Back to Journals » Infection and Drug Resistance » Volume 17
Association Between Hemoglobin-Albumin-Lymphocyte-Platelet Index and Mortality in Hospitalized COVID-19 Omicron BA.2 Infected Patients
Authors Wu W, Lu W , Hong D , Yu X, Xiong L
Received 6 December 2023
Accepted for publication 13 March 2024
Published 12 April 2024 Volume 2024:17 Pages 1467—1476
DOI https://doi.org/10.2147/IDR.S451613
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Wei Wu,1– 4,* Wenbin Lu,5,* Dongmei Hong,1– 4 Xiya Yu,1– 4 Lize Xiong1– 4
1Shanghai Key Laboratory of Anesthesiology and Brain Functional Modulation, Shanghai, 200434, People’s Republic of China; 2Department of Anesthesiology and Perioperative Medicine, Shanghai Fourth People’s Hospital, School of Medicine, Tongji University, Shanghai, 200434, People’s Republic of China; 3Translational Research Institute of Brain and Brain-Like Intelligence, Shanghai Fourth People’s Hospital, School of Medicine, Tongji University, Shanghai, 200434, People’s Republic of China; 4Clinical Research Centre for Anesthesiology and Perioperative Medicine, Tongji University, Shanghai, 200434, People’s Republic of China; 5Faculty of Anesthesiology, Changhai Hospital, Naval Medical University/Second Military Medical University, PLA, Shanghai, 200433, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiya Yu; Lize Xiong, Tel +86-21-55603999 ; +86-21-55603002, Fax +86-21-56660851, Email [email protected]; [email protected]; [email protected]
Background: The hemoglobin-albumin-lymphocyte-platelet (HALP) index is a novel biomarker reflecting systemic inflammation and nutritional status which are important for coronavirus disease 2019 (COVID-19) mortality. However, the association between HALP and mortality in patients with COVID-19 has yet to be investigated.
Methods: A cohort of COVID-19 Omicron BA.2 infected patients admitted to the Shanghai Fourth People’s Hospital, School of Medicine, Tongji University from April 12, 2022 to June 17, 2022 was retrospectively analyzed. Laboratory examinations on hospital admission, including hemoglobin, albumin, and lymphocyte and platelet, were collected. The association between baseline HALP and in-hospital poor overall survival (OS) was assessed using Kaplan–Meier curves, Cox regression models, interaction, and stratified analyses.
Results: A total of 2147 patients with COVID-19 Omicron BA.2 infection were included in the final analyses, and mortality in the hospital was 2.65%. Multivariate analysis indicated that low HALP index was independently associated with in-hospital mortality of COVID-19 patients [hazard ratio (HR) = 2.08; 95% confidence interval (CI) = 1.17– 3.73]. Subgroup analysis demonstrated that low HALP index was an independent risk factor for in-hospital mortality in COVID-19 patients with age ≥ 70 (HR = 2.22, CI = 1.18– 4.15) and severe cases (HR = 2.09, CI = 1.13– 3.86).
Conclusion: HALP index is independently related to in-hospital poor OS for COVID-19 Omicron BA.2 infected patients, especially for age ≥ 70 and severe cases. HALP index on hospital admission is a useful candidate biomarker for identifying high risk of mortality in COVID-19 Omicron BA.2 infected patients.
Keywords: biomarkers, COVID-19, hemoglobin-albumin-lymphocyte-platelet index, mortality, Omicron BA.2
Introduction
Coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Omicron BA.2 has aroused emerging global concerns.1–3 Although there have been major advances in treatment modalities and large-scale vaccination campaigns, patients with severe or critically severe COVID-19 are still prone to a poor prognosis. Previous studies have shown that patients with severe and critically severe COVID-19 were difficult to treat and had a high mortality rate, even in intensive care unit (ICU).4,5 In addition, early detection of severely infected COVID-19 patients was important to identify patients with poor prognosis.6 Therefore, it is crucial to quickly identify risk factors that can predict patient prognosis and make decisions for subsequent treatments in COVID-19 Omicron BA.2 infected patients, especially for severely infected COVID-19 patients.
