), LDL-C (model 4; adjusted HR 0.968, 95% CI 0.952–0.983) were inversely associated with the risk of incident SSc, whereas no significant association was observed between levels of triglyceride (model 4; adjusted HR 1.004, 95% CI 0.998–1.011) and risk of incident SSc. Restricted cubic spline analysis revealed lower risk of incident SSc at higher levels of TC, HDL-C, and LDL-C (Figure 3).
 |
Table 2 Incidence of Systemic Sclerosis According to Lipid Profile in Continuous Variable |
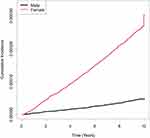 |
Figure 2 Cumulative incidence of SSc over time. Abbreviation: SSc, systemic sclerosis. |
Subgroup Analysis
As the HDL-C levels showed the largest effect size, we further analyzed whether the effect of HDL-C levels is more pronounced in a particular subgroup of individuals (Table 3). HDL-C levels were categorised as Q1 (lowest 25%) and Q2–Q4, and individuals were stratified according to several covariates. The association between higher HDL-C levels (Q2–Q4 vs Q1) and lower risk of incident SSc was more prominent in females than in males (adjusted HR 0.699 vs 0.989, p for interaction = 0.024), in individuals who are not obese (BMI < 25 kg/m2) than in those who are obese (BMI ≥ 25 kg/m2) (adjusted HR 0.675 vs 0.899, p for interaction = 0.032), and in those without type 2 diabetes than in those with type 2 diabetes (adjusted HR 0.720 vs 1.100, p for interaction = 0.042).
 |
Table 3 Subgroup Analysis of the Association Between Levels of HDL-C and Incidence of Systemic Sclerosis |
Discussion
In this study, we observed a significant association between lipid profile and the risk of incident SSc during a mean follow-up of 9.2 years. In particular, higher serum levels of TC, HDL-C, and LDL-C were associated with a lower risk of incident SSc. Among individual lipid parameters, HDL-C had the largest effect size on the risk of incident SSc. To the best of our knowledge, this is the first study reporting the association between lipid profile and the risk of incident SSc.
Although data on the association between lipid profile and the risk of incident SSc are lacking, the association between lipid profile and incidence of other inflammatory diseases, such as rheumatoid arthritis (RA) and inflammatory bowel diseases (IBD), has been reported.28–30 A population-based study showed that TC and LDL-C levels in patients with RA are decreased compared with non-RA individuals five years before the onset of RA.28 Another study reported that HDL-C levels in patients with RA are decreased compared with non-RA individuals 10 years before the onset of RA.29 Regarding IBD, low TC, LDL-C, and HDL-C levels were associated with a higher risk of incident ulcerative colitis over a median follow up of 7.3 years.30 Our data add to the previous knowledge that the inverse association between levels of TC, HDL-C, and LDL-C and incidence of inflammatory diseases also applies to SSc.
In an inflammatory condition, TC, HDL-C, and LDL-C levels decrease.31–33 Therefore, subclinical inflammation might be present in those with low TC, HDL-C, and LDL-C levels at baseline, which led to a later incidence of overt SSc. Moreover, we observed that higher levels of TC, HDL-C, and LDL-C indicate lower risks of incident SSc. Therefore, it is possible that lower TC, HDL-C, and LDL-C levels reflect a higher burden of subclinical inflammation that may have contributed to the higher incidence of overt SSc.
Of the lipid parameters that showed significant association with the risk of incident SSc, HDL-C had the largest effect size, accounting for an approximately 6.4% decrease in the risk of incident SSc per 10 mg/dL increment in levels of HDL-C. In contrast, LDL-C had the smallest effect size, accounting for an approximately 3.2% decrease in risk per 10 mg/dL increment in levels of LDL-C. The effect size of TC (approximately 4.1% decrease in risk per 10 mg/dL increment in levels of TC) was in between that of HDL-C and LDL-C, as expected, considering that the TC includes both HDL-C and LDL-C. HDL-C is distinct from LDL-C in that it has antioxidant and anti-inflammatory activities.34 Moreover, HDL-C has an immune regulatory role, as it removes cholesterol from peripheral cells and decreases cholesterol levels in the cell membrane, resulting in a decreased number of class II major histocompatibility complex molecules and decreased T cell activation.35 Therefore, lower levels of HDL-C, by impairing immune regulation, could have led to a higher risk of incident SSc, which explains the inverse association between HDL-C levels and incidence of SSc. In addition, these characteristics of HDL-C, which are distinct from that of LDL-C, could be a possible explanation for the larger effect size of HDL-C than that of LDL-C.
