Back to Journals » International Journal of General Medicine » Volume 17
Association Between Germline BRCA1/2 Gene Variants and Clinicopathological Features of Ovarian Cancer
Authors Luo Y, Pan R, Rao H , Chen X, Yang H
Received 20 October 2023
Accepted for publication 3 January 2024
Published 11 January 2024 Volume 2024:17 Pages 75—84
DOI https://doi.org/10.2147/IJGM.S445660
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Yu Luo,1,2 Ru Pan,1,2 Hui Rao,2,3 Xing Chen,4 Haikun Yang1,2
1Department of Gynaecology, Meizhou People’s Hospital, Meizhou Academy of Medical Sciences, Meizhou, People’s Republic of China; 2Meizhou Municipal Engineering and Technology Research Center for Molecular Diagnostics of Major Genetic Disorders, Meizhou People’s Hospital, Meizhou, People’s Republic of China; 3Department of Laboratory Medicine, Meizhou People’s Hospital, Meizhou Academy of Medical Sciences, Meizhou, People’s Republic of China; 4Data Center, Meizhou People’s Hospital, Meizhou Academy of Medical Sciences, Meizhou, People’s Republic of China
Correspondence: Haikun Yang, Department of Gynaecology, Meizhou People’s Hospital, Meizhou Academy of Medical Sciences, Add: No. 63 Huangtang Road, Meijiang District, Meizhou, People’s Republic of China, Email [email protected]
Objective: To investigate the relationship between BRCA1/2 gene mutation and clinicopathological features in ovarian cancer patients, so as to develop precise individualized treatment plan for patients.
Methods: Patients diagnosed with ovarian cancer between January 2018 and July 2023 who underwent BRCA1/2 genetic testing were retrospectively analyzed. The clinicopathological characteristics (age, body mass index (BMI), family history of ovarian cancer, pregnancy history, menopause status, tumor size, histopathology, Federation of Gynecology and Obstetrics (FIGO) staging, and ascites) of non-carriers and BRCA1/2 variant carriers were compared. Logistic regression analysis was used to explore the relationship between BRCA1/2 variants and clinicopathological characteristics of ovarian cancer.
Results: A total of 284 ovarian cancer patients were collected, and the subjects were divided into two groups, 197 non-carriers and 87 BRCA1/2 variants carriers. The proportion of serous ovarian carcinoma in BRCA1/2 variant carriers is higher than that in non-BRCA variant carriers (78.2% vs 60.9%, p=0.015). There were 51 patients with BRCA pathogenic or likely pathogenic variant, 22 patients with BRCA likely benign variant, and 14 patients with BRCA variants of uncertain significance (VUS). The proportion of serous ovarian carcinoma in patients with BRCA pathogenic/likely pathogenic variant is higher than that in patients with BRCA likely benign variant and BRCA VUS (94.1% vs 50.0% and 64.3%. p< 0.001). There were no statistically significant differences in BMI, family history of ovarian cancer, pregnancy history, menopause status, maximum diameter of the tumor lesion, FIGO stage, and ascites among patients with different grades of variants. Multivariate logistic regression analysis showed that serous ovarian carcinoma was related to BRCA mutation (Serous carcinoma vs non-serous carcinoma: OR 2.145, 95% CI: 1.044– 4.407) (p=0.038).
Conclusion: Patients with BRCA1 variant develop ovarian cancer at a younger age than those with the BRCA2 variant. The proportion of FIGO stage III–IV in patients with BRCA pathogenic + likely pathogenic variant was significantly higher than those in patients with other variants. Germline BRCA1/2 variants were most frequently identified in serous ovarian carcinoma patients.
