Back to Journals » Journal of Inflammation Research » Volume 16
Association Between Inflammatory Burden Index and Unfavorable Prognosis After Endovascular Thrombectomy in Acute Ischemic Stroke
Authors Du M, Xu L, Zhang X , Huang X , Cao H, Qiu F, Lan W, Jiang H
Received 27 April 2023
Accepted for publication 17 July 2023
Published 19 July 2023 Volume 2023:16 Pages 3009—3017
DOI https://doi.org/10.2147/JIR.S419087
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ning Quan
Mingyang Du,1,* Lili Xu,1,* Xiaohao Zhang,2 Xianjun Huang,3 Hui Cao,1 Feng Qiu,1 Wenya Lan,1 Haibo Jiang1
1Cerebrovascular Disease Center, Nanjing Brain Hospital Affiliated to Nanjing Medical University, Nanjing, Jiangsu Province, 210029, People’s Republic of China; 2Department of Neurology, Nanjing First Hospital, Nanjing Medical University, Nanjing, Jiangsu, 210002, People’s Republic of China; 3Department of Neurology, Yijishan Hospital, Wannan Medical College, Wuhu, Anhui Province, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Mingyang Du, Cerebrovascular Disease Center, Nanjing Brain Hospital Affiliated to Nanjing Medical University, No. 264 Guangzhou Road, Nanjing, Jiangsu Province, 210029, People’s Republic of China, Tel/Fax +86 25-82296000, Email [email protected]
Background: Inflammatory burden index (IBI) is a systemic inflammation indicator that reflects the inflammatory status. We aimed to investigate the prognostic value of IBI after endovascular thrombectomy (EVT) in patients with acute ischemic stroke.
Methods: We enrolled patients treated with EVT from a multicenter cohort between June 2020 and December 2021. The IBI was calculated as C-reaction protein × neutrophil / lymphocyte count. The primary outcome was the unfavorable functional outcome (90-day modified Rankin scale score 3– 6). C-statistics and net reclassification indexes were used to assess the predictive accuracy. Multivariable logistic regression models were used to investigate the association between IBI and unfavorable outcome.
Results: A total of 295 patients (mean age, 64.0 ± 12.8 years; male, 63.7%) were enrolled in this study. In multivariable models, higher IBI levels were associated with an increased risk of 90-day unfavorable outcome after EVT (per 1-SD: odds ratio, 1.754; 95% confidence interval, 1.241– 2.587; P = 0.002). Restricted cubic spline curve displayed a linear relationship between the IBI level and 90-day unfavorable outcome (P for nonlinearity = 0.410). Besides, IBI was a more accurate biomarker for predicting unfavorable outcomes with the highest predictive accuracy and reclassification indexes.
Conclusion: This study demonstrated that higher IBI was associated with an increased risk of 90-day unfavorable outcome in acute ischemic stroke treated with EVT.
Keywords: stroke, thrombectomy, inflammatory burden, systemic, prognosis
Introduction
Endovascular thrombectomy (EVT) has been recognized as an effective therapy for acute ischemic stroke (AIS) with large vessel occlusions in the anterior circulation.1,2 Recently, the evidence from randomized controlled trials has confirmed the efficacy and safety of EVT in patients with large cores.3 However, the efficacy of EVT is limited by early complications, such as symptomatic intracranial hemorrhage (sICH),4 futile recanalization,5 malignant brain edema,6 and early neurology stability.7 Previous studies reported that nearly 50% of the patients treated with EVT remained functional dependence at 90 days.8 Hence, it is urgent to find simple and powerful biomarkers for predicting the prognosis of EVT to prompt the rapid identification of the high-risk patients and improve functional assessments.
Systemic inflammation plays an important role in causing tissue damage after the pathogenic stimuli in different tissues.9 Previous studies had shown that systematic inflammation was associated with the progression of cerebral ischemic lesions through remodeling and infarct resolution in the brain tissue.10,11 Recently, several studies found that lowing systematic inflammation in the acute phase was associated with the improved tissue salvage and reduced risk of vascular events.12,13 Systemic inflammation can be manifested by changes in the peripheral inflammatory parameters, such as neutrophils, lymphocytes, platelets, and C-reactive protein (CRP). Inflammatory burden index (IBI) is calculated from neutrophils, lymphocytes, and CRP.14 Previous studies had shown that IBI was an independent predictor for the prognosis of different malignancies and may be the optimal indicator among various systemic inflammation indicators.14–16 However, few studies are available regarding the prognostic value of IBI for the clinical outcomes of AIS. Hence, we performed a retrospective analysis of a multicenter cohort and aimed to assess the relationship between IBI and unfavorable prognosis in AIS patients treated with EVT.