In the subgroup analysis, the effect of higher HDL-C levels on risk reduction of incident SSc was found to be more robust in females, individuals who are non-obese, and those without type 2 diabetes. Meanwhile, the effect size was smaller than their respective counterparts in males, individuals who are obese, and those with type 2 diabetes and lost statistical significance. The more pronounced effect on females than on males could be attributable to the differences in HDL-C levels according to sex. The HDL-C levels are generally higher in females than in males.36 Indeed, the proportion of individuals with Q2–Q4 HDL-C levels was higher in females (82.0%) than in males (66.9%) (Table 3). Given that female sex is a risk factor of SSc,9 it is important to perceive that females with low HDL-C levels are particularly at a high risk of developing SSc. With regard to obese vs non-obese individuals, obesity itself is associated with chronic inflammation and impaired immune function.37 Hence, the effect of higher HDL-C levels on the risk reduction of incident SSc may be attenuated in obese individuals. Likewise, type 2 diabetes is also recognized as an inflammatory disease,38 and therefore, the effect of higher HDL-C levels could be less pronounced in individuals with type 2 diabetes.
Lipid-lowering agents including statins, ezetimibe, and fenofibrates, have an anti-inflammatory effect, in addition to the lipid-altering effect.39–41 Therefore, it is important to determine whether the effect of HDL-C levels on the incidence of SSc is different according to the use of lipid-lowering agents. In the subgroup analysis, no significant difference in the influence of HDL-C levels on the incidence of SSc was observed between those who did not and did receive lipid-lowering agents (p for interaction = 0.496). This suggests that the use of lipid-lowering agents does not influence the inverse association between HDL-C levels and the risk of incident SSc.
This study has some limitations. First, we lack data on several known risk factors of SSc, such as family history of SSc and exposure to silica.9 Therefore, although we adjusted for a number of covariates in the multivariable model, possibility of bias from unmeasured confounders exist. However, given that the prevalence of SSc is very low (77.7 per 1 million in Koreans)25 and the number of Korean workers occupationally exposed to silica is very small (approximately 150,000, accounting for 0.3% of the Korean population),42 we presume that the influence by these unmeasured confounders is small. Second, as data on the organs involved are not available in the NHIS database, we could not assess whether the lipid profile is associated with a particular manifestation of SSc. Third, as this was a Korean population-based study, generalizability to other ethnic populations remains unclear. Fourth, possibility of bias from running a complete case analysis exists. Finally, as this was an observational study, the exact mechanism by which each lipid parameter affects the incidence of SSc is unclear. Despite these limitations, our findings are robust in that this study was conducted using a large cohort of individuals and novel in that this evaluated the association between the lipid profile and risk of incident SSc for the first time.
Conclusions
In conclusion, higher levels of TC, HDL-C, and LDL-C were associated with a lower risk of incident SSc (Figure 4). The inverse association was most prominent between HDL-C levels and the incidence of SSc, with a 6.4% decrease in risk of incident SSc per 10 mg/dL increment of HDL-C levels. Importantly, our data suggest that levels of TC, HDL-C, and LDL-C could be considered a non-causal biomarker associated with incident SSc, which could aid in detecting individuals at high risk of incident SSc.