Keywords: ovarian cancer, BRCA1, BRCA2, clinicopathological characteristics
Background
Ovarian cancer is one of the most common gynecological cancers worldwide. According to global cancer statistics, ovarian cancer makes up 1.6% of all new cancer cases, accounting for 2.1% of all cancer deaths in 2020.1 Ovarian cancer was the eighth most common causes of cancer-associated death among women, accounting for 4.7% of female mortality rates worldwide.1 In China, an estimated 45,000 new cases and almost 29,000 deaths of ovarian cancer occurred in 2019, and the burden of ovarian cancer increased in women over 40 years old, especially in postmenopausal women.2 Ovarian cancer burden in China is expected to continue to rise with a higher rate than the global level in the next decade.2 Due to the lack of disease-specific symptoms in ovarian cancer, most patients are diagnosed at advanced stages, giving rise to a greatly increased risk of cancer metastasis and early mortality.3,4 Although advances are being made, ovarian cancer remains the most fatal female gynecologic cancer, so that further research into the characteristics of these patients should be performed for early control.
The symptoms of ovarian cancer are non-specific compared with other female cancers that have early warning symptoms.5 Therefore, it is very important to effectively judge the disease development status and severity of ovarian cancer through some predictable indicators. Various risk factors are described to be associated with ovarian cancer, including older age, genetics, family history, nulliparity, and so on.6 Studies have shown that more than 20% of ovarian cancer have a genetic susceptibility, and about 70% of these genetic abnormalities are germline mutations in the breast cancer susceptibility gene, BRCA gene.7 Breast cancer susceptibility gene 1 (BRCA1) and breast cancer susceptibility gene 2 (BRCA2) are two distinct tumor suppressor genes, which play an integral role in response to cellular stress via the activation of DNA repair processes.8–10 BRCA1 gene is located on chromosome 17q21, including 24 exons that encodes 1863 amino acids.11 BRCA1 has two tandem repeat C-terminal domain of BRCA1 (BRCT) domains at the C terminus, including BRCT1 and BRCT2, which are important signaling and protein targeting domains in the DNA damage repair system and are closely related to the important functions of BRCA1.12,13 BRCA2 gene is localized at 13q12-13 with 27 exons and encodes 3418 amino acids.13,14 The C terminal of BRCA2 protein contains five domains, and studies have shown that missense mutations involved in tumorigenesis mainly occur in these five domains in the C terminus of the BRCA2 protein, which play a key role in tumor suppressor function.15 Carriers of mutations in the BRCA1 and BRCA2 genes have an increased risk of ovarian cancer.16–18 In addition, some studies have found that the BRCA1/2 germline mutations are associated with prognosis of ovarian cancer.19,20
Few studies have reported the correlation between BRCA gene mutations and clinicopathological features of ovarian cancer.5,21,22 However, another study has found no correlation of them.23 It is unclear whether BRCA gene mutations are associated with clinicopathological features in ovarian cancer patients, and whether they can guide treatment and early prevention. Therefore, this article explores the clinical and pathologic characteristics, menstruation, and reproductive conditions in germline BRCA1/2 variant carriers, which helps to better understand the tumor characteristics of patients with germline BRCA1/2 variants in the patients with ovarian cancer.
Materials and Methods
Participants
We conducted a retrospective analysis of 284 ovarian cancer patients from our institute who were tested for BRCA1/2 gene mutations between January 2018 and July 2023. The inclusion criteria were as follows: (1) patients diagnosed with ovarian cancer; (2) undergoing BRCA gene testing; and (3) complete clinical data. Exclusion criteria: the genetic test result was of uncertain significance. The clinicopathological and demographic data extracted from the medical records of the patients, including age, body mass index (BMI) of patients, family history of ovarian cancer, pregnancy history, menopause status, maximum diameter of the tumor lesion, histopathology, Federation of Gynecology and Obstetrics (FIGO) staging, and whether the patient is accompanied by ascites. This study was approved by the Ethics Committee of Medicine, Meizhou People’s Hospital, Meizhou Academy of Medical Sciences. All participants signed informed consent in accordance with the Declaration of Helsinki.