A large number of studies have shown that laboratory markers had an important role in the diagnosis, prognosis, and mortality of COVID-19.7–11 Inflammation and nutritional status are also important for COVID-19 mortality. Previous studies have shown that systemic inflammatory biomarkers based on peripheral blood cells, such as derived neutrophil-to-lymphocyte ratio, neutrophil-to-lymphocyte ratio, and systemic immune-inflammation index were reported to predict the prognosis of COVID-19 patients.12–14 In addition, nutritional status, such as hemoglobin and albumin levels, has also been shown to be the useful indicators related to the prognosis of patients with moderate-to-severe COVID-19 patients.15–17
According to recent studies, a novel combined biomarker of hemoglobin-albumin-lymphocyte-platelet (HALP) can reflect both systemic inflammation and nutrition status simultaneously.18,19 It has been shown to be related to survival in patients with cancer and inflammatory diseases.20–22 However, there are no studies on the association between HALP and in-hospital mortality in COVID-19 Omicron BA.2 infected patients. Therefore, this is the first study aimed at exploring the relationship between HALP and mortality in COVID-19 patients.
Materials and Methods
Study Design and Patients
In this retrospective cohort study, COVID-19 Omicron BA.2 infected patients who were admitted to Shanghai Fourth People’s Hospital, School of Medicine, Tongji University from April 12, 2022 to June 17, 2022 were included. The diagnosis of COVID-19 was based on the test for SARS-CoV-2 Omicron BA.2 using real-time reverse transcription polymerase chain reaction. The study obtained the clinical data of 2645 patients and Figure 1 shows the flow diagram. Patients whose age was under 18 years old, having missing data including routine blood examinations and blood albumin, were excluded. The study was approved by the Ethics Committee of the hospital (No. 2022105-001). The requirement for informed consent was waived by the Ethics Commission.
 |
Figure 1 Flowchart of study population. |
Data Collection and Definition
Demographic, clinical, and laboratory data collected included age, gender, comorbidities, laboratory findings (hemoglobin, lymphocyte, platelet, albumin, monocyte, neutrophil, c-reactive protein, and d-dimer), disease severity on admission, length of hospital stay, and the days until in-hospital death. Related treatments during hospitalization were also recorded. To ensure the accuracy of the data, three investigators (DH, WW, and LX) independently reviewed the patient’s medical records.
Techniques for laboratory confirmation of SARS-CoV-2 Omicron BA.2 infection and the criteria for discharge were described in a previous study.13 The Guidelines for the Prevention and Treatment of the Novel Coronavirus (Ninth Edition) in China were used to treat all patients and to classify the severity of the disease into asymptomatic, mild, common, severe, and critically severe.23 High fever referred to axillary temperature ≥38°C. HALP was calculated as hemoglobin (g/L) × albumin (g/L) × lymphocyte (109/L)/platelet (109/L).24 We performed multiple imputation to deal with the missing data whose missing values were less than 30%.
Statistical Analysis
HALP was dichotomized by the optimal cut-off point calculated using maximally selected rank statistics. In addition, we used curve-fitting to assess the linear relationship between HALP and overall survival (OS) after adjusting for age, gender, disease severity, monocyte count, neutrophil count, c-reactive protein, d-dimer, and baseline diseases. Summary statistics of baseline information of all patients stratified by HALP were described as frequencies (percentage) for categorical variables, mean ± SD or medians (quartiles) for continuous variables. The differences were assessed using the χ2 test for categorical variables, Student’s t-test for normally distributed continuous variables, and Mann–Whitney U-test for skewed continuous variables between two groups.
OS was assessed using Kaplan–Meier curves and analyzed by the two-sided Log rank test. We first evaluated the association of HALP as a continuous variable with in-hospital mortality, and then we assessed the relationship when HALP was dichotomized. Univariate and multivariate Cox regression models were used to evaluate these relationships. We selected all variables in the study for model adjustment considering the statistical significance and clinical practice. Model 1 adjusted for age, gender, disease severity, monocyte count, neutrophil count, c-reactive protein, and d-dimer. In model 2, hypertension, diabetes, heart disease, kidney disease, lung disease, brain disease, and malignant tumor were further adjusted.
