Back to Journals » International Journal of Women's Health » Volume 15
Association Between Endometritis and Endometrial Polyp: A Mendelian Randomization Study
Authors Wei L, Zhao Y, Xu S, Zhang C
Received 5 September 2023
Accepted for publication 13 December 2023
Published 20 December 2023 Volume 2023:15 Pages 1963—1970
DOI https://doi.org/10.2147/IJWH.S434299
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Elie Al-Chaer
Longlong Wei,1,2 Yan Zhao,2 Siyue Xu,2 Cuilian Zhang1,2
1Department of Reproductive Medicine Center, People’s Hospital of Zhengzhou University, Zhengzhou, Henan, People’s Republic of China; 2Department of Reproductive Medicine Center, Henan Provincial People’s Hospital, Zhengzhou, Henan, People’s Republic of China
Correspondence: Cuilian Zhang, Email [email protected]
Background: Endometrial polyps (EPs) are one of the most common intrauterine benign tumors, and are an important cause of uterine bleeding and female infertility. Previous studies have suggested that endometritis may contribute to the onset of EPs. This study aims to reveal the causal effect of endometritis on EPs by a two-sample Mendelian randomization (MR) study.
Methods: Utilizing summarized statistics from genome-wide association studies (GWAS) in the European population, we conducted a Mendelian randomization study. In order to select suitable instrumental variables (IVs) that were significantly related to the exposures, a number of quality control approaches were used. For endometritis, 2144 cases and 111,858 controls were included, while for EPs, 2252 cases and 460,758 controls. Utilizing the inverse variance weighted (IVW) as the primary analysis, the data were subjected to a two-sample MR analysis, and the weighted median (WM) technique and MR-Egger regression were carried out additionally. The sensitivity analysis revealed neither heterogeneity nor horizontal pleiotropy.
Results: Four independent single nucleotide polymorphisms (SNPs) from endometritis GWAS as IVs were selected. The IVW data did not agree to a causal association between endometritis and EPs (β=1.11e-04, standard error [SE] =4.88e-04, P = 0.82). Directional pleiotropy did not affect the outcome, according to the MR-Egger regression (intercept = 0.09, P = 0.10); Additionally, it showed no causation association between endometritis and EPs (β= − 3.28e-03, SE = 3.54e-03, P = 0.45). Similar results were obtained using the weighted-median method (β=8.56e-05, SE=5.97e-04, P = 0.89). No proof of heterogeneity and horizontal pleiotropy between IV estimates was discovered.
Conclusion: In conclusion, by large scale genetic data, the results of this MR analysis provided suggestive evidence that the presence of endometritis is not associated with higher EPs risk.
Keywords: two-sample Mendelian randomization, GWAS, endometritis, endometrial polyp
Introduction
Endometrial polyps (EPs) are localized, sessile or pedunculate extensions of the endometrial mucosa that are one of the most common intrauterine benign tumors with a prevalence ranging from 7.8% to 34.9%.1,2 As an important cause of uterine bleeding and female infertility, researches on the etiology of EPs are of great clinical value and could also serve to the reduction of the health insurance burden.3,4 The precise mechanisms involved in the pathogenesis of EPs remain obscure. Currently, it is believed that hormonal variables play a major role in the pathogenesis of EPs.5 Obesity, estrogen therapy and tamoxifen therapy have been recognized as causative factors for EPs via strengthening the routes for estrogen receptor pathways.6,7
Chronic endometritis (CE) is defined as a persistent chronic inflammation of the endometrium, whose diagnosis is based on the presence of plasma cell infiltration within stromal tissue of endometrium.8,9 In 2005, Cicinelli et al demonstrated that CE was associated with small mucosal proliferations (<1 mm in diameter), defined as “micropolyps” firstly.10,11 During the last years, different studies have investigated the correlation between CE and EPs.12–14 However, as there are also accessible opposing data, these suspects have not yet been investigated for confirmation.10 It is vital to define the direction of the correlation and offer genetic evidence between endometritis and EPs because the relationship between the two is far from evident.
A developing statistical technique called Mendelian randomization (MR) studies at the relationship of causation between exposure and result by employing genetic variations as IVs for the exposure.15,16 MR analysis might lessen interference from confounding factors and reverse causation16 since genetic variations are distributed at random during gamete production and conception, which can avoid many of the possible methodological shortcomings of observational research.17
As the relationship between CE and EPs remains unclear, in this study, we identified the causal relationship between endometritis and EPs by using a two-sample MR.