Abbreviations
SSc, Systemic sclerosis; TC, Total cholesterol; HDL-C, High-density lipoprotein cholesterol; LDL-C, Low-density lipoprotein cholesterol; NHIS, National Health Insurance Service; ICD-10, International Classification of Diseases-10th Revision; RID, Rare intractable disease; IRB, Institutional Review Board; BP, Blood pressure; CKD, Chronic kidney disease; GFR, Glomerular filtration rate; HRs, Hazard ratios; CIs, Confidence intervals; BMI, Body mass index; RA, Rheumatoid arthritis; IBD, Inflammatory bowel diseases.
Data Sharing Statement
All data generated or analyzed during this study are included in this article. All data except the results cannot be shared publicly due to the policy of the national health authorities.
Ethics and Consent Statement
This study was approved by the Institutional Review Board (IRB) of Gangnam Severance Hospital (IRB No: 3-2022-0338). Owing to the retrospective nature of this study and the anonymized patient data, the requirement for informed consent was waived and approved by the IRB of Gangnam Severance Hospital. This study complied with the ethical standards in the 1964 Declaration of Helsinki. All data accessed complied with relevant data protection and privacy regulations.
Acknowledgment
The authors thank MID (Medical Illustration & Design) for providing excellent support with medical illustration. The abstract of this paper was presented at the EULAR 2023 European Congress of Rheumatology as a poster presentation with interim findings. The poster’s abstract was published in ‘Poster Abstracts’ in Annals of the Rheumatic Diseases: http://dx.doi.org/10.1136/annrheumdis-2023-eular.2532.
Funding
This work was supported by the research fund of the Rheumatology Research Foundation (RRF-2022-02).
Disclosure
The authors declare that there is no conflict of interest in relation to this article.
References
1. Denton CP, Khanna D. Systemic sclerosis. Lancet. 2017;390(10103):1685–1699. doi:10.1016/s0140-6736(17)30933-9
2. Ruaro B, Smith V, Sulli A, et al. Innovations in the assessment of primary and secondary raynaud’s phenomenon. Front Pharmacol. 2019;10:360. doi:10.3389/fphar.2019.00360
3. Bergamasco A, Hartmann N, Wallace L, Verpillat P. Epidemiology of systemic sclerosis and systemic sclerosis-associated interstitial lung disease. Clin Epidemiol. 2019;11:257–273. doi:10.2147/clep.S191418
4. Tyndall AJ, Bannert B, Vonk M, et al. Causes and risk factors for death in systemic sclerosis: a study from the EULAR Scleroderma Trials and Research (EUSTAR) database. Ann Rheum Dis. 2010;69(10):1809–1815. doi:10.1136/ard.2009.114264
5. Nihtyanova SI, Tang EC, Coghlan JG, Wells AU, Black CM, Denton CP. Improved survival in systemic sclerosis is associated with better ascertainment of internal organ disease: a retrospective cohort study. Qjm. 2010;103(2):109–115. doi:10.1093/qjmed/hcp174
6. Murdaca G, Contatore M, Gulli R, Mandich P, Puppo F. Genetic factors and systemic sclerosis. Autoimmun Rev. 2016;15(5):427–432. doi:10.1016/j.autrev.2016.01.016
7. Makino T, Jinnin M. Genetic and epigenetic abnormalities in systemic sclerosis. J Dermatol. 2016;43(1):10–18. doi:10.1111/1346-8138.13221
8. Silman AJ, Newman J. Genetic and environmental factors in scleroderma. Curr Opin Rheumatol. 1994;6(6):607–611. doi:10.1097/00002281-199411000-00010
9. Abbot S, Bossingham D, Proudman S, de Costa C, Ho-Huynh A. Risk factors for the development of systemic sclerosis: a systematic review of the literature. Rheumatol Adv Pract. 2018;2(2):rky041. doi:10.1093/rap/rky041
10. Pauling JD, McGrogan A, Snowball J, McHugh NJ. Epidemiology of systemic sclerosis in the UK: an analysis of the Clinical Practice Research Datalink. Rheumatology. 2021;60(6):2688–2696. doi:10.1093/rheumatology/keaa680
11. Delisle VC, Hudson M, Baron M, Thombs BD; And The Canadian Scleroderma Research Group A. Sex and time to diagnosis in systemic sclerosis: an updated analysis of 1129 patients from the Canadian scleroderma research group registry. Clin Exp Rheumatol. 2014;32(6 Suppl 86):10–14.