Finally, a sub-group analysis of age, gender, and disease severity was conducted to examine interaction and stratified analyses. Each subgroup adjusted for all the factors (age, gender, disease severity, monocyte count, neutrophil count, c-reactive protein, d-dimer, hypertension, diabetes, heart disease, kidney disease, lung disease, brain disease, and malignant tumor) in the multivariable Cox regression, except for the stratification factor itself. Age was adjusted to 70 according to the optimal cut-off point calculated using maximally selected rank statistics. We defined severe and critically severe patients as severe group, and asymptomatic, mild, and common patients as non-severe group in the subgroup of disease severity. The HALP ≥18.06 was defined as high HALP group, which was the reference for low HALP (HALP < 18.06).
The software packages R (http://www.R-project.org, The R Foundation) and Free Statistics software versions 1.7.1 were used to perform all statistical analyses. Statistical differences were considered significant at P<0.05.
Results
Characteristics of the Patients
Of 2147 COVID-19 Omicron BA.2 infected patients included in the final analyses, the mean age of the cohort was 73.67 ± 15.26 years, and 58.17% of the participants were female. The overall number of in-hospital mortality was 57 (2.65%). Table 1 compares the baseline demographic, clinical, laboratory data, and comorbidities of included patients stratified by HALP. Compared with patients in the higher HALP, those in the lower HALP were older, more likely to have elevated disease severity and brain disease. In addition, patients with low HALP were more likely to develop high fever, be transferred to the ICU, and receive primary care, antibiotics, and assisted ventilation. The incidence of in-hospital mortality significantly increased in patients with low HALP compared to high HALP (7.95% vs 1.70%). Besides, decreased HALP was found along with elevated disease severity in COVID-19 Omicron BA.2 infected patients (Supplementary Figure 1).
 |
Table 1 Demographics and Clinical Characteristics Stratified by HALP |
Association of HALP with Mortality in COVID-19 Patients
The cut-off point of HALP was 18.06 calculated using maximally selected rank statistics. The linear correlation between HALP and the mortality of the patients is shown in Supplementary Figure 2. The univariate Cox regression models between baseline information and mortality in Table 2 indicates that age (HR = 1.05, 95% CI, 1.03–1.08), low HALP (HR = 4.59, 95% CI, 2.72–7.73), neutrophil count (HR = 1.18, 95% CI, 1.13–1.23), c-reactive protein (HR = 1.01, 95% CI, 1.00–1.02), d-dimer (HR = 1.02, 95% CI, 1.01–1.03), severe cases (HR = 26.84, 95% CI, 3.63–198.27), critically severe cases (HR = 33.25, 95% CI, 4.50–245.54) were positively correlated with mortality. The hemoglobin (HR = 0.98, 95% CI, 0.97–0.99), albumin (HR = 0.85, 95% CI, 0.81–0.89), and lymphocyte count (HR = 0.42, 95% CI, 0.25–0.72) was negatively correlated with mortality. In addition, Kaplan–Meier curves indicated that patients with a low HALP had an unfavorable OS (Figure 2).
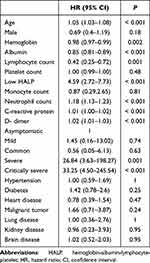 |
Table 2 The Unadjusted Association Between Baseline Variables and In-Hospital Mortality |
 |
Figure 2 Kaplan–Meier curves of mortality for COVID-19 Omicron BA.2 infected patients. |
Table 3 shows the results of multivariate Cox regression models evaluating the association between HALP and in-hospital mortality. HALP was independently associated with in-hospital mortality regarding of continuous variable (HR = 0.96, 95% CI, 0.94–0.97) or categorical variable (HR = 4.59, 95% CI, 2.72–7.73 for HALP < 18.06) in the unadjusted model. Moreover, the relationship of HALP with in-hospital mortality was not affected after adjusting for confounding factors. HALP as a continuous variable was negatively correlated with mortality (HR = 0.98, 95% CI, 0.96–0.99) in Model 2. Meanwhile, low HALP was an independent risk factor (HR = 2.08, 95% CI, 1.17–3.73) for in-hospital mortality in Model 2.
 |
Table 3 The Multivariate Cox Analysis of HALP Associated with In-Hospital Mortality |
Subgroup Analysis
A forest plot of the results for HALP in the sub-groups (Figure 3) indicated that low HALP was an independent risk factor for mortality in COVID-19 Omicron BA.2 infected patients aged ≥70 (HR = 2.22, 95% CI = 1.18–4.15), those who were severe (HR = 2.09, 95% CI = 1.13–3.86).
