Back to Journals » International Journal of General Medicine » Volume 17
Association Between Family History in Patients with Primary Gout and Left Ventricular Diastolic Function: A Cross-Sectional Study
Authors Wen W , Lei P , Dang W, Ma L, Hu J, Liu J
Received 21 November 2023
Accepted for publication 20 February 2024
Published 3 April 2024 Volume 2024:17 Pages 1311—1322
DOI https://doi.org/10.2147/IJGM.S450951
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Vinay Kumar
Wen Wen,1,* Ping Lei,1,* Wantai Dang,2 Liwen Ma,1 Jing Hu,1 Jian Liu1
1Department of Ultrasound, First Affiliated Hospital, Clinical Medical College of Chengdu Medical College, Chengdu, People’s Republic of China; 2Department of Rheumatology, First Affiliated Hospital, Clinical Medical College of Chengdu Medical College, Chengdu, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jian Liu, Department of Ultrasound, First Affiliated Hospital of Chengdu Medical College, 278 Baoguang Avenue, Xindu District, Chengdu, Si Chuan, 610500, People’s Republic of China, Email [email protected]
Objective: This study aimed to employ echocardiography for measuring the markers of left ventricular (LV) diastolic function to investigate the effects of family history of gout on the LV diastolic function in patients with primary gout.
Methods: Two hundred and eighty-four patients with primary gout who visited the Department of Rheumatology and Immunology of the First Affiliated Hospital of Chengdu Medical College from September 2020 to July 2022 were selected and their family history of gout, general information, and laboratory markers were recorded. Parameters of LV diastolic function were measured via echocardiography. The correlation between family history and LV diastolic function markers was analyzed using univariate and multivariate regression and the receiver operating characteristic (ROC) curve analyses.
Results: LV diastolic function parameters, peak early mitral diastolic velocity (E)/peak late mitral diastolic velocity (A), and early septal mitral annulus diastolic motion velocity (Sepe’), early lateral mitral annulus diastolic motion velocity (Late’) and their mean (e’), were significantly lower in patients with familial primary gout, while left atrial volume index (LAVI) and E/e’ were markedly elevated in patients with sporadic primary gout. In patients with family history, the proportion of grade ≥ 2 LV diastolic insufficiency was distinctly higher than that in patients without family history (41.6% vs 12.3%). Even after adjusting for confounding variables, LAVI, E/A, Sepe’, Late’, e’, E/e’ were obviously associated with family history of gout. The area under ROC of family history combined with SUA level for identifying grade ≥ 2 LV diastolic insufficiency in patients with primary gout was 0.872 (P< 0.05).
Conclusion: Family history of gout was closely related to echocardiographic LV diastolic function parameters in patients with gout, what is more, family history of gout combined with SUA level was found to be a valuable indicator for discriminating grade ≥ 2 LV diastolic insufficiency in patients with primary gout.
Keywords: primary gout, family history, echocardiography, left ventricular diastolic function
Introduction
According to the statistics of the World Health Organization, about 3.9% of people in the world are suffering from gout, while in China, the incidence of gout ranges from 1% to 3%. Gout is more common in men than in women, and in population-based studies from Europe and North America, the male to female sex ratio has been reported to range between 2:1 and 4:1. However, the sex ratio in studies from Asia is much higher, at approximately 8:1.1 Gout is commonly associated with cardiovascular disease, hypertension, obesity, diabetes, dyslipidemia and chronic kidney disease. These conditions can complicate the management of gout and contribute to premature mortality. Of those, cardiovascular disease is the major cause of increased mortality in patients with gout. On the other hand, reciprocally, gout is a potential risk factor and a poor prognostic factor for cardiovascular disease. Growing evidence has indicated that persistent elevated uric acid may have direct negative effects on cardiac function, further leading to clinical heart failure, especially heart failure patients with preserved ejection fraction (HFpEF).2–4
In total, ~20% of patients with gout are reported to have family history. Genetic predispositions are known to play an important role in the pathogenesis of gout.5 In genome-wide association studies for gout, about 183 loci have been identified that contribute to high or low serum uric acid (SUA) and 55 of these loci are associated with the risk of gout. The identified loci explain approximately 7.7% of the variation in SUA concentrations, suggesting a genetic predisposition to gout. A previous segregation and linkage analysis of familial gout reveal an autosomal-arbitrary major gene model, which indicate a genetic basis for familial gout.6 Findings from twin studies also show that the heritability of serum urate is 45–73%.7 Although the familial aggregation of gout is influenced by both genetic and lifestyle/biological factors, accumulating evidences showed that the magnitude of familial risk increased with increasing genetic relatedness. The risk was highest among individuals with more than one affected first-degree relatives, followed by siblings, then offspring.8 These findings suggest that a genetic factor plays a substantial role in the familial aggregation of gout.