Materials and Methods
Study Design
The MR study is based on three predominant hypotheses (1) There is a strong and persistent correlation between certain IVs and exposures. (2) There is no correlation between confounding factors and IVs. (3) IVs affect results directly through exposures, but not through other mechanisms. Genetic variants are frequently utilized as IVs, due to their well-defined nature and resistance to alteration by environmental factors, thereby avoiding reverse causation.18
The GWAS ID “finn-b-N14_INFLUTH” of the IEU Open GWAS database was used to acquire endometritis data by the R package TwoSampleMR (v 0.5.6).19,20 The Pan-UK Biobank website provided the GWAS summary statistics for EPs among Europeans in the UK Biobank.21 Endometritis data included 2144 cases and 111,858 controls, and the data of EPs included 2252 cases and 460,758 cases. The diagnosis of endometritis is defined by N71 in the International Classification of Diseases, 10th Revision (ICD-10). The diagnosis of EPs is defined by N84 in ICD-10.
Figure 1 illustrates the conceptual and analytical flow of this study.
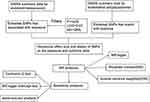 |
Figure 1 Illustrates the conceptual and analytical flow of this study. |
Genetic Variants Selection and Data Sources
We investigated relationships between endometritis and EPs using instrumental variables. To choose suitable instrumental variables, we used a rigorous quality control process. First of all, only SNPs with p-values lower than 5e-08 were considered to have a substantial link with endometritis in order to fulfill the correlation between SNP and exposure. Unfortunately, only a small number of SNPs at this level were connected to endometritis, therefore the genetic instruments were adjusted at P<1e-05.22–24 In order to completely eradicate linkage disequilibrium (LD), we simultaneously set the standard as r2 = 0.001 and the breadth of the LD area to 10,000 kb. The 1000 Genomes Project’s European reference panel, which was used to assess LD between SNPs, served as the basis for the clumping phase. When harmonizing data of the exposure and outcome, we additionally eliminated SNPs with non-concordant alleles and palindrome SNPs with unclear strands. Last but not least, the F statistic was calculated as a measure of the strength of the instruments.25 The F value of each SNP was calculated using the formula (F =2/SE2), and SNPs with F value <10 were excluded in order to make sure that the SNPs stated above did not generate mild instrumental bias.25,26 All IVs selection and quality control procedures are completed using the R package TwoSampleMR (v0.5.6).19
Statistical Analysis
In order to establish the causal relationship between endometritis and EPs, MR analysis was performed mainly using the WM, MR-Egger, and IVW approaches. In the main analyses, the IVW approach was used as the primary statistical model because it permitted all SNPs to exhibit a random degree of horizontal pleiotropy and produced estimates that were more precise than those from the two methods mentioned above. However, it assumes that all genetic variants are valid instrumental variables, an assumption that may not hold in practice.27 Thus, other robust methods which do not require all genetic variants to be valid IVs were also employed to give consistent estimates of a causal parameter.28,29 Specifically, the WM and MR-Egger were used.
Sensitivity Analysis
Firstly, we used the MR-Egger regression and the causal estimates of fixed effects IVW technique to detect heterogeneity.30 Cochran’s Q statistics were used to measure the heterogeneities, and a P-value below 0.05 was deemed significant heterogeneity. The intercept term in MR-Egger regression may show the directional horizontal pleiotropy in the causal estimations.19 Additionally, the R-based package MRPRESSO was used to build MR Pleiotropy RE Sidual Sum and Outlier (MR-PRESSO), which detects and corrects for horizontal pleiotropy by eliminating outliers. In addition, we conducted a “leave-one-out” analysis in which each SNP was eliminated in turn, and then MR analysis was done on the remaining SNPs to identify possibly outlying IVs.
Results
Instrumental Variables Selection
Four independent SNPs from endometritis GWASs as IVs were obtained for MR analysis. These SNPs were rs10255973, rs1053102, rs11578938, rs62366729. Furthermore, the MRPRESSO test failed to identify outlier IVs (Table 1). No outliers were discovered after MR-PRESSO analysis.
 |
Table 1 Instrumental SNPs from GWAS on Endometritis and Endometrial Polyp |
Mendelian Randomization Analyze
The results of the MR analysis generally revealed that endometritis and EPs are not causally related.
Endometritis on EPs (IVW method: odds ratio (OR) = 1.00, 95% confidence interval (CI) 0.99–1.00, P = 0.82) were not genetically associated. The MR estimates determined using weighted-median, and MR-Egger regression methods were consistent (MR-egger method: OR = 0.99, 95% CI: 0.98–1.00, P = 0.45; weighted-median method: OR = 1.00, 95% CI: 0.99–1.00, P = 0.89) (Figure 2).