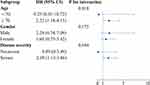 |
Figure 3 Association between HALP and in-hospital mortality in sub-groups for COVID-19 Omicron BA.2 infected patients. |
Discussion
To the best of our knowledge, this is the first retrospective cohort study investigating the relationship of HALP and in-hospital mortality in COVID-19 Omicron BA.2 infected patients. The present study included 2147 COVID-19 patients, and the total in-hospital mortality was 2.65%. We showed that low HALP significantly associated with an increased risk of poor OS during hospitalization after adjusting for confounding factors. Moreover, low HALP was stably and consistently discriminative for risk stratification in patients aged ≥70, those who were severe according to the sub-groups analysis.
Several studies have reported that inflammatory responses play an important role in the prognosis of COVID-19 patients.25–27 The innate and acquired immunity impaired in COVID-19 patients can result in lymphocyte stimulation and dysregulation.28,29 Besides, platelet stimulation produces related inflammatory factors, which have an important role in regulating immunity and inflammation during the disease.30,31 Therefore, lymphocyte dysregulation and platelet stimulation can contribute to poor prognosis of COVID-19 patients. In the present study, we found that lower lymphocyte count was associated with in-hospital death other than platelet count.
In addition, recent studies have shown that malnutrition status was associated with poor OS in COVID-19 patients.32,33 Serum albumin has anti-inflammatory, nutritional, and blood rheological properties, preventing platelet activation and aggregation.34,35 Malnutrition or hyper-catabolism can cause hypoalbuminemia, and systemic inflammation and increased cytokine release can also inhibit albumin production.36,37 Albumin has been reported as a reliable biomarker of prognosis in critically ill patients.38 In addition, it has been reported that anemia was an independent prognostic factor for survival in patients with cancer.39,40 Moreover, low hemoglobin can affect the oxygen supply which exacerbates hypoxia due to lung injury in COVID-19 patients.41 Consistent with previous studies, we found that hypoalbuminemia and low hemoglobin were related to poor OS in COVID-19 Omicron BA.2 infected patients.
However, the definite causes and pathophysiological mechanisms underlying the relationship between HALP and poor prognosis remain unclear. Based on the above data, it can be said that the HALP, calculated using hemoglobin, lymphocyte, platelet, and albumin values, is a comprehensive body reserve involving malnutrition, liver reserve, inflammation, and coagulation reserve and may be more useful than one variable in assessing the outcome of diseases. Moreover, HALP is simple and easy to calculate, and is convenient for clinical use, which is not affected by individual subjectivity. Previous studies have shown that HALP was associated with prognosis in cancer and inflammatory diseases.42,43 However, the association of HALP with mortality in COVID-19 Omicron BA.2 infected patients has yet to be investigated. The present study showed that low HALP index was independently related to in-hospital death in patients. Besides, the association did not change in patients aged ≥70 and those who were severe.
There are several clinical implications and strengths in the results of this study. HALP index can be easily and inexpensively applied to identify COVID-19 Omicron BA.2 infected patients with a high risk of mortality at an early stage. Thus, related therapies to increase HALP index may reduce in-hospital mortality. We performed strict methods of statistical adjustment to reduce the possible confounding in this observational study which was susceptible to multiple confounding factors. Besides, in order to verify the consistency of the association between HALP and all-cause mortality during hospitalization, we conducted the analyses in different subgroups of gender, age, and disease severity.
There are some limitations that should not be ignored. First, this is a retrospective study which may result in bias during data collection. Second, the sample of deaths may not have been large enough to increase validity and decrease the risk of overfitting. Third, the effect of some treatments before hospital admission on the outcome of HALP was not excluded. Finally, to test the association between HALP and in-hospital mortality, external patients with COVID-19 especially for other ethnicities need to be evaluated in the future.
Conclusion
In conclusion, low HALP index at hospital admission was an independent risk factor for in-hospital mortality of COVID-19 Omicron BA.2 infected patients in this retrospective cohort study. Future clinical research is needed to verify our findings in external patients and reduce the effects related to decreased HALP index in order to improve treatments and increase OS.