Given that both genetic factors and inflammatory component contributed to the complex mechanism of gout pathogenesis, it may be possible that these factors influence cardiac dysfunction. Individuals with a family history of gout may yield a greater impact on cardiac insufficiency compared to non-genetically predisposed persons. However, to the best of our knowledge, no such study has so far been conducted. Echocardiography has an irreplaceable function in the assessment of cardiac function, particularly diastolic function (ventricular relaxation and filling).9 Therefore, a comprehensive investigation is needed to explore the effect of family history of gout on left ventricular (LV) diastolic function by measuring LV diastolic function parameters. Accordingly, the presented study enrolled 284 patients with primary gout and collected their demographic characteristics, chronic comorbidities, disease characteristics, and multiple biochemical indexes, aiming to investigate the diagnostic value of family history of gout in LV diastolic insufficiency.
Methods
Patients
The study was a cross-sectional study involving 284 patients diagnosed with primary gout and 60 healthy controls. Patients with primary gout who presented for the first time to the Department of Rheumatology and Immunology, the First Affiliated Hospital of Chengdu Medical College from September 2020 to July 2022 were enrolled. Inclusion criteria: Diagnostic criteria for gout proposed by the American College of Rheumatology (ACR) and the European League Against Rheumatism (EULAR) in 2015; Standard uric acid lowering therapy was not performed. Exclusion criteria: (1) Patients with diabetes, hypertension, kidney diseases and secondary gout. (2) Patients with past valvular heart disease, cardiomyopathy, coronary artery disease. (3) Patients with arrhythmia and pacemaker implantation. (4) Previous history of cardiac surgery. (5) Patients with autoimmune diseases or severe infections. (6) Patients who have been infected with the COVID-19. The healthy control group was recruited from the physical examination center of our hospital, and the recruitment criteria were as follows: no history of tumor, chronic disease, cardiovascular disease, physical examination, electrocardiogram, echocardiogram results were normal. All participants signed informed consent forms, and the study received approval from the First Affiliated Hospital of Chengdu Medical College’s Medical Ethics Committee (2019CYFYHEC-BA-34).
Identification of Gout Family History
Only individuals who had an identifiable biological father and mother were enrolled. In addition to the proband, there was more than one case of gout in the family line within three generations of direct or collateral relatives, namely, familial gout patient. Specifically, family member within three generations of direct or collateral relatives of the proband (defined as the individual diagnosed with primary gout) were categorized as “exposed” from the date of diagnosis of the first affected case in the family. The second family member to be diagnosed was defined as the first “familial case”, and so forth. The diagnostic information of the familial case was obtained from the hospital medical electronic records system. The system distinctly contained family relationship information of all enrolled patients.
Echocardiography
The examination was performed by senior cardiovascular sonographers (Associate professor, working for more than ten years) who were blinded to the patients’ clinical information. An EPIQ 7C color Doppler ultrasonography (Philips, Washington, USA) with an S5-1 probe was used and frequency was 1–5 MHz.