Sensitivity Test
With endometritis as exposure factors, the sensitivity analysis revealed that neither the Cochran’s Q statistic nor the MR-Egger regression intercept was significant (p > 0.05), showing neither heterogeneity nor horizontal pleiotropy (Table 2). In addition, “leave-one-out” analysis did not identify the existence of outlier IVs, confirming the robustness of our findings. MR-PRESSO methods were also conducted for sensitivity analyses in MR analysis.
 |
Table 2 Heterogeneity Test and Horizontal Pleiotropy Test |
Discussion
According to our knowledge, this is the first MR research to examine the relationship between endometritis and EPs. For MR studies, we assessed causality using three dominant estimate approaches: IVW, WM, and MR-Egger regression. The results of these three analyses mentioned above were consistent and our findings demonstrated a non-genetic connection between endometritis and EPs utilizing large-scale GWAS data for MR analysis. Besides, the sensitivity test confirmed the robustness of our findings.
Our MR studies contribute valuable affirmative evidence to the ongoing discourse on the relationship between endometritis and EPs.
EPs are a common benign gynecological disease with high recurrence, often manifested as abnormal uterine bleeding (AUB), infertility and risk of endometrial cancer.31,32 In case of endometrial cancer, assisted reproductive technologies can assist patients in achieving their fertility goals if a fertility-preserving approach is not possible.33–36
Although endometrial physiology and pathology have been better understood during the past ten years, the etiology of EPs is still not completely understood.37–39 It is meaningful to clarify the specific pathogenesis of EPs for the prevention and treatment of this disease. Recently, some studies suggest a possible relationship between chronic inflammation and the development of EPs, and the same condition may occur in polyps of many mucosal tissues across the human body (including lower gastrointestinal tract, the urinary tract and upper respiratory tract).40–43 The endometritis is one of the most common inflammation of uterus. Endometritis can adversely affect embryo implantation by decreasing endometrial tolerance through multiple pathways.44–48 In case of women with untreated endometritis, the frozen-embryo transfer protocol would result in better pregnancy outcomes.49,50 As a result, we selected endometritis as an exposure variable to investigate the causal relationship.
Some previous studies have found that a higher prevalence of endometritis in the endometrial polyp group, and proposed that endometritis was considered as another factor in the development of EPs.47–49 It should be noted that the selection of participants in these observational studies above was of specialized populations, such as those with infertility or abnormal uterine bleeding. Thus, these findings may not be generalizable. Besides, other studies debate these viewpoints. A prospective cross-sectional study by Kitaya et al reported no plasma cell infiltration which is seen as a characteristic manifestation of endometritis within samples from EPs.14 In addition, compared to EP tissue, the density of plasma cells was much larger in micropolyps. Another study by Resta L et al also revealed that there exist obvious differences in the expression of growth factors between micropolyps and EPs.51 According to reports, just little more than one-third (33.7%) of polyps form as a result of inflammation.52 Consistent with these above findings, the results of our study did not support a causal relationship between endometritis and EPs. One explanation for these contradicting findings might be that endometrial macropolyps (also known as EPs) and micropolyps have distinct etiologies and pathophysiology.14 Endometrial micropolyps (one of the characteristic manifestations of chronic endometritis) may grow in an inflammatory milieu which have been observed to co-exist with endometritis and exhibit an aberrant local mononuclear cell composition, whereas endometrial macropolyps (ie, EPs) are hypothesized to form under an estrogen-sensitive situation.10 In addition, it has been speculated that single versus multiple polyps may also have different pathogenesis. Only multiple EP were independently related with endometritis, according to Luyan Guo et al, while the single polyp did not significantly correlate with inflammation.53 This is another explanation for the non-causal relationship between endometritis and EPs in our study.
One of the advantage of this study is that there exists no randomized controlled trials (RCT) of endometritis in EPs, and the observational studies that have been conducted in the past are susceptible to several inevitable confounding variables. The observed association of endometritis with EPs, however, may be due to bias or confounding factors inherent to observational studies, reverse causation, small study numbers and sizes, and selection biases. Limited by the inherent shortcomings of traditional observational studies, the current evidence is insufficient to draw firm conclusions. Because the MR approach is based on the idea that genetic variations are randomly distributed during gamete production and conception, it is less vulnerable to confounding bias and reverse causality.16 In addition, we employed two separate large-scale datasets (UK Biobank and IEU Open GWAS database) for the endometritis and EPs data. Finally, to assess the validity of the assumptions surrounding the IVs, we used a variety of additional studies for sensitivity analyses.