BRCA1/2 Testing
Approximately 2 mL of peripheral blood was collected in a tube containing EDTA, and genomic DNA was extracted according to the QIAamp DNA Blood Mini Kit instructions (Qiagen, Germany). The genomic DNA samples were sent to CapitalBio (Beijing, China) and subjected to next-generation sequencing on the Ion Proton instrument (Life Technologies). All procedures were performed according to the standard operating procedures of the Life Technology Company. The sequencing results were compared with the BRCA1 (NM_007294.3) and BRCA2 (NM_000059.3) reference sequences for variant detection. According to the Human Genome Variation Society (HGVS) guidelines, there are five grades of variants: pathogenic variants, likely pathogenic variants, variants of uncertain significance (VUS), likely benign variants, and benign variants.13
This study divided ovarian cancer patients who had been tested for BRCA1/2 gene variants into two groups: (1) BRCA1/2 variant/variants carriers, (2) non-BRCA1/2 variant/variants carriers. The demographic data and clinicopathological characteristics of the two groups of patients were tabulated, and the two groups of patients were compared.
Statistical Analysis
SPSS statistical software version 26.0 was used for data analysis. Qualitative variables were presented using frequencies and percentages (N, %). The χ2 test or Fisher’ s exact test was used to compare frequencies of qualitative variables. Univariate and multivariate logistic regression analyses were performed to evaluate the relationship between clinical and pathological parameters of ovarian cancer and BRCA mutation. Values of odds ratios (ORs) and 95% confidence intervals (95% CI) were calculated to measure the strength of the associations by logistic regression. All p values were two-sided, and p<0.05 was considered statistically significant.
Results
Clinical and Pathological Characteristics of Ovarian Cancer Patients
All clinical and pathological data of the 284 ovarian cancer patients are summarized in Table 1. There were 139 (48.9%) and 145 (51.1%) patients with <55 years old and ≥55 years old, respectively. Most of the patients were diagnosed after with pregnancy history (95.1%) or in a menopausal status (about 64.1%). The patients with maximum diameter of tumor lesion ≥ 5cm accounted for 68.0%. Most of the patients were diagnosed at stage III–IV (184/284, 64.8%), while 79 patients were diagnosed at stage I–II (79/284, 27.8%). There were 180 (63.4%) patients with ascites. The proportion of serous ovarian carcinoma in BRCA1/2 variant carriers is higher than that in non-BRCA variant carriers (78.2% vs 60.9%, p=0.015). There were no statistically significant differences in age, BMI, family history of ovarian cancer, pregnancy history, menopause status, maximum diameter of the tumor lesion, FIGO stage, and ascites between non-BRCA1/2 variant/variants carriers and BRCA1/2 variant/variants carriers.
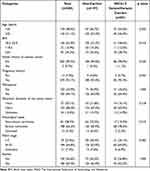 |
Table 1 Clinical Characteristics in gBRCA1/2 Variant/Variants Carriers and Non-Carriers |
Comparison of the Clinical Characteristics of BRCA Variant Carriers According to Different Grades of Variants
In this study, there were 51 patients with BRCA pathogenic or likely pathogenic variant, 22 patients with BRCA likely benign variant, and 14 patients with BRCA VUS. The proportion of serous ovarian carcinoma in patients with BRCA pathogenic or likely pathogenic variant is higher than that in patients with BRCA likely benign variant and BRCA VUS (94.1% vs 50.0% and 64.3%. p<0.001). The proportion of FIGO stage III–IV in patients with BRCA pathogenic + likely pathogenic variant was significantly higher than those in patients carried BRCA likely benign variant and BRCA VUS (82.4% vs 54.5% and 42.9%. p=0.009). There were no statistically significant differences in BMI, family history of ovarian cancer, pregnancy history, menopause status, maximum diameter of the tumor lesion, and ascites among patients with different grades of variants (Table 2). In this study, 45 patients carried the BRCA1 variant, 38 patients carried the BRCA2 variant, and 4 patients carried both the BRCA1 and BRCA2 gene variants. The patients with BRCA1 variant were younger than the patients with BRCA2 variant, accounting for 66.7% (30/45) and 42.1% (16/38) in patients younger than 55 years, respectively. Patients with the BRCA1 gene variant had a higher proportion of cases younger than 55 years of age than those with the BRCA2 gene variant (p=0.021). The histological subtypes were classified into serous ovarian cancer and non-serous ovarian cancer. In the patients with BRCA1 variant, the histological subtype was mainly serous ovarian carcinoma (91.1%) compared with patients with BRCA2 variant (60.5%). There were no statistically significant differences in BMI, family history of ovarian cancer, pregnancy history, menopause status, maximum diameter of the tumor lesion, FIGO stage, and ascites between patients with BRCA1 variant and BRCA2 variant (Table 2).