Methods
Data that support the findings of this study are available from the corresponding authors upon reasonable request.
Study Population
This study was a retrospective cohort study with prospective data collection. We enrolled patients diagnosed with AIS and treated with EVT from 3 comprehensive centers: Nanjing brain hospital, Nanjing first hospital, and Yijishan Hospital between June 2020 and December 2021. This study was performed in accordance with the 1964 Helsinki Declaration and was approved by the ethics committees of each participating center (2019-kyy121–01). All patients or their legally authorized representatives provided the written informed consents.
Patients were included according to the following criteria: (1) aged ≥18 years; (2) diagnosis of AIS due to large vessel occlusion in the anterior circulation; (3) treated with EVT within 6 hours of stroke onset or met the DAWN or DEFUSE criteria within the extended time window;17,18 (4) pre-stroke modified Rankin Scale score (mRS) score <2. We excluded patients if they: (1) had missing routine blood examinations and follow up information; (2) had active infections within three days before treatment; (3) had chronic inflammatory diseases or taking corticosteroids; (4) had autoimmune diseases or a history of tumors.
Data Collection
Demographic and clinical data, medial history, laboratory data, procedural parameters and vital signs were retrospectively collected. Stroke severity was assessed by the National Institutes of Health Stroke Scale (NIHSS).19 Computed tomography was routinely performed at admission and repeated within 24 hours after EVT or at any time of symptom deterioration. Radiological findings were independently reviewed by two experienced neurologists blinded to this study. Cerebral ischemia was assessed by the Alberta Stroke Program Early CT score (ASPECTS).20 Collateral circulation was assessed by the American Society of Interventional and Therapeutic Neuroradiology / Society of Interventional Radiology collateral vessel grading system. Successful recanalization was defined as modified Thrombolysis in Cerebral Ischemia score of 2b or 3. Symptomatic intracranial hemorrhage (sICH) was defined according to the European Cooperative Acute Stroke Study (ECASS-III) criteria.21
Endovascular Treatment
Intravenous thrombolysis was initiated within 4.5 hours after the onset of symptoms. Therapeutic decisions of bridging therapy or direct EVT were based on the judgement of physicians. EVT was performed by experienced interventionists using stent retrievers, aspiration thrombectomy, or the combination of both techniques.
Systemic Inflammatory Indicators
We routinely collected laboratory results, including routine blood, blood chemistry, and other serological test from peripheral venous blood samples before EVT and intravenous thrombolysis treatment. The systemic inflammatory indicators were calculated with the following equations from laboratory results: IBI = CRP × (neutrophil count / lymphocyte count), neutrophil-to-lymphocyte ratio (NLR) = neutrophil count / lymphocyte count; platelet-to-lymphocyte ratio (PLR) = platelet count / lymphocyte count, systemic-immune-inflammation index (SII) = platelet count × (neutrophil count / lymphocyte count).
Follow Up and Clinical Outcome
In this study, the functional statuses of the enrolled patients were systematically assessed by trained neurologists via clinical interviews or phone calls at 90 days after the index stroke. The primary outcome was the unfavorable outcome of patients treated with EVT, which was defined as a mRS score of 3–6 at 90 days.
Statistical Analyses
Data were expressed as frequencies and percentages for categorical variables and mean ± standard deviation or median (interquartile range, [IQR]) for continuous variables. We test the normality using the Kolmogorov–Smirnov test. Differences between groups were compared with chi-square tests or Fisher’s exact tests for categorical variables t-test or Mann–Whitney U-test for continuous variables as appropriate.
We used the receiver operative characteristic curve and Delong test to evaluate the predictive accuracy of systematic inflammatory indicators for unfavorable outcome after EVT. The optimal cut-off value of IBI was determined using the maximally selected rank statistic method.22 Multivariable logistic regression models were used to investigate the association between IBI (per 1-SD increase and quartiles) and unfavorable outcome after EVT. Model 1 was an unadjusted model. Model 2 was adjusted for age, sex, and potential predictors for unfavorable outcome such as: hypertension, diabetes mellitus, atrial fibrillation, hyperlipidemia, coronary heart disease, heart failure, smoking, drinking, NIHSS score, ASPECTS score and intravenous thrombolysis treatment. Model 3 was adjusted for variables with P <0.10 in univariate analyses and other inflammatory biomarkers using the back-ward elimination method.
