Back to Journals » Pharmacogenomics and Personalized Medicine » Volume 16
Association Between HTRA1, GAS6 and IFNGR2 Gene Polymorphisms and Stroke Susceptibility in the Chinese Han Population
Authors Zhang F, Peng H, Fu C, Deng Y, Zhang M, Li W, Zhong J, Zhou Q, Huang L, Xiao S, Zhao J
Received 16 February 2023
Accepted for publication 20 June 2023
Published 7 July 2023 Volume 2023:16 Pages 717—727
DOI https://doi.org/10.2147/PGPM.S408911
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Martin H Bluth
Fan Zhang,1,* Hao Peng,1,* Chuanyi Fu,1 Yidong Deng,1 Mao Zhang,1 Wenan Li,1 Jian Zhong,1 Qing Zhou,1 Li Huang,1 Shuli Xiao,1 Jiannong Zhao2
1Department of Cerebrovascular Disease, Hainan Affiliated Hospital of Hainan Medical University, Hainan General Hospital, Haikou, Hainan, 570311, People’s Republic of China; 2Department of Neurosurgery, Hainan Medical University, Hainan Affiliated Hospital of Hainan Medical University, Hainan General Hospital, Haikou, Hainan, 571199, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jiannong Zhao, Department of Neurosurgery, Hainan Medical University, Hainan Affiliated Hospital of Hainan Medical University, Hainan General Hospital, Haikou, 571199, Hainan, People’s Republic of China, Tel/Fax +86-13876673888, Email [email protected]
Background: Stroke has a high disability rate, and 30% of stroke cases have an unknown cause. Accurate diagnosis and treatment of stroke requires consideration of several rare heritable and non-heritable factors.
Objective: This study aimed to evaluate the impacts of three genetic polymorphisms (rs369149111 in HTRA1, rs1803628 in GAS6 and rs9808753 in IFNGR2) on stroke susceptibility among the Chinese Han population.
Methods: Three single nucleotide polymorphisms (SNPs) from 623 stroke cases and 572 healthy controls were genotyped by the Agena MassARRAY platform. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated by logistic regression analysis to evaluate the associations of three SNPs with stroke susceptibility. Additionally, SNP-SNP interactions were analyzed by multifactor dimensionality reduction (MDR).
Results: As demonstrated by the overall analysis, rs9808753 in IFNGR2 (allele: OR = 1.25, 95% CI = 1.06– 1.47, p = 0.007; homozygous: OR = 1.59, 95% CI = 1.14– 2.23, p = 0.007; dominant: OR = 1.31, 95% CI = 1.02– 1.67, p = 0.032; recessive: OR = 1.42, 95% CI = 1.05– 1.91, p = 0.022; additive: OR = 1.26, 95% CI = 1.07– 1.48, p = 0.007) was associated with an increased susceptibility to stroke. Besides, stratification analysis suggested that rs9808753 was associated with an increased risk of stroke in subgroup aged ≤ 64 years, males and drinkers (p < 0.05). And rs1803628 in GAS6 was significantly associated with an increased susceptibility to stroke in non-smokers (p < 0.05).
Conclusion: A risk-increasing effect of IFNGR2 rs980875 on stroke was detected in this study, which further broadens the understanding of the relationship between genetic polymorphisms and stroke susceptibility.
Keywords: IFNGR2, polymorphisms, stroke, susceptibility, case-control
Introduction
Stroke, a kind of common cerebrovascular disease, is a major global health problem and the second leading cause of disability and death in the world nowadays.1–3 The main types of stroke include ischemic stroke (87%), cerebral hemorrhage (10%) and subarachnoid hemorrhage (3%).2 In addition, hypertension, atherosclerosis, or heart diseases possibly lead to complications such as stroke.4 Statistically, there were 12.2 million new strokes and 101 million epidemic strokes worldwide in 2019, and the number of cases will rise in the coming years.5 By 2050, there are projected to be approximately 200 million stroke survivors worldwide, with more than 30 million new strokes and 12 million stroke deaths each year thereafter.6
In fact, as a highly heterogeneous disease, stroke can be caused by extremely diverse factors. A study on the Swedish Twin Registry has concluded that stroke is significantly inherited, and monozygotic twins are more likely to develop strokes than dizygotic twins.7,8 The heritability of stroke is approximately 40%, as estimated by two related studies.9,10 Currently, increasing evidence supports the role of genetic factors in determining stroke risk,11 and there is growing interest in identifying other genetic factors for stroke, such as single nucleotide polymorphisms (SNPs). However, few genetic loci for stroke have been found. Therefore, investigating the genetic risk factors for stroke is vital to promote the discovery of new therapeutic targets and optimize prevention strategies.12
The high temperature requirement serine peptidase A1 (HTRA1) gene, belonging to the HTRA protein family and located on chromosome 10 (10q26), encodes a thermostable serine protease.13 HTRA1 can be involved in a variety of physiological processes, including maintenance of mitochondrial homeostasis, cell signaling, and apoptosis.14 Meanwhile, as a serine enzyme mediating cell signaling, HTRA1 also plays an important role in vascular integrity, skeletal development and osteogenesis.15 Remarkably, previous studies have shown that HTRA1 mutations are closely related to the occurrence of stroke, including small vessel ischemic stroke16 and lacunar stroke.17 Moreover, it has been reported that HTRA1 mutations may contribute to stroke susceptibility.14 However, at present, little is known about the specific role of the rs369149111 polymorphism of HTRA1 in stroke susceptibility.