Data Sharing Statement
The data supporting the findings of this study can be obtained from the corresponding author (Xiya Yu) according to reasonable request, and the corresponding author/s can be directly contacted for further inquiry.
Ethics Statement
The study concerning human participants was reviewed and approved by the ethics committee at Shanghai Fourth People’s Hospital. The requirement for informed consent was waived by the ethics committee. The study project conforms to the ethical guidelines of the Declaration of Helsinki. In order to publish any potentially identifiable images or data contained in this article, written informed consent was obtained from the individual(s).
Acknowledgments
We thank all those who supported and participated in the study, including our staff, patients, and their family members.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Major Program of National Natural Science Foundation of China (82293640 and 82293643), the Research Initiation Foundation of Shanghai Fourth People’s Hospital (sykyqd05901), the Discipline Boosting Program of Shanghai Fourth People’s Hospital (SY-XKZT-2022-1003), and the Innovative Program of First Affiliated Hospital of Naval Medical University (2020YXK013).
Disclosure
The authors state that the study was conducted without any commercial or financial relationships and potential conflicts of interest.
References
1. Yuan HY, Liang J, Hossain MP. Impacts of social distancing, rapid antigen test and vaccination on the Omicron outbreak during large temperature variations in Hong Kong: a modelling study. J Infect Public Health. 2022;15(12):1427–1435. doi:10.1016/j.jiph.2022.10.026
2. Khan Z, Ali SA, Mohsin M, Parvin F, Shamim SK, Ahmad A. A district-level vulnerability assessment of next COVID-19 variant (Omicron BA.2) in Uttarakhand using quantitative SWOT analysis. Environ Dev Sustain. 2022;1–30. doi:10.1007/s10668-022-02727-3
3. Strasser ZH, Greifer N, Hadavand A, Murphy SN, Estiri H. Estimates of SARS-CoV-2 Omicron BA.2 Subvariant Severity in New England. JAMA Network Open. 2022;5(10):e2238354. doi:10.1001/jamanetworkopen.2022.38354
4. Liu Y, Yang Y, Zhang C, et al. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci. 2020;63(3):364–374. doi:10.1007/s11427-020-1643-8
5. Lippi G, Plebani M, Henry BM. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a meta-analysis. Int J Clin Chem. 2020;506:145–148. doi:10.1016/j.cca.2020.03.022
6. Tahir Huyut M, Huyut Z, Ilkbahar F, Mertoğlu C. What is the impact and efficacy of routine immunological, biochemical and hematological biomarkers as predictors of COVID-19 mortality? Int Immunopharmacol. 2022;105:108542. doi:10.1016/j.intimp.2022.108542
7. Huyut MT, Huyut Z. Effect of ferritin, INR, and D-dimer immunological parameters levels as predictors of COVID-19 mortality: a strong prediction with the decision trees. Heliyon. 2023;9(3):e14015. doi:10.1016/j.heliyon.2023.e14015
8. Huyut MT, Huyut Z. Forecasting of oxidant/antioxidant levels of COVID-19 patients by using expert models with biomarkers used in the diagnosis/prognosis of COVID-19. Int Immunopharmacol. 2021;100:108127.
9. Mertoglu C, Huyut MT, Arslan Y, Ceylan Y, Coban TA. How do routine laboratory tests change in coronavirus disease 2019? Scand J Clin Lab Invest. 2021;81(1):24–33. doi:10.1080/00365513.2020.1855470
10. Velichko A, Huyut MT, Belyaev M, Izotov Y, Korzun D. Machine learning sensors for diagnosis of COVID-19 disease using routine blood values for internet of things application. Sensors. 2022;22(20):7886. doi:10.3390/s22207886
11. Huyut MT, Ilkbahar F. The effectiveness of blood routine parameters and some biomarkers as a potential diagnostic tool in the diagnosis and prognosis of Covid-19 disease. Int Immunopharmacol. 2021;98:107838. doi:10.1016/j.intimp.2021.107838
12. Bilge M, Akilli IK, Karaayvaz EB, Yesilova A, Kart Yasar K. Comparison of systemic immune-inflammation index (SII), early warning score (ANDC) and prognostic nutritional index (PNI) in hospitalized patients with malignancy, and their influence on mortality from COVID-19. Infect Agent Cancer. 2021;16(1):60. doi:10.1186/s13027-021-00400-4
13. Qiu W, Shi Q, Chen F, Wu Q, Yu X, Xiong L. The derived neutrophil to lymphocyte ratio can be the predictor of prognosis for COVID-19 Omicron BA.2 infected patients. Front Immunol. 2022;13:1065345. doi:10.3389/fimmu.2022.1065345
14. Liu Y, Du X, Chen J, et al. Neutrophil-to-lymphocyte ratio as an independent risk factor for mortality in hospitalized patients with COVID-19. J Infect. 2020;81(1):e6–e12. doi:10.1016/j.jinf.2020.04.002
15. Hung KC, Ko CC, Wang LK, et al. Association of prognostic nutritional index with severity and mortality of hospitalized patients with COVID-19: a systematic review and meta-analysis. Diagnostics. 2022;12(7):1.