We used the restricted cubic spline curve with 4 knots (5th, 35th, 65th, and 95th percentiles) to assess the nonlinear relationship between IBI and unfavorable outcome adjusted for covariates finally included in the model 3,23 and the relationship was linear when the P value was greater than 0.05. Subgroup analysis was used to assess the robustness of the association between IBI and unfavorable outcome according to age, sex, NIHSS score, and intravenous thrombolysis. Furthermore, we used the net improvement index (NRI) to evaluate the improvement of the predictive accuracy after adding each systematic inflammatory indicator into model 3, respectively,24 which can compare the predictive accuracy between the original model and the original model added with systematic inflammatory indicators.
Statistical analyses were performed using R version 4.2.2. (R Foundation, Vienna, Austria), and a two-sided P value <0.05 was considered to be statistically significant.
Results
A total of 295 patients were enrolled in this study after excluding 18 patients with missing routine blood examinations and follow up information, 11 patients with active infections within three days, 4 patients with chronic inflammatory diseases and 3 patients with autoimmune diseases or hematological tumors. The baseline characteristics of patients were shown in Table 1. The mean age was 64.0 ± 12.8 years and 188 (63.7%) patients were male. The median value of IBI was 40.2 [12.6, 141.2] mg/L. 101 (34.2%) patients received intravenous thrombolysis treatment and 250 (84.7%) had successful recanalization. 23 (7.8%) patients developed sICH and 131 (44.4%) patients had 90-day unfavorable outcome after EVT. Compared to patients with favorable outcome, those with 90-day unfavorable outcome were older, had a higher proportion of hypertension, diabetes mellitus, atrial fibrillation, heart failure, internal carotid artery occlusion and successful recanalization, had lower ASPECTS scores, had higher systolic blood pressures, NIHSS scores, neutrophil counts, CRP, fasting blood glucose, IBI, NLR, and SII (all P <0.05).
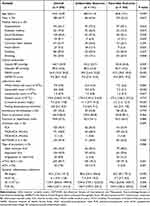 |
Table 1 Baseline Characteristics of Patients Overall and by Functional Outcomes After EVT |
Among the systematic inflammatory indicators, the IBI had the highest C-statistic (0.658; 95% CI, 0.598–0.659; Figure 1) for predicting 90-day unfavorable outcome after EVT. The results of the Delong test between IBI and other inflammatory biomarkers were 0.112 for NLR, 0.038 for SII, 0.002 for PLR. The optimal cut-off value of IBI was 23.59 mg/L in the maximally selected rank statistic method. Univariable analyses revealed that age, hypertension, diabetes mellitus, atrial fibrillation, heart failure, systolic blood pressure, NIHSS score, ASPECTS score, Neutrophil count, CRP, fasting blood glucose, occlusion site, collateral circulation, successful recanalization, type of procedure, and sICH were significant predictors for 90-day unfavorable outcome after EVT (all P <0.05; Supplementary Table 1).
In multivariable analyses, IBI was significantly associated with unfavorable outcome in model 1 (per 1-SD: odds ratio [OR], 1.805; 95% CI, 1.336–2.578; P <0.001; quartile 3 versus quartiles 1: OR, 3.218; 95% CI, 1.799–5.861; P <0.001), model 2 (per 1-SD: OR, 1.945; 95% CI, 1.372–2.861; P <0.001; quartile 3 versus quartiles 1: OR, 2.908; 95% CI, 1.485–5.810; P = 0.002), and model 3 adjusted for the selected variables after the backward elimination method: age, fasting blood glucose, heart failure, NIHSS score, ASPECTS score, mTICI, and sICH (per 1-SD: OR, 1.754; 95% CI, 1.241–2.587; P = 0.002; quartile 3 versus quartiles 1: OR, 2.564; 95% CI, 1.296–5.158; P = 0.007; Table 2 and Supplementary Table 2).
 |
Table 2 Multivariable Analyses for the Association Between IBI and Unfavorable Outcomes After EVT |
The multivariable restricted cubic spline curve showed a linear and increasing relationship between IBI and the risk of 90-day unfavorable outcome after EVT (P for nonlinearity = 0.410; Figure 2). The reclassification index analysis revealed that IBI had the highest improvement of the predictive accuracy compared with other systematic inflammatory indicators (NRI, 0.332; 95% CI, 0.029–0.635; P = 0.036; Table 3). In addition, the association between IBI and 90-day unfavorable outcome was similar across different subgroups stratified by age, sex, NIHSS score and intravenous thrombolysis treatment (all P for interaction >0.05; Figure 3).