Despite the strengths, a few limitations should not to be ignored. First off, the study only used a small amount of SNPs as IVs, which might weaken the power to detect associations. Secondly, due to limitations of GWAS data, this study did not stratify confounders of EPs, such as age, BMI, diabetes and so on. Thirdly, the generalizability of our findings is still up in the air because all of our participants were of European descent. Finally, more clinical data and fundamental mechanistic investigations are required to support the database resources.
Conclusions
In conclusion, by large scale genetic data, this work overcame limitations inherent to observational studies and provided valuable suggestive evidence that the presence of endometritis is not associated with higher EPs risk. Future large-scale randomized controlled trials are needed to clarify the underlying mechanisms of EPs and point to new therapies against occurrence and recurrence of EPs in the future.
Data Sharing Statement
The GWAS summary statistics for endometritis are available on the IEU Open GWAS database (https://gwas.mrcieu.ac.uk/). The Pan-UK Biobank website (https://pan.ukbb.broadinstitute.org/) provided the GWAS summary statistics for endometrial polyp among Europeans in the UK Biobank.
Ethics Approval and Informed Consent
This study was reviewed and approved by the Institutional Review Board and Ethics Committee of Henan Provincial People’s Hospital, China.
Acknowledgments
We would like to thank the researchers and participants in the UK Biobank consortia. We also appreciate the IEU Open GWAS database for providing the data set.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Henan Provincial Medical Science and Technology Tackling Program Provincial–Ministerial Co-construction Project (SBGJ202001002).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Fagioli R, Vitagliano A, Carugno J, Castellano G, De Angelis MC, Di Spiezio Sardo A. Hysteroscopy in postmenopause: from diagnosis to the management of intrauterine pathologies. Climacteric. 2020;23(4):360–368. doi:10.1080/13697137.2020.1754387
2. Luerti M, Vitagliano A, Di Spiezio Sardo A, Angioni S, Garuti G, De Angelis C; Italian School of Minimally Invasive Gynecological Surgery Hysteroscopists Group. Effectiveness of hysteroscopic techniques for endometrial polyp removal: the Italian multicenter trial. J Minim Invasive Gynecol. 2019;26(6):1169–1176. doi:10.1016/j.jmig.2018.12.002
3. Rackow BW, Jorgensen E, Taylor HS. Endometrial polyps affect uterine receptivity. Fertil Steril. 2011;95(8):2690–2692. doi:10.1016/j.fertnstert.2010.12.034
4. Lasmar RB, Dias R, Barrozo PR, Oliveira MA, Coutinho Eda S, da Rosa DB. Prevalence of hysteroscopic findings and histologic diagnoses in patients with abnormal uterine bleeding. Fertil Steril. 2008;89(6):1803–1807. doi:10.1016/j.fertnstert.2007.05.045
5. Kosei N, Zakharenko N, Herman D. Endometrial polyps in women of reproductive age: clinical and pathogene-tic variations. Georgian Med News. 2017;273:16–22.
6. Serhat E, Cogendez E, Selcuk S, Asoglu MR, Arioglu PF, Eren S. Is there a relationship between endometrial polyps and obesity, diabetes mellitus, hypertension? Arch Gynecol Obstet. 2014;290(5):937–941. doi:10.1007/s00404-014-3279-4
7. Wong AWY, Chan SSC, Yeo W, Yu MY, Tam WH. Prophylactic use of levonorgestrel-releasing intrauterine system in women with breast cancer treated with tamoxifen: a randomized controlled trial. Obstet Gynecol. 2013;121(5):943–950. Erratum in: Obstet Gynecol. 2013 Sep;122(3):698. doi:10.1097/AOG.0b013e31828bf80c
8. Li Y, Xu S, Yu S, et al. Diagnosis of chronic endometritis: how many CD138+ cells/HPF in endometrial stroma affect pregnancy outcome of infertile women? Am J Reprod Immunol. 2021;85(5):e13369. doi:10.1111/aji.13369
9. Bouet PE, El Hachem H, Monceau E, Gariépy G, Kadoch IJ, Sylvestre C. Chronic endometritis in women with recurrent pregnancy loss and recurrent implantation failure: prevalence and role of office hysteroscopy and immunohistochemistry in diagnosis. Fertil Steril. 2016;105(1):106–110. doi:10.1016/j.fertnstert.2015.09.025