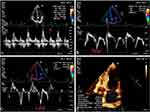
LV end-diastolic and LV end-systolic volumes were measured in apical four-chamber cardiac section and apical two-chamber cardiac section by transthoracic two-dimensional echocardiography, and the left atrial volume index (LAVI) was calculated by the biplane Simpson method (Figure 1d). Peak mitral valve velocities in early diastole (E) and late diastole (A) were measured using a pulsed Doppler (Figure 1a), and E/A was calculated. Tissue Doppler measurements of septal mitral annular early diastolic motion velocity (Sepe’) and lateral mitral annular early diastolic motion velocity (Late’) were recorded (Figure 1b and c), and the mean of mitral annular septal and lateral early diastolic motion velocities (e’) were calculated to determine E/e’.
The ACR assessment markers for LV diastolic function was combined with the Chinese Medical Association’s Clinical Application Guidelines for Echocardiographic Assessment of Cardiac Systolic and Diastolic Function (2020), which contains the following main reference markers for abnormal LV diastolic function in patients with normal LVEF: (1) mitral annular e’ velocity (septal e’ < 7 cm/s or lateral wall e’ < 10 cm/s); (2) mean E/e’ > 14; (3) LAVI > 34 mL/m²; and (4) maximum tricuspid regurgitation velocity > 2.8 m/s. If two or three of these three markers are negative or if only two markers are available and are negative, this suggests normal left atrial pressure and grade 1 diastolic insufficiency; if two or three of these three markers are positive or if only two markers are available and are positive, this suggests increased left atrial pressure and grade 2 diastolic insufficiency.
Clinical Data
Family history of gout and general data (age, disease duration, height, weight, and BMI) of all participants were collected. Laboratory tests were performed within 24 hours of echocardiography. Creatinine (CREA), serum uric acid (SUA), cystatin C (CysC), β2-microglobulin (β2-MG), triglyceride (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C) were collected.
Statistical Analysis
Non-normally distributed variables were expressed as median (quartiles) using the Mann–Whitney U-test; categorical data were compared using the chi-square test; the relationship among demographic statistics, laboratory markers, and LV diastolic function was analyzed using Spearman correlation analysis and point-biserial correlation; the relationships between family history and LV diastolic function parameters and between family history and LV diastolic insufficiency grade were analyzed using linear regression, then a receiver operating characteristic (ROC) curve was plotted and areas under the curve (AUC) were determined. Regarding the calculation of the study size, the general guidance of limiting variable inclusion to 1 variable per 10 outcome events was used. All statistical analyses were performed using SPSS v21.0 (IBM, Armonk, NY, USA). A difference with two-tailed P-value <0.05 was considered significant.
Results
Comparison of general data, laboratory markers, and LV diastolic function parameters between the primary gout and the control groups
The mean age of the 284 patients with primary gout was 42.3(33.2~53.8) years and the mean age of the 60 controls was 44.6(39.7~62.0) years. Of those, 244 are male and 40 are female (male to female ratio was 6.1:1). CREA, SUA, CysC, β2MG, and TG levels increased in patients with primary gout than in controls (all P < 0.05); HDL-C levels decreased in patients with primary gout than in controls (P = 0.003). There were no statistical differences in BMI, TC and LDL-C levels between primary gout patients and controls (Table 1).
 |
Table 1 Comparison of General Data, Laboratory Markers, and Left Ventricular Diastolic Function Parameters Between the Primary Gout Group and the Control Group |
Compared to the control group, E/A, Sepe’, Late’, and e’ were distinctly lower in patients with primary gout (all P < 0.001), whereas E/e’ and LAVI were obviously higher (all P < 0.001). Further, LV diastolic insufficiency in the primary gout patients and the control group was graded according to the American Society of Echocardiography guidelines according to four markers of LV diastolic insufficiency, and the results indicated that all cases of LV diastolic insufficiency in the control group were of grade 1, and 80.3% of the patients in the primary gout group experienced grade 1 LV diastolic insufficiency and 19.7% had grade ≥2 (Table 1).