Growth-arrest specific gene 6 (GAS6), containing fifteen exons and spanning 43.8 kb, is located on chromosome 13q34.18 Related studies have elucidated that GAS6 can be expressed in vascular smooth muscle cells (VSMCs) and involved in the regulation of vascular homeostasis.19 VSMCs are key regulators in maintaining vascular homeostasis, and VSMC dysfunction is a common cause of stroke.20 Therefore, we speculated that GAS6 may affect stroke susceptibility by regulating VSMC function. At present, studies have revealed the relationship between GAS6 variants and disease risk, such as the role of rs1803628 in preeclampsia risk.21 However, little information reveals the relationship between rs1803628 and stroke susceptibility.
Interferon gamma receptor 2(IFNGR2), located on chromosome 21q22.11, is the second subunit of the IFN-γ receptor. Some studies have demonstrated that IFNGR2 gene polymorphisms are associated with the risk of many diseases, such as viremias22 and marginal zone B-cell lymphoma.23 And IFNGR2 (rs9808753) has been identified to be significantly related to the risk of multiple sclerosis (MS).24 An increased risk of stroke has been reported in MS patients. For example, one cohort study showed that the risk of stroke remained increased in the MS cohort compared with the control group after adjusting for confounding variables.25 However, little is known about the effect of IFNGR2 rs9808753 on stroke susceptibility, especially in the Chinese population.
Consequently, a case-control study was carried out to explore the association between HTRA1-rs369149111, GAS6-rs1803628 and IFNGR2-rs9808753 and stroke susceptibility, trying to provide a new perspective for the prevention, diagnosis and treatment of stroke.
Materials and Methods
Study Subjects
Power analysis was performed to determine the required sample size with the relevant parameters (Effect size=0.2, α=0.05, Power=0.9 and case/control=0.995) before the study. Based on the power analysis, the case and control groups should consist of at least 526 and 528 individuals, respectively. All of 1195 unrelated participants (623 stroke cases and 572 controls) were enrolled from Hainan General Hospital. Stroke cases were newly diagnosed based on the World Health Organization diagnosis criteria and were confirmed by professionals using cranial magnetic resonance imaging (MRI) or computed tomography. Patients with tumors, systemic inflammatory diseases or other serious illnesses were excluded. The controls were selected randomly from healthy subjects in Hainan General Hospital. At the same time, we conducted MRI for controls to exclude the asymptomatic stroke. The study was approved by the Ethics Committee of Hainan General Hospital. All participants signed informed consent forms and completed questionnaires about their basic information before entering the study.
Data Collection, DNA Extraction and SNP Genotyping
In this study, three SNPs (rs369149111 in HTRA1, rs1803628 in GAS6, and rs9808753 in IFNGR2) were selected for genotyping. The minor allele frequencies (MAFs) of the above SNPs from the Southern Han Chinese population in the 1000 Genomes Project (https://www.internationalgenome.org/) database were rs369149111-T = 0.186, rs1803628-A = 0.219 and rs9808753-G = 0.433, respectively, which are all greater than 0.05. The peripheral blood (5 mL) was collected from patients and healthy controls in EDTA-coated tubes. The samples were centrifuged and stored at −80℃ for following analyses. The demographic and clinical symptoms were obtained from medical records and questionnaires. Primer design was performed by Agena MassARRAY Assay Design 3.0 software. The GoldMag Genomic DNA Purification kit (GoldMag Co., Ltd., Xi’an, China) was utilized to extract genomic DNA. The DNA concentration was estimated by NanoDrop 2000 (Thermo Scientific, Waltham, Massachusetts, USA). Agena MassARRAY (Agena Bioscience, San Diego, CA, USA) was applied to genotype SNPs. Ten percent of the samples were genotyped repeatedly, and the concordance rate was 100%.