 |
Table 2 Comparison of the Clinical Characteristics of BRCA Variant Carriers According to Different Grades of Variants |
Comparison of the Clinical Characteristics of BRCA Variant Carriers According to Different Grades of Variants of BRCA1 and BRCA2, Respectively
In the patients with BRCA1 variant, the proportion of serous ovarian carcinoma in patients with pathogenic or likely pathogenic variant is higher than that in patients with likely benign variant and VUS (97.1% vs 60.0% and 87.5%. p=0.030). The proportion of III–IV stage in patients with pathogenic or likely pathogenic variant is higher than those in patients with likely benign variant and VUS (82.9% vs 60.0% and 37.5%. p=0.037). In the patients with BRCA2 variant, patients with the BRCA2 VUS had a higher proportion of cases younger than 55 years of age than those with other grades of BRCA2 variants (p=0.046). The proportion of serous ovarian carcinoma in patients with pathogenic or likely pathogenic variant is higher than that in patients with likely benign variant and VUS (87.5% vs 47.1% and 33.3%. p=0.005). There were no statistically significant differences in BMI, family history of ovarian cancer, pregnancy history, menopause status, maximum diameter of the tumor lesion, FIGO stage, and ascites among patients with different grades of variants in BRCA1 and BRCA2, respectively (Table 3).
 |
Table 3 Comparison of the Clinical Characteristics of BRCA Variant Carriers According to Different Grades of Variants of BRCA1 and BRCA2, Respectively |
Logistic Regression of Clinical Characteristics Related to BRCA Gene Variant in Ovarian Cancer
Logistic regression analysis was performed to determine which clinical features are associated with BRCA mutation. Univariate logistic regression showed that serous ovarian carcinoma was related to BRCA mutation (serous carcinoma vs non-serous carcinoma: odds ratio (OR) 2.133, 95% confidence interval (CI): 1.157–3.934) (p=0.015). Univariate logistic regression analysis showed no correlation between the other clinical characteristics and BRCA variant. Multivariate logistic regression analysis showed that serous ovarian carcinoma was related to BRCA mutation (serous carcinoma vs non-serous carcinoma: OR 2.145, 95% CI: 1.044–4.407) (p=0.038) (Table 4). That is to say, germline BRCA1/2 variants were most frequently identified in serous ovarian carcinoma patients.