Comparison of general data, laboratory markers, and LV diastolic function parameters between familial primary gout and sporadic primary gout groups
Two hundred and eighty-four patients with primary gout were divided into familial primary gout group (n=89) and sporadic primary gout group (n=195) according to family history of gout. Patients in familial primary gout group were younger but had longer disease duration than that in sporadic primary gout group. The levels of SUA, TG, TC, and LDL-C were higher in the familial primary gout group than in the sporadic primary gout group (all P < 0.05), while the levels of HDL-C were lower than in the sporadic primary gout group (P = 0.009). There were no statistically significant differences in BMI, CREA, CysC, and β2MG levels between the familial primary gout group and the sporadic primary gout group (Table 2).
 |
Table 2 Comparison of General Data, Laboratory Parameters, and LV Diastolic Function Parameters Between the Familial Primary Gout Group and the Sporadic Primary Gout Group |
Compared to the sporadic primary gout group, patients with familial primary gout had lower E/A, Sepe’, Late’, and e’ (all P < 0.05), but elevated E/e’ and LAVI (all P < 0.05). Significant LV diastolic insufficiency was observed in patients with familial primary gout. Specifically, 41.6% of patients with familial primary gout had grade ≥2 diastolic insufficiency and 58.4% had grade <2 insufficiency, whereas 87.7% of patients with sporadic primary gout had grade <2 diastolic function and 12.3% had grade ≥2 diastolic function (Table 2).
Correlation between LV diastolic function parameters and family history of gout, general information, and laboratory markers
Correlation analysis between LV diastolic function parameters and family history of gout, general data, and laboratory markers showed that E/e’, LAVI, and LV diastolic insufficiency grade were positively correlated with family history of gout (all P < 0.001), and E/A, Sepe’, Late’, and e’ were negatively correlated with family history of gout (all P < 0.001), which were presented in Table 3. In addition, E/A was negatively correlated with age, disease course, BMI, and CysC; E/e’ was positively correlated with age, disease course, and SUA. Moreover, LAVI related to BMI, SUA, and CysC positively, meanwhile, LV diastolic insufficiency classification related to disease course, CREA, SUA, CysC, and TG positively and related to HDL-C negatively.
 |
Table 3 Correlation Between Family History, General Information, Laboratory Markers, and Echocardiographic Parameters in the Familial Primary Gout Group and the Sporadic Primary Gout Group |
Regression Analysis of LV Diastolic Function Parameters and Family History of Gout
Linear regression analysis (Table 4) indicated that E/A, Sepe’, Late’, and e’ remained significant negative correlation with family history of gout (all P < 0.05), and E/e’ and LAVI were positively correlated with family history of gout (all P < 0.05). As shown in Table 4, these negative and positive correlations were maintained when adjusted for age, BMI, disease course, CREA, SUA, CysC, β2MG, TC, and HDL-C.
 |
Table 4 Correlation Between Echocardiographic Parameters and Family History of Gout |
The capability of family history of gout, SUA and the combination of both to identify grade ≥2 LV diastolic insufficiency
Further, we constructed ROC curves to explore the identification performance of family history and SUA for grade ≥2 LV diastolic insufficiency in patients with primary gout. As shown in Figure 2, the AUC of family history of gout and SUA for discriminating grade ≥2 LV diastolic insufficiency was 0.714 and 0.845, respectively (both P<0.05). What is more, the combination of family history of gout and SUA, presented a good value in discriminating grade ≥2 LV diastolic insufficiency (AUC=0.872, P<0.05), which was more valuable than family history of gout alone.
 |
Figure 2 AUC of family history of gout, SUA and the combination of both predicting the grade ≥2 LV diastolic insufficiency. |
Discussion
In this study, we investigated the effects of family history of gout on LV diastolic function in patients with primary gout and the identification value of family history of gout on grade ≥2 higher LV diastolic insufficiency in patients with primary gout. The results showed that family history of gout was closely associated with LV diastolic insufficiency, supported by the following points: (1) LV diastolic function parameters, E/A, Sepe’, Late’, and e’ were lower and E/e’ and LAVI measured by echocardiography were higher in patients with family history of gout than those without that; (2) The proportion of grade ≥2 LV diastolic insufficiency was increased in patients with family history of gout; (3) E/e’, LAVI, and LV diastolic insufficiency grades were positively correlated with family history of gout, while E/A, Sepe’, Late’, and e’ were negatively correlated with family history of gout; (4) ROC curve analysis indicated that family history of gout could identify grade ≥2 LV diastolic insufficiency in patients with primary gout, and the identification ability was better when combined with SUA level.