Statistical Analysis
The statistical analysis was conducted by SPSS 22.0 statistical package (SPSS, Chicago, IL, USA). Student’s t-test and the chi-square test were carried out to assess the differences of variables. Fisher’s exact test was used to evaluate Hardy-Weinberg equilibrium (HWE) in the control group. The potential functions of three SNPs were predicted by HaploReg v4.1. The association between polymorphisms and stroke susceptibility was assessed by odds ratios (ORs) and 95% confidence intervals (CIs) using logistic regression models with PLINK 1.07 (Harvard, Boston, MA, USA). Multifactor dimensionality reduction (MDR) software (version 3.0.2) was used to assess the effect of SNP-SNP interactions on stroke susceptibility. The p-value < 0.05 was thought of statistical significance.
Results
Features of Subjects
The characteristics of all subjects are illustrated in Table 1. A total of 623 stroke patients (64.05 ± 10.58 years) and 572 healthy controls (64.12 ± 5.50 years) were included in the study. No significant differences in age (p = 0.884), gender (p = 0.111), drinking (p = 0.108), and smoking (p = 0.054) between cases and controls were found. The MAFs of all candidate SNPs were greater than 0.05 (Table 2). In addition, GAS6-rs1803628 and IFNGR2-rs9808753 were both functionally associated with SiPhy cons, Enhancer histone marks (Table 2).
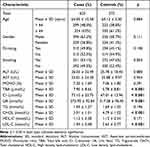 |
Table 1 Demographic Characteristic of the Cases with Stroke and Controls |
 |
Table 2 Basic Information About SNPs and Association with Risk of Stroke in Allele Model |
Association Between Three Candidate SNPs and Stroke Susceptibility
The associations between the three SNPs and stroke susceptibility under different genetic models are presented in Table 2 and Table 3. There were no significant associations of rs369149111 and rs1803628 with stroke susceptibility under the allelic and other genetic models. By contrast, the minor allele-G of rs9808753 was related to an increased risk of stroke (OR = 1.25, 95% CI: 1.06–1.47, p = 0.007). Furthermore, rs9808753 exerted an risk-increasing effect on stroke in the Chinese Han population under the homozygous (OR = 1.59, 95% CI: 1.14–2.23, p = 0.007), dominant (OR = 1.31, 95% CI: 1.02–1.67, p = 0.032), recessive (OR = 1.42, 95% CI: 1.05–1.91, p = 0.022), and additive (OR = 1.26, 95% CI: 1.07–1.48, p = 0.007) models.
 |
Table 3 Genetic Model Analyses of the Association Between Three SNPs and the Risk of Stroke (Adjusted by Age and Gender) |
Analyses Stratified by Age, Gender, Drinking Status and Smoking Status
To further examine the effects of three SNPs on stroke susceptibility, the stratification analyses based on age, gender, drinking status and smoking status were conducted (Table 4). For subjects aged ≤ 64 years, rs9808753 was correlated with an increased susceptibility to stroke under the homozygous model (OR = 1.65, 95% CI: 1.01–2.71, p = 0.046) and additive model (OR = 1.29, 95% CI: 1.01–1.65, p = 0.042). For males, the risk-increasing effect of rs9808753 on stroke susceptibility was found under the homozygous (OR = 1.82, 95% CI: 1.17–2.84, p = 0.008), recessive (OR = 1.61, 95% CI: 1.09–2.39, p = 0.017) and additive (OR = 1.33, 95% CI: 1.07–1.65, p = 0.009) modes. For subjects who drink alcohol, rs9808753 was correlated with the rising stroke susceptibility under the homozygous (OR = 2.21, 95% CI: 1.30–3.74, p = 0.003), dominant (OR = 1.53, 95% CI: 1.08–2.17, p = 0.018), recessive (OR = 1.83, 95% CI: 1.13–2.95, p = 0.014), and additive (OR = 1.46, 95% CI: 1.14–1.87, p = 0.003) modes. For non-smoking subjects, rs1803628 was correlated with an increased risk of stroke under the heterozygous (OR = 1.45, 95% CI: 1.01–2.07, p = 0.042), dominant (OR = 1.49, 95% CI: 1.06–2.09, p = 0.023) and additive (OR = 1.41, 95% CI: 1.05–1.89, p = 0.021) models. The analyses stratified by age, gender, drinking status and smoking status showed no significant association between rs369149111 and stroke susceptibility under all genetic models.