 |
Table 4 Multivariate Logistic Regression of Clinical Characteristics Related to BRCA Gene Variant in Ovarian Cancer |
Discussion
The risk of ovarian cancer in female lifetime is about 2% and is the leading cause of death from any gynecologic malignancy.24 The prognosis of ovarian cancer remains relatively poor, especially in low-resource settings. Therefore, it is important to continuously examine the burden of ovarian cancer to identify domain differences.25 Therefore, it is important to educate women and health care providers about the risk factors for ovarian cancer. However, the signs and symptoms of ovarian cancer historically have been nonspecific and vague. Studies have indicated that there have multiple environmental and genetic factors for ovarian cancer. The most intensively studied risk factors have been family history, pregnancy history, oral contraceptive use, menopause, body mass index (BMI), and number of pregnancies.26–28
Moreover, mutations of BRCA1 and BRCA2 are mainly associated with a genetic risk of ovarian cancer, and can increase the risk of ovarian cancer from 1.6% to 40% and 18%, respectively.29,30 BRCA1 and BRCA2 are autosomal dominant genes that are the most studied genes among mutations associated with hereditary ovarian cancer syndrome.11,31 Mutations in BRCA1 and BRCA2 account for hereditary breast and ovarian cancer syndrome in a majority of families and 14% of epithelial ovarian cancer cases.32 Different populations may have different characteristics of BRCA1/2 gene variants. Zhang et al found that the prevalence and spectrum of variants in BRCA1/2 genes in the population which this study was conducted were different from those of other nationalities.33 BRCA1 and BRCA2 are the tumor suppressor genes, and highly penetrating mutations in these genes result in a loss of tumor suppressor function and thus an increased risk of ovarian cancer.34 Understanding whether the unique clinical and pathological features of ovarian cancer are associated with the BRCA1/2 gene mutation is essential to mitigate prognosis differences in the female population.
As far as the current literature reports are concerned, the relationship between BRCA gene and ovarian cancer is mostly studied in the aspects of treatment effect, prognosis, and recurrence of ovarian cancer.19,35 There is relatively little research on the relationship between BRCA germline variants and clinicopathological features of ovarian cancer patients. In this study, germline BRCA1/2 variants were most frequently identified in serous ovarian carcinoma patients. Vera M Witjes et al showed that germline BRCA1/2 pathogenic variants were most frequently identified in high-grade serous ovarian carcinoma patients.36 A study from an Israeli population showed that BRCA variant carriers had a higher rate of serous cancer than non-carriers.37 Patients with germline BRCA variants were frequently observed in ampulla type and FIGO I/II stage fallopian tube cancers in Japanese women.22 In addition, the age of patients carried BRCA variant was significantly lower than that of BRCA wild-type patients.38 BRCA1/2 variants were significantly associated with age, family history, and FIGO stage, according to a study from China.39 But this study did not get similar results. However, Li et al found no significant differences in age-of-onset, FIGO stage, pathological type, and family disease history between patients with BRCA1/2 mutation and others.40
Ovarian serous carcinoma is the most common histological type of ovarian epithelial carcinoma, accounting for 30–70% of the entire ovarian cancer, and has a high degree of clinical malignancy.41 Norquist et al studied p53 imprinted and tubal intraepithelial carcinoma in patients with inherited BRCA1 mutation, and found that there was loss of heterozygosity of wild-type allele BRCA1 in tubal intraepithelial carcinoma, but not in p53 imprinted, suggesting loss of BRCA gene function after TP53 gene mutation, and it may be a key event that drives cells to become cancerous.42 Chromosome instability is one of the causes of tumorigenesis and plays an important role in the occurrence and development of ovarian tumors. Singer et al performed a single nucleotide polymorphism (SNP) analysis on the DNA of serous ovarian tumors and found that there was an imbalance of the upper genes of chromosomes 1p, 5q, 8p, 18q, 22q and Xp.43 The insufficiency or absence of BRCA function can lead to the defect of homologous recombination (DHR) repair, which leads to chromosome instability.44,45
The study has some limitations. First, the number of research objects in this study is relatively small, which leads to some deviations in the results. Second, we only studied the germline variants of BRCA genes in ovarian cancer patients, and did not compare the variants of BRCA genes in somatic cells of these patients. Third, this study was limited to the relationship between BRCA1/2 gene variants and clinicopathological features of ovarian cancer patients, and the association between BRCA1/2 gene variants and treatment response and prognosis in patients with ovarian cancer was not analyzed. Therefore, future studies need to collect more cases for comprehensive analysis.