Under normal circumstances, the production and excretion of uric acid in the human body are in relative equilibrium and the occurrence of hyperuricemia and gout inevitably disrupts this equilibrium. Epidemiological studies have revealed that elevated uric acid increases the risk of cardiovascular diseases (including coronary heart disease, stroke, congestive heart failure, hypertension, and atrial fibrillation) in populations.10 In addition, both high uric acid levels and gout are associated with an increased risk of death in patients with gout, and this is mostly attributed to adverse cardiovascular events.11 The pathogenesis of gout and cardiovascular disease system involvement is complex and has not been fully elucidated to date, but most scholars believe that it is mainly an interaction between inflammatory processes, oxidative stress, and genetic factors.1 Increased uric acid production promotes LV hypertrophy and cardiac fibrosis by stimulating inflammation and oxidative stress, which leads to diastolic dysfunction.12 Moreover, uric acid leads to the deposition of monosodium urate (MSU) crystals in joint tissues, which causes macrophage activation, thereby inducing the expression of various pro-inflammatory cytokines and proteases that are dependent on signaling pathways (such as NF-κB, JAK/STAT) and activating nucleotide-binding oligomerization domain (NOD)-like receptor protein 3 (NLRP3). NLRP3 inflammasomes rapidly recruit neutrophils and release large amounts of pro-inflammatory mediators, such as interleukin (IL)-1β, IL-6, tumor necrosis factor-α, and other inflammatory cytokines, forming a positive feedback loop of inflammation and interacting with the local microenvironment. This is amplified through a cascade and ultimately triggers a severe inflammatory response leading to adverse cardiovascular outcomes.1,13,14
However, this theory that inflammation occurs due to high SUA levels and activation of the intrinsic immune system by MSU crystals does not fully explain some clinical problems of gout. For example, during acute gout attacks, most patients have elevated blood uric acid levels, but some patients with gouty arthritis do not have elevated blood uric acid levels; moreover, some patients exhibit remarkable finding of gout stones in the articular cavity but do not develop a gout attack.15 It is well known that genetic factors have important effects on the development of gout.16 A previous segregation and linkage analysis of familial gout revealed an autosomal-arbitrary major gene model, which indicated a genetic basis for familial gout.6 Moreover, findings in the relevant literature have identified a series of genes associated with the development of gout that directly affect SUA levels, gout-related immunity, inflammation, or urate transport. For example, genes that closely relate to uric acid synthesis, hypoxanthine-guanine phosphoribosyltransferase 1 and phosphoribosylpyrophosphate synthase 1, and genes that affect uric acid secretion and reabsorption, adenosine triphosphate-binding cassette transmembrane transporter protein G superfamily member 2 (ABCG2) and solute carrier family 2 member 9. Based on the genotyping of 387 Han Chinese male patients with gout and 576 controls in China via polymerase-chain reaction restriction fragment length polymorphisms, the investigators suggested that IL-8 251T/A and IL-12B 1188A/C gene mutations are host susceptibility factors for gout development. In a separate case–control study of 400 gout patients and 582 controls, it was reported that IL-23R rs7517847 G/T gene mutation may be correlated with gout in Han Chinese men in China. In addition, another studies have reported that non-coding regions are present in approximately 90% of susceptibility risk genes in autoimmune diseases, and non-coding RNAs participate in disease development mainly by recruiting epigenetic regulators or transcription factors to activate or silence transcriptional processes.17 A close association between non-coding RNA and the pathogenesis of gout has been found in the ongoing exploration, implying that non-coding RNAs may play an important role in gout pathogenesis. In summary, genetic mechanisms are involved in the development of gout. With the rapid advances in gout genetics research, we can predict the occurrence of gout at the genetic level and guide personalized medication use. Therefore, the data on the family history of patients with gout can be included in the assessment of gout diagnosis and treatment to provide more targeted and precise treatment for patients.