16. Fernandes AL, Reis BZ, Murai IH, Pereira RMR. Prognostic nutritional index and oxygen therapy requirement associated with longer hospital length of stay in patients with moderate to severe COVID-19: multicenter prospective cohort analyses. Front Nutr. 2022;9:802562. doi:10.3389/fnut.2022.802562
17. Bodolea C, Nemes A, Avram L, et al. Nutritional risk assessment scores effectively predict mortality in critically ill patients with severe COVID-19. Nutrients. 2022;14(10):1.
18. Zhao Z, Yin XN, Wang J, Chen X, Cai ZL, Zhang B. Prognostic significance of hemoglobin, albumin, lymphocyte, platelet in gastrointestinal stromal tumors: a propensity matched retrospective cohort study. World J Gastroenterol. 2022;28(27):3476–3487. doi:10.3748/wjg.v28.i27.3476
19. Cong L, Hu L. The value of the combination of hemoglobin, albumin, lymphocyte and platelet in predicting platinum-based chemoradiotherapy response in male patients with esophageal squamous cell carcinoma. Int Immunopharmacol. 2017;46:75–79. doi:10.1016/j.intimp.2017.02.027
20. Han H, Hu S, Du J. Predictive value of the hemoglobin-albumin-lymphocyte-platelet (HALP) index for ICU mortality in patients with acute exacerbations of chronic obstructive pulmonary disease (AECOPD). Int Emerg Med. 2022;18(1):85–96. doi:10.1007/s11739-022-03132-4
21. Güç ZG, Alacacıoğlu A, Kalender ME, et al. HALP score and GNRI: simple and easily accessible indexes for predicting prognosis in advanced stage NSCLC patients. The İzmir oncology group (IZOG) study. Front Nutr. 2022;9:905292. doi:10.3389/fnut.2022.905292
22. Xu M, Chen L, Hu Y, et al. The HALP (hemoglobin, albumin, lymphocyte, and platelet) score is associated with early-onset post-stroke cognitive impairment. Neurol Sci. 2022;2022:1.
23. Hou YC, Su WL, Chao YC. COVID-19 illness severity in the elderly in relation to vegetarian and non-vegetarian diets: a single-center experience. Front Nutr. 2022;9:837458. doi:10.3389/fnut.2022.837458
24. Jiang P, Kong W, Gong C, et al. Predicting the recurrence of operable cervical cancer patients based on Hemoglobin, Albumin, Lymphocyte, and Platelet (HALP) score and classical clinicopathological parameters. J Inflamm Res. 2022;15:5265–5281. doi:10.2147/JIR.S383742
25. Lu N, Gu T, Tian X, et al. Acetylshikonin inhibits inflammatory responses and papain-like protease activity in murine model of COVID-19. Signal Transduct Target Ther. 2022;7(1):371. doi:10.1038/s41392-022-01220-7
26. Prasad AS, Malysa A, Bepler G, Fribley A, Bao B. The mechanisms of zinc action as a potent anti-viral agent: the clinical therapeutic implication in COVID-19. Antioxidants. 2022;11(10):1862. doi:10.3390/antiox11101862
27. Yalcinkaya M, Liu W, Islam MN, et al. Modulation of the NLRP3 inflammasome by Sars-CoV-2 envelope protein. Sci Rep. 2021;11(1):24432. doi:10.1038/s41598-021-04133-7
28. Zheng M, Gao Y, Wang G, et al. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell Mol Immunol. 2020;17(5):533–535.