Conclusions
This study analyzed the relationship of BRCA1/2 variants and clinical and pathological characteristics of ovarian cancer patients. Patients with BRCA1 variant develop ovarian cancer at a younger age than those with the BRCA2 variant, and BRCA2 VUS carriers develop the disease at a younger age than other grades of BRCA2 variant carriers. The proportion of FIGO stage III–IV in patients with BRCA pathogenic + likely pathogenic variant was significantly higher than those in patients with other variants. It should be mentioned that germline BRCA1/2 variants were most frequently identified in serous ovarian carcinoma patients. BRCA1/2 mutation detection should be performed in patients with serous ovarian carcinoma to evaluate the prognosis of clinical treatment.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics Approval
All participants were informed on the study procedures and goals and the study obtained written informed consent from all the participants. We confirm that all methods were performed in accordance with relevant guidelines and regulations. This study was approved by the Human Ethics Committees of Meizhou People’s Hospital (Clearance No.: MPH-HEC 2022-C-100).
Acknowledgments
The authors would like to thank their colleagues who were not listed in the authorship of the Department of Gynaecology, Meizhou People’s Hospital, for their helpful comments on the manuscript.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by the Science and Technology Program of Meizhou (Grant No.: 2019B0202001), Medical and Health Research Project of Meizhou (2020-B-37).
Disclosure
The authors declare that they have no competing interests.
References
1. Sung H, Ferlay J, Siegel RL. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Wang Y, Wang Z, Zhang Z, Wang H, Peng J, Hong L. Burden of ovarian cancer in China from 1990 to 2030: a systematic analysis and comparison with the global level. Front Public Health. 2023;11:1136596. doi:10.3389/fpubh.2023.1136596
3. Urban RR, He H, Alfonso R, Hardesty MM, Gray HJ, Goff BA. Ovarian cancer outcomes: predictors of early death. Gynecol Oncol. 2016;140(3):474–480. doi:10.1016/j.ygyno.2015.12.021
4. Sambasivan S. Epithelial ovarian cancer: review article cancer treatment and research communications. Cancer Treat Res Commun. 2022;33:100629. doi:10.1016/j.ctarc.2022.100629
5. Ali AT, Al-Ani O, Al-Ani F. Epidemiology and risk factors for ovarian cancer. Prz Menopauzalny. 2023;22(2):93–104. doi:10.5114/pm.2023.128661
6. Phung MT, Pearce CL, Meza R, Jeon J. Trends of ovarian cancer incidence by histotype and race/ethnicity in the United States 1992–2019. Cancer Res Commun. 2023;3(1):1–8. doi:10.1158/2767-9764.CRC-22-0410
7. Toss A, Tomasello C, Razzaboni E, et al. Hereditary ovarian cancer: not only BRCA 1 and 2 genes. Biomed Res Int. 2015;2015:341723. doi:10.1155/2015/341723
8. Filippini SE. Breast cancer genes: beyond BRCA1 and BRCA2. Front Biosci. 2013;18(4):1358–1372. doi:10.2741/4185
9. Narod SA, Foulkes WD. BRCA1 and BRCA2: 1994 and beyond. Nat Rev Cancer. 2004;4(9):665–676. doi:10.1038/nrc1431