Although LV structure and systolic function have not been significantly altered in patients with early gout, studies have found that gout can lead to LV diastolic dysfunction.18 Diastolic dysfunction is the cardinal component for the diagnosis of HFpEF. Identifying these gout patients with diastolic dysfunction in an early clinical stage is important, aiming to prevent heart failure development with preserved ejection fraction. Echocardiography is currently the first-line tool for the noninvasive assessment of LV diastolic function and the echocardiographic criteria for the assessment of LV diastolic function are well established.19 Of these parameters (LAVI, E/A, Sepe’, Late’, e’, E/e’), LAVI by body surface area is an important parameter and a robust predictor of advanced cardiovascular diseases.20 The left atrium stores blood during LV systole acts as a conduit during early LV diastole, and acts as an auxiliary pump for the left ventricle during late diastole. Decreased left atrial storage function and auxiliary pump function may reflect progressive deterioration of LV diastolic insufficiency. In the present study, LAVI was obviously greater in patients with familial primary gout compared to those with sporadic primary gout. Increased LAVI is the result of pressure and volume overload and is an important indicator of LV diastolic insufficiency grade and an important indicator of left atrial remodeling.21 Some investigators found that LAVI was elevated in all time phases in patients with gout and was more pronounced in gout than in hyperuricemia.22 Moreover, peak left atrial longitudinal strain and left atrial longitudinal strain rate were reduced in patients with gout, and the severity of gout was independently and negatively correlated with left atrial longitudinal strain rate in late ventricular diastole.18 These findings and the results of the current study indicated that increased left atrial volume, decreased deep left atrial longitudinal myocardial contractile function, and particularly left atrial auxiliary pump function were main manifestations of LV diastolic dysfunction in patients with gout.
In this study, we have also shown that, compared with patients with sporadic gout, patients with familial gout are characterized by younger in age, longer course, increased SUA concentration and abnormal blood lipid levels (increases in TG, TC and LDL-C levels, and decreased in HDL-C level). We found that elevated SUA was strongly associated with LV diastolic dysfunction, even though the elevated uric acid levels were low. This finding is consistent with the results of previous studies. As mentioned previously,23 dysfunction of many genes leads to uric acid transport disorders resulting in hyperuricemia. For example, the ABCG2 gene, a well-established risk locus for gout, encodes a uric acid transporter that plays a key role in renal and intestinal uric acid excretion.1 Simultaneously, abnormal ABCG2 gene releases IL-8 from endothelial cells, which induces an inflammatory response.24 In the present study, LV diastolic function was worse in patients with familial primary gout than in those with sporadic primary gout. The present study reported that genetic factors may contribute to this condition, however, other factors should not be ignored. First, in our study, patients with familial primary gout were younger and had a longer disease course. Second, TC, LDL-C and TG concentrations were higher in patients with familial primary gout than in those with sporadic primary gout. As is well known, age and dyslipidemia are established traditional risk factors for cardiovascular diseases and important risk factors for LV diastolic dysfunction as well. Notably, the highlight of this study is that, family history is strongly associated with parameters of LV diastolic insufficiency in patients with primary gout, and this association remained significant even after correction for age, disease course, TC, and HDL-C. Thus, the importance of family history assessment should not be underestimated. What is more, to the best of our knowledge, this is the first study to examine the identification performance of family history combined with SUA level for grade ≥2 LV diastolic insufficiency in patients with primary gout. The result showed that the combination of family history of gout and SUA, presented a good value in discriminating grade ≥2 LV diastolic insufficiency. These findings reinforce the importance of family history of gout patients in LV diastolic insufficiency risk.