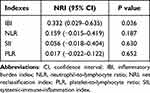 |
Table 3 Net Reclassification Index for Systemic Inflammatory Indicators |
Discussion
In this multicenter study of 295 patients with AIS treated with EVT, we investigated the association between systemic inflammation indicators and 90-day unfavorable outcome. We found that IBI was positively related to the risk of unfavorable outcome after adjusting for important prognostic covariates and might be considered a target for attenuation therapy for systemic inflammation. Compared with other inflammatory markers, IBI was a more accurate biomarker for predicting unfavorable outcomes with the highest area under the curve and reclassification indexes.
Previous studies had shown that systemic inflammation was closely associated with clinical prognosis of AIS patients. The proportion of patients with 90-day unfavorable outcome in our study (44.4%) was comparable to previous findings. Yao et al retrospectively evaluated the association between dynamic inflammatory markers and unfavorable outcome of AIS patients who achieved complete reperfusion after EVT. They found that the NLR measured at 24h and 3–7 day were independent predictors for 90-day unfavorable outcome, which could improve the diagnostic accuracy of the conventional characteristics.25 Chen et al found that platelet volume markers were correlated with stroke severity and NLR levels were independent predictors of 90-day unfavorable outcome after EVT.23 Pikija et al found that NLR was an independent predictor for intracranial hemorrhage after EVT and the post-stroke immune system may influence the pathophysiological development of intracranial hemorrhage.24
BI is a newly developed biomarker that comprehensively reflects the inflammatory status. Previous studies suggested that IBI may be a better choice for predicting the prognosis of patients with different malignancies. Song et al systemically assessed the prognostic ability of IBI for the primary hepatocellular carcinoma and compared IBI with traditional inflammatory indicators, such as NLR, PLR, and SII. They found that IBI was the optimal predictor compared with other indicators with area under the curve of 0.698.16 Xie et al explored the association between IBI and the overall survival rate of 6359 patients with common cancers and demonstrated that the grading system based on stratified IBI levels could function as the reference for evaluating the clinical prognosis of cancers.14 Xie et al also re-evaluated results in patients with non-small cell lung cancer and proposed that IBI was the optimal inflammatory biomarker among systemic inflammation-related biomarkers.
The advantage of IBI over other inflammatory indicators may lie in its measurement of the balance between immune and acute inflammation by combining hematological biomarkers of neutrophils, lymphocytes and CRP.26 The possible mechanisms of IBI and unfavorable prognosis might be explained as follows. First, neutrophils can prompt the release of free oxygen radicals and matrix metalloproteinase-9, which is an important predictor for hemorrhage transformation and secondary brain injury.27–29 Second, cerebral ischemic tissues can release inflammatory cytokines and chemokines, which guides the concentration of the leukocytes and may have neuroprotective effect on the neurological function.10,30 Third, CPR is a well-established inflammatory biomarker and is related to the ischemia-reperfusion injury, post-stroke infection, and inflammatory stimuli.31,32
To the best of our knowledge, this was the first study to investigate the association between IBI and unfavorable outcome after EVT. However, this study had several limitations. First, this was a multi-center study with a small sample size, which might generate biases due to the differences in the treatment preferences and experiences of each participating center. Second, systemic inflammatory indicators were only measured at a single time point before EVT and intravenous thrombolysis, and the dynamic changes of these indicators would be affected by intravenous thrombolysis. Third, several potentially prognostic indicators were not available due to the retrospective design of this study, such as calcitonin, interleukin-1 and calprotectin. Finally, larger prospective cohorts were warranted to further explore the role of IBI in guiding the treatment of lowing systemic inflammation in AIS patients treated with EVT.