 |
Table 4 Distribution of SNPs in Different Age, Gender, Smoking Status, Drinking Status and Its Association with Risk of Stroke |
MDR Analysis of the Role of SNP-SNP Interaction in Stroke Susceptibility
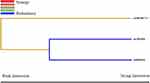
The SNP-SNP interaction was analyzed by MDR. Figure 1 is a dendrogram about the analysis of the SNP-SNP interaction. Different colors represent the synergistic or redundant effects of SNP-SNP interaction on stroke susceptibility. The brown line indicates that candidate SNPs have no synergistic or redundant effect in regulating stroke susceptibility. The blue line indicates that candidate SNPs have a redundant effect on stroke susceptibility (Figure 1). Additionally, as presented by Table 5, the rs9808753 single locus was regarded as the best model to predict stroke risk, with the highest testing balanced accuracy of 0.529 and good cross-validation consistency (10/10).
 |
Table 5 Summary of SNP-SNP Interactions on the Risk of Stroke Analyzed by MDR Method |
Difference in Indicators Based on the Genotypes of Selected SNPs
We also assessed the impact of three candidate SNPs on the level of indicators under different genotypes. As shown in Table 6, the levels of alanine transaminase (ALT, p < 0.001), aspartate aminotransferase (AST, p < 0.001), and monocytes ratio (MONO, p = 0.018) had significant differences under different genotypes of rs1803628. Differences in total cholesterol levels (CHOL, p = 0.014) under different genotypes of rs9808753 were also detected. Whereas, there was no significant difference between rs369149111 and the level of indicators (Table S1).
 |
Table 6 Clinical Characteristics of Stroke Patients Based on the Genotypes of Selected SNPs |
Discussion
To our knowledge, this study is the first to assess the relationship between three selected SNPs and stroke susceptibility in the Chinese Han population. Our results showed that rs369149111 was unrelated with stroke susceptibility in overall and stratification analyses. The rs1803628 was related to stroke susceptibility only in the non-smoking group. The rs9808753 was significantly associated with a higher stroke susceptibility under the allelic, dominant, recessive and additive models, and the relationship between rs9808753 and stroke susceptibility was age-, gender- and drinking dependent. Collectively, the results broadened our knowledge on the effects of SNPs on stroke susceptibility, and provided new clues for the screening of high-risk groups and early detection and diagnosis of the disease.
The occurrence of stroke is related to many factors. In our results, rs1803628 was related to an increased stroke susceptibility only in the non-smoking subgroup. This is inconsistent with previous studies that has identified smoking as a risk factor for stroke.26 This may be related to the random selection of the study population. In addition, we also noticed that rs9808753 was significantly associated with a higher stroke susceptibility under the allelic, dominant, recessive and additive models. Particularly, the effect of rs9808753 on stroke susceptibility might be correlated with age, gender and drinking, according to the results of stratification analyses. It has been reported that age is a major risk factor for stroke occurrence, and the older the age, the higher the stroke risk.2 However, our results suggested that rs9808753 was associated with an increased susceptibility of stroke in people younger than 64 years of age, which may be related to study participants and their lifestyles. It could also mean that stroke is becoming more common at a younger age. Similar to our findings, Mao et al have pointed out that gene polymorphisms are associated with stroke risk in Chinese males27 and Caucasian males.28 In addition, males are historically considered to be more susceptible to stroke than females,29 which is consistent with our findings. This is likely because of the higher risk of dyslipidemia, diabetes, myocardial infarction and peripheral artery disease in males, as well as their unhealthy living habits, such as smoking and alcohol consumption. Just as Millwood et al have found that alcohol consumption is a risk factor for stroke.30 All in all, these findings may suggest that genetic susceptibility to stroke differs by age, gender, drinking and smoking status in genetic association studies.
The detection of stroke-related clinical indicators is very important for the prevention, diagnosis and treatment of stroke. Our study found a significant difference in CHOL level under the different genotypes of rs9808753. As elucidated by Wang et al, CHOL value is associated with an elevated risk of incident stroke and ischemic stroke, which is partly consistent with our results.31 Furthermore, we found the levels of ALT, AST and MONO had significant differences under the different genotypes of rs1803628. However, no significant associations of AST and ALT with stroke were found,32 which may be related to the fact that rs1803628 was a risk factor for stroke only in non-drinking subjects.