It has to be admitted that there are some unavoidable limitations in the current study. First, this is a single-center study and is limited to a regional population. Second, this is a cross-sectional study that does not determine the causality of disease occurrence and progression. Third, although our study excluded diseases that can traditionally cause LV diastolic insufficiency, such as hypertension and diabetes mellitus, there are still other factors that may cause LV diastolic insufficiency that have not been fully considered. Fourth, new technologies such as more sensitive blood flow vector imaging, stress echocardiography, and ultrasound instantaneous wave intensity are needed, and the integrated use of multiple echocardiographic techniques needs to be considered in future studies, to further reveal in-depth structural and functional damage to the cardiovascular system in patients with gout.
Conclusions
Family history of gout in primary gout patients was significantly associated with LV diastolic function parameters. LV diastolic function was worse in patients with family history of gout than those without family history. Additionally, family history of gout is a valuable indicator for discriminating grade ≥2 LV diastolic insufficiency in patients with primary gout. A combination of family history and SUA level in patients with gout had a better identification performance. Early clinical determination and intervention of LV diastolic insufficiency can be achieved by asking patients with gout if they have family history of gout.
Ethics Statement
Approval of the Ethics Committee of First Affiliated Hospital of Chengdu Medical College’s Medical Ethics Committee (2019CYFYHEC-BA-34) was obtained, and the written informed consents were obtained from all the participants. The study conformed to the Declaration of Helsinki and collected the required data from the clinical records without clinical intervention to protect patients’ privacy.
Acknowledgments
The authors thank the study subjects for their participation and supporting.
Funding
This research was funded by the National Natural Science Foundation of China (No. 81800446); Key Projects of Health Commission of Sichuan Province (No. 19ZD004); Key R&D Projects of Chengdu Science and Technology Bureau (No. 2022-YF05-01414-SN) and Scientific Research Project of The First Affiliated Hospital of Chengdu Medical College (No. CYFY-GQ18).
Disclosure
The authors declare that they have no competing interests.
References
1. Dalbeth N, L GA, Gaffo A, et al. Gout[J]. Lancet. 2021;397(10287):1843–1855. doi:10.1016/S0140-6736(21)00569-9
2. D AR, N BF, B KW, et al. Gout and coronary heart disease: the Framingham study[J]. J Clin Epidemiol. 1988;41(3):237–242. doi:10.1016/0895-4356(88)90127-8
3. Shimizu T, Yoshihisa A, Kanno Y, et al. Relationship of hyperuricemia with mortality in heart failure patients with preserved ejection fraction[J]. Am J Physiol Heart Circ Physiol. 2015;309(7):H1123–H1129. doi:10.1152/ajpheart.00533.2015
4. Vaduganathan M, J GS, P AA, et al. Relation of serum uric acid levels and outcomes among patients hospitalized for worsening heart failure with reduced ejection fraction (from the efficacy of vasopressin antagonism in heart failure outcome study with tolvaptan trial)[J]. Am J Cardiol. 2014;114(11):1713–1721. doi:10.1016/j.amjcard.2014.09.008
5. J MT, Dalbeth N, A SE, et al. An update on the genetics of hyperuricaemia and gout[J]. Nat Rev Rheumatol. 2018;14(6):341–353. doi:10.1038/s41584-018-0004-x
6. H WW, J CS, N WT, et al. Complex segregation and linkage analysis of familial gout in Taiwanese aborigines[J]. Arthritis Rheum. 2004;50(1):242–246. doi:10.1002/art.11441
7. Krishnan E, Lessov-Schlaggar CN, Krasnow RE, et al. Nature versus nurture in gout: a twin study[J]. Am J Med. 2012;125(5):499–504. doi:10.1016/j.amjmed.2011.11.010
8. Kim KH, Choi IA, Kim HJ, et al. Familial risk of gout and interaction with obesity and alcohol consumption: a population-based cohort study in Korea[J]. Arthritis Care Res. 2023;75(9):1955–1966. doi:10.1002/acr.25095
9. Zhang X, Lu Q, Zhang Z, et al. Value of three-dimensional speckle tracking echocardiography to assess left ventricular function in hyperuricemia patients[J]. Clin Rheumatol. 2018;37(9):2539–2545. doi:10.1007/s10067-018-4132-0
10. F KC, J GM, Zhang W, et al. Global epidemiology of gout: prevalence, incidence and risk factors[J]. Nat Rev Rheumatol. 2015;11(11):649–662. doi:10.1038/nrrheum.2015.91
11. Disveld I, Zoakman S, Jansen T, et al. Crystal-proven gout patients have an increased mortality due to cardiovascular diseases, cancer, and infectious diseases especially when having tophi and/or high serum uric acid levels: a prospective cohort study[J]. Clin Rheumatol. 2019;38(5):1385–1391. doi:10.1007/s10067-019-04520-6
12. Wang H, Zhang H, Sun L, et al. Roles of hyperuricemia in metabolic syndrome and cardiac-kidney-vascular system diseases[J]. Am J Transl Res. 2018;10(9):2749–2763.