In conclusion, our study showed that higher IBI was an independent predictor for unfavorable outcome after EVT. These results suggested that IBI can be helpful to assess the inflammatory burden of AIS patients, and may provide individual references for anti-inflammatory treatments such as recombinant human IL-1 receptor antagonist,33 minocycline,34 and fingolimod.35
Funding
Nanjing Medical Science and Technique Development Foundation: YKK21113.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387(10029):1723–1731. doi:10.1016/S0140-6736(16)00163-X
2. Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344–e418. doi:10.1161/STR.0000000000000211
3. Huo X, Ma G, Tong X, et al. Trial of endovascular therapy for acute ischemic stroke with large infarct. N Engl J Med. 2023;388(14):1272–1283. doi:10.1056/NEJMoa2213379
4. Hao Y, Yang D, Wang H, et al. Predictors for symptomatic intracranial hemorrhage after endovascular treatment of acute ischemic stroke. Stroke. 2017;48(5):1203–1209. doi:10.1161/STROKEAHA.116.016368
5. Deng G, Xiao J, Yu H, et al. Predictors of futile recanalization after endovascular treatment in acute ischemic stroke: a meta-analysis. J Neurointerv Surg. 2022;14(9):881–885. doi:10.1136/neurintsurg-2021-017963
6. Huang X, Yang Q, Shi X, et al. Predictors of malignant brain edema after mechanical thrombectomy for acute ischemic stroke. J Neurointerv Surg. 2019;11(10):994–998. doi:10.1136/neurintsurg-2018-014650
7. Kim JM, Bae JH, Park KY, et al. Incidence and mechanism of early neurological deterioration after endovascular thrombectomy. J Neurol. 2019;266(3):609–615. doi:10.1007/s00415-018-09173-0
8. van de Graaf RA, Samuels N, Chalos V, et al. Predictors of poor outcome despite successful endovascular treatment for ischemic stroke: results from the MR CLEAN Registry. J Neurointerv Surg. 2022;14(7):660–665. doi:10.1136/neurintsurg-2021-017726
9. Lattanzi S, Norata D, Divani AA, et al. Systemic inflammatory response index and futile recanalization in patients with ischemic stroke undergoing endovascular treatment. Brain Sci. 2021;11(9):1164. doi:10.3390/brainsci11091164
10. Macrez R, Ali C, Toutirais O, et al. Stroke and the immune system: from pathophysiology to new therapeutic strategies. Lancet Neurol. 2011;10(5):471–480. doi:10.1016/S1474-4422(11)70066-7
11. Zang N, Lin Z, Huang K, et al. Biomarkers of unfavorable outcome in acute ischemic stroke patients with successful recanalization by endovascular thrombectomy. Cerebrovasc Dis. 2020;49(6):583–592. doi:10.1159/000510804
12. Tardif J-C, Kouz S, Waters DD, et al. Efficacy and safety of low-dose colchicine after myocardial infarction. N Engl J Med. 2019;381(26):2497–2505. doi:10.1056/NEJMoa1912388
13. Ridker PM, MacFadyen JG, Everett BM, Libby P, Thuren T, Glynn RJ; CANTOS Trial Group. Relationship of C-reactive protein reduction to cardiovascular event reduction following treatment with canakinumab: a secondary analysis from the CANTOS randomised controlled trial. Lancet. 2018;391(10118):319–328. doi:10.1016/S0140-6736(17)32814-3
14. Xie H, Ruan G, Ge Y, et al. Inflammatory burden as a prognostic biomarker for cancer. Clin Nutr. 2022;41(6):1236–1243. doi:10.1016/j.clnu.2022.04.019
15. Xie H, Ruan G, Wei L, et al. The inflammatory burden index is a superior systemic inflammation biomarker for the prognosis of non-small cell lung cancer. J Cachexia Sarcopenia Muscle. 2023;14(2):869–878. doi:10.1002/jcsm.13199
16. Song R, Ni H, Huang J, et al. Prognostic value of inflammation-immunity-nutrition score and inflammatory burden index for hepatocellular carcinoma patients after hepatectomy. J Inflamm Res. 2022;15:6463–6479. doi:10.2147/JIR.S386407
17. Nogueira RG, Jadhav AP, Haussen DC, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018;378(1):11–21. doi:10.1056/NEJMoa1706442