It should be noted that this study has several limitations. First, all subjects were enrolled from the same hospital, so there is a selection bias. Second, due to missing information on smoking and alcohol consumption in some subjects, only age and gender were selected for adjustment in this study. Finally, the association of IFNGR2 rs9808753 with increased stroke susceptibility in this study was not functionally tested. In future studies, we will expand the sample size, improve the sample information, adjust the risk factors, and perform corresponding functional tests to further analyze the relationship between IFNGR2 rs9808753 and stroke susceptibility to make our study more convincing.
Conclusion
In summary, we found that IFNGR2-rs9808753 is significantly associated with an increased risk of stroke in the Han Chinese population, suggesting that IFNGR2 variants may be biomarkers for the early detection and diagnosis of stroke.
Data Sharing Statement
All data generated or analyzed during this study are included in this published article and its Supplementary Information.
Ethics Approval and Consent to Participate
This study was approved by the Ethics Committee of Hainan General Hospital and conformed to the ethical principles of the Declaration of Helsinki. All participants signed informed consent forms before participating in this study.
Acknowledgment
We thank all authors for their contributions and support. We are also grateful to all subjects for providing blood samples.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The study was funded by the Major Science and Technology Plan Projects in Hainan Province (No. ZDKJ202004).
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Feigin VL, Forouzanfar MH, Krishnamurthi R, et al. Global and regional burden of stroke during 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet. 2014;383(9913):245–254. doi:10.1016/s0140-6736(13)61953-4
2. Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics--2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29–e322. doi:10.1161/cir.0000000000000152
3. Zhao L, Fang J, Zhou M, et al. Interaction between COX-1 and COX-2 increases susceptibility to ischemic stroke in a Chinese population. BMC Neurol. 2019;19(1):291. doi:10.1186/s12883-019-1505-1
4. Rubattu S, Giliberti R, Volpe M. Etiology and pathophysiology of stroke as a complex trait. Am J Hypertens. 2000;13(10):1139–1148. doi:10.1016/s0895-7061(00)01249-8
5. Béjot Y. Forty years of descriptive epidemiology of stroke. Neuroepidemiology. 2022;56(3):157–162. doi:10.1159/000525220
6. Brainin M, Feigin VL, Norrving B, et al. Global prevention of stroke and dementia: the WSO declaration. Lancet Neurol. 2020;19(6):487–488. doi:10.1016/s1474-4422(20)30141-1
7. Flossmann E, Schulz UG, Rothwell PM. Systematic review of methods and results of studies of the genetic epidemiology of ischemic stroke. Stroke. 2004;35(1):212–227. doi:10.1161/01.str.0000107187.84390.aa
8. de Faire U, Friberg L, Lundman T. Concordance for mortality with special reference to ischaemic heart disease and cerebrovascular disease. A study on the Swedish Twin Registry. Prev Med. 1975;4(4):509–517. doi:10.1016/0091-7435(75)90036-5
9. Holliday EG, Maguire JM, Evans TJ, et al. Common variants at 6p21.1 are associated with large artery atherosclerotic stroke. Nat Genet. 2012;44(10):1147–1151. doi:10.1038/ng.2397
10. Bevan S, Traylor M, Adib-Samii P, et al. Genetic heritability of ischemic stroke and the contribution of previously reported candidate gene and genomewide associations. Stroke. 2012;43(12):3161–3167. doi:10.1161/strokeaha.112.665760
11. Khasanova LT, Stakhovskaya LV, Koltsova EA, et al. енетические особенности церебрального инсульта [Genetic characteristics of stroke]. Zh Nevrol Psikhiatr Im S S Korsakova. 2019;119(12.Vyp. 2):65–72. Russian. doi:10.17116/jnevro201911912265
12. Chauhan G, Debette S. Genetic risk factors for ischemic and hemorrhagic stroke. Curr Cardiol Rep. 2016;18(12):124. doi:10.1007/s11886-016-0804-z