10. Polyak K. Breast cancer gene discovery. Expert Rev Mol Med. 2002;4(18):1–18. doi:10.1017/S146239940200491X
11. Miki Y, Swensen J, Shattuck-Eidens D, et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science. 1994;266(5182):66–71. doi:10.1126/science.7545954
12. Deng CX, Scott F. Role of the tumor suppressor gene brca1 in genetic stability and mammary gland tumor formation. Oncogene. 2000;19(8):1059–1064. doi:10.1038/sj.onc.1203269
13. Bhattacharyya A, Ear US, Koller BH, Weichselbaum RR, Bishop DK. The breast cancer susceptibility gene BRCA1 is required for subnuclear assembly of rad51 and survival following treatment with the DNA cross-linking agent cisplatin. J Biol Chem. 2000;275(31):23899–23903. doi:10.1074/jbc.C000276200
14. Pellegrini L, Yu DS, Lo T, et al. Insights into DNA recombination from the structure of a RAD51-BRCA2 complex. Nature. 2002;420(6913):287–293. doi:10.1038/nature01230
15. Jasin M. Homologous repair of DNA damage and tumorigenesis: the BRCA connection. Oncogene. 2002;21(58):8981–8993. doi:10.1038/sj.onc.1206176
16. King MC, Marks JH, Mandell JB. Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science. 2003;302(5645):643–646. doi:10.1126/science.1088759
17. van der Kolk DM, de Bock GH, Leegte BK, et al. Penetrance of breast cancer, ovarian cancer and contralateral breast cancer in BRCA1 and BRCA2 families: high cancer incidence at older age. Breast Cancer Res Treat. 2010;124(3):643–651. doi:10.1007/s10549-010-0805-3
18. Mavaddat N, Peock S, Frost D, et al. Cancer risks for BRCA1 and BRCA2 mutation carriers: results from prospective analysis of EMBRACE. J Natl Cancer Inst. 2013;105(11):812–822. doi:10.1093/jnci/djt095
19. Yoshida R. Hereditary breast and ovarian cancer (HBOC): review of its molecular characteristics, screening, treatment, and prognosis. Breast Cancer. 2021;28(6):1167–1180. doi:10.1007/s12282-020-01148-2
20. Huang Y-W. Association of BRCA1/2 mutations with ovarian cancer prognosis: an updated meta-analysis. Medicine. 2018;97(2):e9380. doi:10.1097/MD.0000000000009380
21. McAlpine JN, Porter H, Köbel M, et al. BRCA1 and BRCA2 mutations correlate with TP53 abnormalities and presence of immune cell infiltrates in ovarian high-grade serous carcinoma. Mod Pathol. 2012;25(5):740–750. doi:10.1038/modpathol.2011.211
22. Sakurada S, Watanabe Y, Tokunaga H, et al. Clinicopathologic features and BRCA mutations in primary fallopian tube cancer in Japanese women. Jpn J Clin Oncol. 2018;48(9):794–798. doi:10.1093/jjco/hyy095
23. Kim YT, Nam EJ, Yoon BS, et al. Germline mutations of BRCA1 and BRCA2 in Korean sporadic ovarian carcinoma. Gynecol Oncol. 2005;99(3):585–590. doi:10.1016/j.ygyno.2005.06.058
24. Gupta KK, Gupta VK, Naumann RW. Ovarian cancer: screening and future directions. Int J Gynecol Cancer. 2019;29(1):195–200. doi:10.1136/ijgc-2018-000016
25. Cabasag CJ, Fagan PJ, Ferlay J. Ovarian cancer today and tomorrow: a global assessment by world region and human development index using GLOBOCAN 2020. Int J Cancer. 2022;151(9):1535–1541. doi:10.1002/ijc.34002