12. Sakkas LI, Simopoulou T, Katsiari C, Bogdanos D, Chikanza IC. Early systemic sclerosis-opportunities for treatment. Clin Rheumatol. 2015;34(8):1327–1331. doi:10.1007/s10067-015-2902-5
13. Melissaropoulos K, Kraniotis P, Bogdanos D, Dimitroulas T, Sakkas L, Daoussis D. Targeting very early systemic sclerosis: a case-based review. Rheumatol Int. 2019;39(11):1961–1970. doi:10.1007/s00296-019-04357-x
14. Gogulska Z, Smolenska Z, Turyn J, Mika A, Zdrojewski Z. Lipid Alterations in Systemic Sclerosis. Front Mol Biosci. 2021;8:761721. doi:10.3389/fmolb.2021.761721
15. Chen W, Wang Q, Zhou B, Zhang L, Zhu H. Lipid Metabolism Profiles in Rheumatic Diseases. Front Pharmacol. 2021;12:643520. doi:10.3389/fphar.2021.643520
16. Han X. Lipidomics for studying metabolism. Nat Rev Endocrinol. 2016;12(11):668–679. doi:10.1038/nrendo.2016.98
17. Robinson G, Pineda-Torra I, Ciurtin C, Jury EC. Lipid metabolism in autoimmune rheumatic disease: implications for modern and conventional therapies. J Clin Invest. 2022;132(2). doi:10.1172/jci148552
18. Bruckdorfer KR, Hillary JB, Bunce T, Vancheeswaran R, Black CM. Increased susceptibility to oxidation of low-density lipoproteins isolated from patients with systemic sclerosis. Arthritis Rheum. 1995;38(8):1060–1067. doi:10.1002/art.1780380807
19. Borba EF, Borges CT, Bonfá E. Lipoprotein profile in limited systemic sclerosis. Rheumatol Int. 2005;25(5):379–383. doi:10.1007/s00296-004-0580-8
20. Kotyla PJ, Gozdzik J, Lewicki M, Kotulska AT, Kucharz EJ. Serum lipid profile in patients with systemic sclerosis: relationship to the thyreometabolic state. Rheumatol Int. 2006;26(6):583–584. doi:10.1007/s00296-005-0075-2
21. Kim HB, Kim A, Kim Y, et al. Associations of serum monocyte-to-high-density lipoprotein cholesterol ratio with digital ulcers and skin fibrosis in patients with systemic sclerosis. Scand J Rheumatol. 2021;50(3):231–238. doi:10.1080/03009742.2020.1837237
22. Ferraz-Amaro I, Delgado-Frías E, Hernández-Hernández V, et al. HDL cholesterol efflux capacity and lipid profile in patients with systemic sclerosis. Arthritis Res Ther. 2021;23(1):62. doi:10.1186/s13075-021-02443-9
23. Kim JA, Yoon S, Kim LY, Kim DS. Towards actualizing the value potential of Korea health insurance review and assessment (HIRA) data as a resource for health research: strengths, limitations, applications, and strategies for optimal use of HIRA data. J Korean Med Sci. 2017;32(5):718–728. doi:10.3346/jkms.2017.32.5.718
24. Kwon OC, Han K, Chun J, et al. Effects of immune-mediated inflammatory diseases on cardiovascular diseases in patients with type 2 diabetes: a nationwide population-based study. Sci Rep. 2022;12(1):11548. doi:10.1038/s41598-022-15436-8
25. Kang GW, Jung KH, Lee YS, et al. Incidence, prevalence, mortality and causes of death in systemic sclerosis in Korea: a nationwide population-based study. Br J Dermatol. 2018;178(1):e37–e39. doi:10.1111/bjd.15838
26. Hong S, Park JH, Han K, Lee CB, Kim DS, Yu SH. Association between obesity and cardiovascular disease in elderly patients with diabetes: a retrospective cohort study. J Clin Endocrinol Metab. 2022;107(2):e515–e527. doi:10.1210/clinem/dgab714