13. Grigaitė J, Šiaurytė K, Audronytė E, et al. Novel in-frame deletion in HTRA1 gene, responsible for stroke at a young age and dementia-a case study. Genes. 2021;12(12):1955. doi:10.3390/genes12121955
14. Tian Y, Tang W, Yang S, et al. HTRA1 variants and the interaction with smoking confer the genetic susceptibility to ischemic stroke. Int J Med Sci. 2021;18(8):1840–1847. doi:10.7150/ijms.45856
15. Liu JY, Zhu YC, Zhou LX, et al. HTRA1-related autosomal dominant cerebral small vessel disease. Chin Med J. 2020;134(2):178–184. doi:10.1097/cm9.0000000000001176
16. Liu G, Zhang H, Liu B, et al. Rs2293871 regulates HTRA1 expression and affects cerebral small vessel stroke and Alzheimer’s disease. Brain. 2019;142(11):e61. doi:10.1093/brain/awz305
17. Tan RYY, Traylor M, Megy K, et al. How common are single gene mutations as a cause for lacunar stroke? A targeted gene panel study. Neurology. 2019;93(22):e2007–e2020. doi:10.1212/wnl.0000000000008544
18. Wu CS, Hu CY, Chan CJ, et al. Genetic polymorphism of the growth arrest-specific 6 gene is associated with cutaneous vasculitis in Taiwanese patients with systemic lupus erythematosus. Clin Rheumatol. 2012;31(10):1443–1448. doi:10.1007/s10067-012-2027-z
19. Clauser S, Meilhac O, Bièche I, et al. Increased secretion of Gas6 by smooth muscle cells in human atherosclerotic carotid plaques. Thromb Haemost. 2012;107(1):140–149. doi:10.1160/th11-05-0368
20. Klose R, Prinz A, Tetzlaff F, et al. Loss of the serine protease HTRA1 impairs smooth muscle cells maturation. Sci Rep. 2019;9(1):18224. doi:10.1038/s41598-019-54807-6
21. Ye L, Guan L, Fan P, et al. Association study between GAS6 gene polymorphisms and risk of preeclampsia in Chinese population. Eur J Obstet Gynecol Reprod Biol. 2017;211:122–126. doi:10.1016/j.ejogrb.2017.02.014
22. Huang HH, Shih WL, Li YH, et al. Hepatitis B viraemia: its heritability and association with common genetic variation in the interferon gamma signalling pathway. Gut. 2011;60(1):99–107. doi:10.1136/gut.2010.207670
23. Chen Y, Zheng T, Lan Q, et al. Cytokine polymorphisms in Th1/Th2 pathway genes, body mass index, and risk of non-Hodgkin lymphoma. Blood. 2011;117(2):585–590. doi:10.1182/blood-2010-07-295097
24. Lill CM, Luessi F, Alcina A, et al. Genome-wide significant association with seven novel multiple sclerosis risk loci. J Med Genet. 2015;52(12):848–855. doi:10.1136/jmedgenet-2015-103442
25. Tseng CH, Huang WS, Lin CL, et al. Increased risk of ischaemic stroke among patients with multiple sclerosis. Eur J Neurol. 2015;22(3):500–506. doi:10.1111/ene.12598
26. Chen Z, Peto R, Zhou M, et al. Contrasting male and female trends in tobacco-attributed mortality in China: evidence from successive nationwide prospective cohort studies. Lancet. 2015;386(10002):1447–1456. doi:10.1016/s0140-6736(15)00340-2
27. Mao Y, Yang L, Chen Q, et al. The influence of CYP1A1 and CYP1A2 polymorphisms on stroke risk in the Chinese population. Lipids Health Dis. 2020;19(1):221. doi:10.1186/s12944-020-01370-z
28. Hendrix P, Berger A, Person TN, et al. Sex-specific association of RAGE and HMGB1 genotype variations with susceptibility to ischemic stroke in Caucasians. J Clin Neurosci. 2021;94:328–331. doi:10.1016/j.jocn.2021.11.001
29. Reeves MJ, Bushnell CD, Howard G, et al. Sex differences in stroke: epidemiology, clinical presentation, medical care, and outcomes. Lancet Neurol. 2008;7(10):915–926. doi:10.1016/s1474-4422(08)70193-5
30. Millwood IY, Li L, Smith M, et al. Alcohol consumption in 0.5 million people from 10 diverse regions of China: prevalence, patterns and socio-demographic and health-related correlates. Int J Epidemiol. 2013;42(3):816–827. doi:10.1093/ije/dyt078
31. Wang Y, Wang A, Zuo Y, et al. Two-year change in serum total cholesterol is associated with incident ischemic stroke: results from the kailuan study. Front Neurol. 2021;12:710083. doi:10.3389/fneur.2021.710083
32. Ruban A, Daya N, Schneider ALC, et al. Liver enzymes and risk of stroke: the Atherosclerosis Risk in Communities (ARIC) study. J Stroke. 2020;22(3):357–368. doi:10.5853/jos.2020.00290
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.