26. Daly M, Obrams GI. Epidemiology and risk assessment for ovarian cancer. Semin Oncol. 1998;25(3):255–264.
27. Phung MT, Muthukumar A, Trabert B, et al. Effects of risk factors for ovarian cancer in women with and without endometriosis. Fertil Steril. 2022;118(5):960–969. doi:10.1016/j.fertnstert.2022.07.019
28. Husby A, Wohlfahrt J, Melbye M. Pregnancy duration and ovarian cancer risk: a 50-year nationwide cohort study. Int J Cancer. 2022;151(10):1717–1725. doi:10.1002/ijc.34192
29. Slatnik CL, Duff E. Ovarian cancer: ensuring early diagnosis. Nurse Pract. 2015;40(9):47–54. doi:10.1097/01.NPR.0000450742.00077.a2
30. Tschernichovsky R, Goodman A. Risk-reducing strategies for ovarian cancer in BRCA mutation carriers: a balancing Act. Oncologist. 2017;22(4):450–459. doi:10.1634/theoncologist.2016-0444
31. O’Donovan PJ, Livingston DM. BRCA1 and BRCA2: breast/ovarian cancer susceptibility gene products and participants in DNA double-strand break repair. Carcinogenesis. 2010;31(6):961–967. doi:10.1093/carcin/bgq069
32. Andrews L, Mutch DG. Hereditary ovarian cancer and risk reduction. Best Pract Res Clin Obstet Gynaecol. 2017;41:31–48. doi:10.1016/j.bpobgyn.2016.10.017
33. Zhang Y, Wu H, Yu Z, et al. Germline variants profiling of BRCA1 and BRCA2 in Chinese Hakka breast and ovarian cancer patients. BMC Cancer. 2022;22(1):842. doi:10.1186/s12885-022-09943-0
34. Paik HJ, Jung YJ, Kim DI, et al. Clinicopathological features of BRCA1/2 mutation-positive breast cancer. Oncology. 2021;99(8):499–506. doi:10.1159/000515790
35. Chelariu-Raicu A, Coleman RL. Breast cancer (BRCA) gene testing in ovarian cancer. Chin Clin Oncol. 2020;9(5):63. doi:10.21037/cco-20-4
36. Witjes VM, van Bommel MHD, Ligtenberg MJL, et al. Probability of detecting germline BRCA1/2 pathogenic variants in histological subtypes of ovarian carcinoma. A Meta-Analysis Gynecol Oncol. 2022;164(1):221–230. doi:10.1016/j.ygyno.2021.10.072
37. Helpman L, Zidan O, Friedman E. Young Israeli women with epithelial ovarian cancer: prevalence of BRCA mutations and clinical correlates. J Gynecol Oncol. 2017;28(5):e61. doi:10.3802/jgo.2017.28.e61
38. Kubelac P, Vlad C, Berindan Neagoe I, Irimie A, Achimas Cadariu P. The clinical features associated with mutated BRCA1 and 2 genes in ovarian cancer patients. J BUON. 2019;24(4):1538–1543.
39. Bu H, Chen J, Li Q, et al. BRCA mutation frequency and clinical features of ovarian cancer patients: a report from a Chinese study group. J Obstet Gynaecol Res. 2019;45(11):2267–2274. doi:10.1111/jog.14090
40. Li L, Chen F, Lin A, Wang D, Shi Y, Chen G. Detection of BRCA1/2 mutation and analysis of clinicopathological characteristics in 141 cases of ovarian cancer. Comput Math Methods Med. 2021;2021:4854282. doi:10.1155/2021/4854282
41. Hollis RL. Molecular characteristics and clinical behaviour of epithelial ovarian cancers. Cancer Lett. 2023;555:216057. doi:10.1016/j.canlet.2023.216057
42. Norquist BM, Garcia RL, Allison KH, et al. The molecular pathogenesis of hereditary ovarian carcinoma: alterations in the tubal epithelium of women with BRCA1 and BRCA2 mutations. Cancer. 2010;116(22):5261–5271. doi:10.1002/cncr.25439
43. Singer G, Kurman RJ, Chang HW, Cho SK, Shih Ie M. Diverse tumorigenic pathways in ovarian serous carcinoma. Am J Pathol. 2002;160(4):1223–1228. doi:10.1016/s0002-9440(10)62549-7
44. Prat J, D’Angelo E, Espinosa I. Ovarian carcinomas: at least five different diseases with distinct histological features and molecular genetics. Hum Pathol. 2018;80:11–27. doi:10.1016/j.humpath.2018.06.018
45. Zhao W, Wiese C, Kwon Y, Hromas R, Sung P. The BRCA tumor suppressor network in chromosome damage repair by homologous recombination. Annu Rev Biochem. 2019;88(1):221–245. doi:10.1146/annurev-biochem-013118-111058
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