29. Sun Y, Zou Y, Wang H, Cui G, Yu Z, Ren Z. Immune response induced by novel coronavirus infection. Front Cell Infect Microbiol. 2022;12:988604. doi:10.3389/fcimb.2022.988604
30. Mabrouk M, Guessous F, Naya A, Merhi Y, Zaid Y. The pathophysiological role of platelet-derived extracellular vesicles. Semin Thromb Hemost. 2022;49(3):279–283. doi:10.1055/s-0042-1756705
31. Carestia A, Godin LC, Jenne CN. Step up to the platelet: role of platelets in inflammation and infection. Thromb Res. 2023;231:182–194. doi:10.1016/j.thromres.2022.10.001
32. Can B, Senturk Durmus N, Olgun Yıldızeli S, Kocakaya D, Ilhan B, Tufan A. Nutrition risk assessed by nutritional risk screening 2002 is associated with in-hospital mortality in older patients with COVID-19. Nutr Clin Pract. 2022;37(3):605–614. doi:10.1002/ncp.10860
33. Liu H, Zhou L, Wang H, et al. Malnutrition is associated with hyperinflammation and immunosuppression in COVID-19 patients: a prospective observational study. Nutr Clin Pract. 2021;36(4):863–871. doi:10.1002/ncp.10679
34. Ozcan Cetin EH, Könte HC, Temizhan A. Blood viscosity should not be overlooked when evaluating the fibrinogen to albumin ratio. Angiology. 2019;70(5):465–466. doi:10.1177/0003319718822244
35. Baratta F, Bartimoccia S, Carnevale R, Stefanini L, Angelico F, Del Ben M. Oxidative stress mediated platelet activation in patients with congenital analbuminemia: effect of albumin infusion. J Thromb Haemost. 2021;19(12):3090–3094. doi:10.1111/jth.15545
36. Lucijanic M, Veletic I, Rahelic D, et al. Assessing serum albumin concentration, lymphocyte count and prognostic nutritional index might improve prognostication in patients with myelofibrosis. Wiener klinische Wochenschrift. 2018;130(3–4):126–133. doi:10.1007/s00508-018-1318-z
37. Fang CJ, Saadat GH, Butler BA, Bokhari F. The geriatric nutritional risk index is an independent predictor of adverse outcomes for total joint arthroplasty patients. J Arthroplasty. 2022;37(8):S836–S841. doi:10.1016/j.arth.2022.01.049
38. Jin X, Li J, Sun L, et al. Prognostic value of serum albumin level in critically ill patients: observational data from large intensive care unit databases. Front Nutr. 2022;9:770674. doi:10.3389/fnut.2022.770674
39. Fang Y, Sun X, Zhang L, Xu Y, Zhu W. Hemoglobin/red blood cell distribution width ratio in peripheral blood is positively associated with prognosis of patients with primary Hepatocellular carcinoma. Med Sci Monit. 2022;28:e937146.
40. Belcher DA, Ju JA, Baek JH, et al. The quaternary state of polymerized human hemoglobin regulates oxygenation of breast cancer solid tumors: a theoretical and experimental study. PLoS One. 2018;13(2):e0191275. doi:10.1371/journal.pone.0191275
41. Patiño-Aldana AF, Ruíz Sternberg ÁM, Pinzón Rondón ÁM, Molano-Gonzalez N, Rodriguez lima DR. Interaction effect between hemoglobin and hypoxemia on COVID-19 mortality: an observational study from Bogotá, Colombia. Int J Gene Med. 2022;15:6965–6976. doi:10.2147/IJGM.S371067
42. Solmaz S, Uzun O, Sevindik OG, et al. The effect of haemoglobin, albumin, lymphocyte and platelet score on the prognosis in patients with multiple myeloma. Int J Lab Hematol. 2023;45(1):13–19. doi:10.1111/ijlh.13958
43. Tian M, Li Y, Wang X, et al. The Hemoglobin, Albumin, Lymphocyte, and Platelet (HALP) score is associated with poor outcome of acute ischemic stroke. Front Neurol. 2021;11:610318. doi:10.3389/fneur.2020.610318
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