27. Kwak SK, Kim JH. Statistical data preparation: management of missing values and outliers. Korean J Anesthesiol. 2017;70(4):407–411. doi:10.4097/kjae.2017.70.4.407
28. Myasoedova E, Crowson CS, Kremers HM, Fitz-Gibbon PD, Therneau TM, Gabriel SE. Total cholesterol and LDL levels decrease before rheumatoid arthritis. Ann Rheum Dis. 2010;69(7):1310–1314. doi:10.1136/ard.2009.122374
29. van Halm VP, Nielen MM, Nurmohamed MT, et al. Lipids and inflammation: serial measurements of the lipid profile of blood donors who later developed rheumatoid arthritis. Ann Rheum Dis. 2007;66(2):184–188. doi:10.1136/ard.2006.051672
30. Soh H, Im JP, Han K, et al. Crohn’s disease and ulcerative colitis are associated with different lipid profile disorders: a nationwide population-based study. Aliment Pharmacol Ther. 2020;51(4):446–456. doi:10.1111/apt.15562
31. Rosenson RS. Myocardial injury: the acute phase response and lipoprotein metabolism. J Am Coll Cardiol. 1993;22(3):933–940. doi:10.1016/0735-1097(93)90213-k
32. Vermont CL, den Brinker M, Kâkeci N, et al. Serum lipids and disease severity in children with severe meningococcal sepsis. Crit Care Med. 2005;33(7):1610–1615. doi:10.1097/01.ccm.0000171272.50888.ad
33. Akgün S, Ertel NH, Mosenthal A, Oser W. Postsurgical reduction of serum lipoproteins: interleukin-6 and the acute-phase response. J Lab Clin Med. 1998;131(1):103–108. doi:10.1016/s0022-2143(98)90083-x
34. Negre-Salvayre A, Dousset N, Ferretti G, Bacchetti T, Curatola G, Salvayre R. Antioxidant and cytoprotective properties of high-density lipoproteins in vascular cells. Free Radic Biol Med. 2006;41(7):1031–1040. doi:10.1016/j.freeradbiomed.2006.07.006
35. Norata GD, Pirillo A, Ammirati E, Catapano AL. Emerging role of high density lipoproteins as a player in the immune system. Atherosclerosis. 2012;220(1):11–21. doi:10.1016/j.atherosclerosis.2011.06.045
36. Davis CE, Williams DH, Oganov RG, et al. Sex difference in high density lipoprotein cholesterol in six countries. Am J Epidemiol. 1996;143(11):1100–1106. doi:10.1093/oxfordjournals.aje.a008686
37. Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. 2006;6(10):772–783. doi:10.1038/nri1937
38. Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. 2011;11(2):98–107. doi:10.1038/nri2925
39. Pirro M, Simental-Mendía LE, Bianconi V, Watts GF, Banach M, Sahebkar A. Effect of statin therapy on arterial wall inflammation based on 18F-FDG PET/CT: a systematic review and meta-analysis of interventional studies. J Clin Med. 2019;8(1):118. doi:10.3390/jcm8010118
40. Ju HB, Zhang FX, Wang S, et al. Effects of fenofibrate on inflammatory cytokines in diabetic retinopathy patients. Medicine. 2017;96(31):e7671. doi:10.1097/md.0000000000007671
41. Ghanim H, Green K, Abuaysheh S, et al. Ezetimibe and simvastatin combination inhibits and reverses the pro-inflammatory and pro-atherogenic effects of cream in obese patients. Atherosclerosis. 2017;263:278–286. doi:10.1016/j.atherosclerosis.2017.06.010
42. Min YS, Kim MG, Ahn YS. Rheumatoid arthritis in silica-exposed workers. Int J Environ Res Public Health. 2021;18(23). doi:10.3390/ijerph182312776
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