18. Jovin TG, Chamorro A, Cobo E, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015;372(24):2296–2306. doi:10.1056/NEJMoa1503780
19. Brott T, Adams HP, Olinger CP, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke. 1989;20(7):864–870. doi:10.1161/01.STR.20.7.864
20. Barber PA, Demchuk AM, Zhang J, Buchan AM. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. Lancet. 2000;355(9216):1670–1674. doi:10.1016/S0140-6736(00)02237-6
21. Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359(13):1317–1329. doi:10.1056/NEJMoa0804656
22. Hothorn T, Lausen B. On the exact distribution of maximally selected rank statistics. Comput Stat Data Anal. 2003;43(2):121–137. doi:10.1016/S0167-9473(02)00225-6
23. Chen Z, He Y, Su Y, Sun Y, Zhang Y, Chen H. Association of inflammatory and platelet volume markers with clinical outcome in patients with anterior circulation ischaemic stroke after endovascular thrombectomy. Neurol Res. 2021;43(6):503–510. doi:10.1080/01616412.2020.1870359
24. Pikija S, Sztriha LK, Killer-Oberpfalzer M, et al. Neutrophil to lymphocyte ratio predicts intracranial hemorrhage after endovascular thrombectomy in acute ischemic stroke. J Neuroinflammation. 2018;15(1):319. doi:10.1186/s12974-018-1359-2
25. Feng Y, Bai X, Li W, et al. Postoperative neutrophil-lymphocyte ratio predicts unfavorable outcome of acute ischemic stroke patients who achieve complete reperfusion after thrombectomy. Front Immunol. 2022;13:963111. doi:10.3389/fimmu.2022.963111
26. Zhu H, Cao X. NLR members in inflammation-associated carcinogenesis. Cell Mol Immunol. 2017;14(5):403–405. doi:10.1038/cmi.2017.14
27. Duan Z, Wang H, Wang Z, et al. Neutrophil-lymphocyte ratio predicts functional and safety outcomes after endovascular treatment for acute ischemic stroke. Cerebrovasc Dis. 2018;45(5–6):221–227. doi:10.1159/000489401
28. Gong P, Liu Y, Gong Y, et al. The association of neutrophil to lymphocyte ratio, platelet to lymphocyte ratio, and lymphocyte to monocyte ratio with post-thrombolysis early neurological outcomes in patients with acute ischemic stroke. J Neuroinflammation. 2021;18(1):51. doi:10.1186/s12974-021-02090-6
29. Yamamoto Y, Osanai T, Nishizaki F, et al. Matrix metalloprotein-9 activation under cell-to-cell interaction between endothelial cells and monocytes: possible role of hypoxia and tumor necrosis factor-α. Heart Vessels. 2012;27(6):624–633. doi:10.1007/s00380-011-0214-5
30. Rust R, Grönnert L, Schwab ME. Inflammation after stroke: a local rather than systemic response? Trends Neurosci. 2018;41(12):877–879. doi:10.1016/j.tins.2018.09.011
31. Seo W-K, Seok H-Y, Kim JH, et al. C-reactive protein is a predictor of early neurologic deterioration in acute ischemic stroke. J Stroke Cerebrovasc Dis. 2012;21(3):181–186. doi:10.1016/j.jstrokecerebrovasdis.2010.06.002
32. Mechtouff L, Debs N, Frindel C, et al. Association of blood biomarkers of inflammation with penumbra consumption after mechanical thrombectomy in patients with acute ischemic stroke. Neurology. 2022;99(18):e2063–e2071. doi:10.1212/WNL.0000000000201038
33. Emsley HCA, Smith CJ, Georgiou RF, et al. Acute Stroke Investigators. A randomised Phase II study of interleukin-1 receptor antagonist in acute stroke patients. J Neurol Neurosurg Psychiatry. 2005;76(10):1366–1372. doi:10.1136/jnnp.2004.054882
34. Padma Srivastava MV, Bhasin A, Bhatia R, et al. Efficacy of minocycline in acute ischemic stroke: a single-blinded, placebo-controlled trial. Neurol India. 2012;60(1):23–28. doi:10.4103/0028-3886.93584
35. Zhu Z, Fu Y, Tian D, et al. Combination of the immune modulator fingolimod with alteplase in acute ischemic stroke: a pilot trial. Circulation. 2015;132(12):1104–1112. doi:10.1161/CIRCULATIONAHA.115.016371
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