13. He J, Yang Y, Peng DQ. Monosodium urate (MSU) crystals increase gout associated coronary heart disease (CHD) risk through the activation of NLRP3 inflammasome[J]. Int J Cardiol. 2012;160(1):72–73. doi:10.1016/j.ijcard.2012.05.083
14. Chen CC, Hsu YJ, Lee TM. Impact of elevated uric acid on ventricular remodeling in infarcted rats with experimental hyperuricemia[J]. Am J Physiol Heart Circ Physiol. 2011;301(3):H1107–H1117. doi:10.1152/ajpheart.01071.2010
15. Chen SL, Chen JR, Yang SW. Painless gouty tophus in the nasal bridge: a case report and literature review[J]. Medicine. 2019;98(11):e14850. doi:10.1097/MD.0000000000014850
16. Scott JT. Gout[J]. Baillieres Clin Rheumatol. 1987;1(3):525–546. doi:10.1016/s0950-3579(87)80043-2
17. Xu F, Jin L, Jin Y, et al. Long noncoding RNAs in autoimmune diseases[J]. J Biomed Mater Res A. 2019;107(2):468–475. doi:10.1002/jbm.a.36562
18. L PK, C LJ, L LC, et al. Impact of gout on left atrial function: a prospective speckle-tracking echocardiographic study[J]. PLoS One. 2014;9(9):e108357. doi:10.1371/journal.pone.0108357
19. F NS, A SO, P AC, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American society of echocardiography and the European Association of cardiovascular imaging[J]. J Am Soc Echocardiogr. 2016;29(4):277–314. doi:10.1016/j.echo.2016.01.011
20. K WL, T KJ, Schuster A, et al. Quantification of left atrial volume and phasic function using cardiovascular magnetic resonance imaging-comparison of biplane area-length method and Simpson’s method[J]. Int J Cardiovasc Imaging. 2017;33(11):1761–1769. doi:10.1007/s10554-017-1160-9
21. P AC, M GJ, S GM, et al. Estimation of left ventricular filling pressures using two-dimensional and Doppler echocardiography in adult patients with cardiac disease. additional value of analyzing left atrial size, left atrial ejection fraction and the difference in duration of pulmonary venous and mitral flow velocity at atrial contraction[J]. J Am Coll Cardiol. 1993;22(7):1972–1982. doi:10.1016/0735-1097(93)90787-2
22. L PK, C LJ, L LC, et al. The effects of gout on left atrial volume remodelling: a prospective echocardiographic study[J]. Rheumatology. 2014;53(5):867–874. doi:10.1093/rheumatology/ket444
23. Merriman TR. An update on the genetic architecture of hyperuricemia and gout[J]. Arthritis Res Ther. 2015;17(1):98. doi:10.1186/s13075-015-0609-2
24. J CC, C TC, H YJ, et al. ABCG2 contributes to the development of gout and hyperuricemia in a genome-wide association study[J]. Sci Rep. 2018;8(1):3137. doi:10.1038/s41598-018-21425-7
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.