10. Cicinelli E, Resta L, Nicoletti R, Zappimbulso V, Tartagni M, Saliani N. Endometrial micropolyps at fluid hysteroscopy suggest the existence of chronic endometritis. Hum Reprod. 2005;20(5):1386–1389. doi:10.1093/humrep/deh779
11. Cicinelli E, Resta L, Nicoletti R, et al. Detection of chronic endometritis at fluid hysteroscopy. J Minim Invasive Gynecol. 2005;12(6):514–518. doi:10.1016/j.jmig.2005.07.394
12. Mylonas I, Makovitzky J, Fernow A, et al. Expression of the inhibin/activin subunits alpha (alpha), beta-A (betaA) and beta-B (betaB) in benign human endometrial polyps and tamoxifen-associated polyps. Arch Gynecol Obstet. 2005;272(1):59–66. doi:10.1007/s00404-004-0666-2
13. Mollo A, Stile A, Alviggi C, et al. Endometrial polyps in infertile patients: do high concentrations of interferon-gamma play a role? Fertil Steril. 2011;96(5):1209–1212. doi:10.1016/j.fertnstert.2011.08.001
14. Kitaya K, Tada Y, Taguchi S, Funabiki M, Hayashi T, Nakamura Y. Local mononuclear cell infiltrates in infertile patients with endometrial macropolyps versus micropolyps. Hum Reprod. 2012;27(12):3474–3480. doi:10.1093/humrep/des323
15. Grover S, Del Greco MF, Stein CM, Ziegler A. Mendelian randomization. Methods Mol Biol. 2017;1666:581–628. doi:10.1007/978-1-4939-7274-6_29
16. Verduijn M, Siegerink B, Jager KJ, Zoccali C, Dekker FW. Mendelian randomization: use of genetics to enable causal inference in observational studies. Nephrol Dial Transplant. 2010;25(5):1394–1398. doi:10.1093/ndt/gfq098
17. Smith GD, Ebrahim S. ‘Mendelian randomization’: can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol. 2003;32(1):1–22. doi:10.1093/ije/dyg070
18. Burgess S, Small DS, Thompson SG. A review of instrumental variable estimators for Mendelian randomization. Stat Methods Med Res. 2017b;26:2333–2355. doi:10.1177/0962280215597579
19. Hemani G, Zheng J, Elsworth B, et al. The MR-Base platform supports systematic causal inference across the human phenome. Elife. 2018;7:e34408. doi:10.7554/eLife.34408
20. Elsworth B, Lyon M, Alexander T, et al. The MRC IEU OpenGWAS data infrastructure. BioRxiv. 2020;2020:8.
21. Pan-UK Biobank. Pan-ancestry genetic analysis of the UK Biobank; 2020. Available from: https://pan.ukbb.broadinstitute.org.
22. Chen J, Ruan X, Yuan S, et al. Antioxidants, minerals and vitamins in relation to Crohn’s disease and ulcerative colitis: a Mendelian randomization study. Aliment Pharmacol Ther. 2023;57(4):399–408. doi:10.1111/apt.17392
23. Kurilshikov A, Medina-Gomez C, Bacigalupe R, et al. Large-scale association analyses identify host factors influencing human gut microbiome composition. Nat Genet. 2021;53(2):156–165. doi:10.1038/s41588-020-00763-1
24. Xie J, Huang H, Liu Z, et al. The associations between modifiable risk factors and nonalcoholic fatty liver disease: a comprehensive Mendelian randomization study. Hepatology. 2023;77(3):949–964. doi:10.1002/hep.32728
25. Li B, Martin EB. An approximation to the F distribution using the chi-square distribution. Comput Stat Data Anal. 2002;40(1):21–26.
26. Georgakis MK, Gill D, Rannikmäe K, et al. Genetically determined levels of circulating cytokines and risk of stroke. Circulation. 2019;139(2):256–268. doi:10.1161/CIRCULATIONAHA.118.035905
27. Burgess S, Bowden J, Fall T, Ingelsson E, Thompson SG. Sensitivity analyses for robust causal inference from Mendelian randomization analyses with multiple genetic variants. Epidemiology. 2017a;28:30–42.
28. Burgess S, Zuber V, Gkatzionis A, Foley CN. Modal-based estimation via heterogeneity-penalized weighting: model averaging for consistent and efficient estimation in Mendelian randomization when a plurality of candidate instruments are valid. Int J Epidemiol. 2018;47:1242–1254. doi:10.1093/ije/dyy080
29. Burgess S, Thompson SG. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur J Epidemiol. 2017;32:377–389.
30. Bowden J, Del Greco MF, Minelli C, Davey Smith G, Sheehan N, Thompson J. A framework for the investigation of pleiotropy in two-sample summary data Mendelian randomization. Stat Med. 2017;36(11):1783–1802. doi:10.1002/sim.7221
31. Liu J, Liang Y, Ouyang J, Yang S. Analysis of risk factors and model establishment of recurrence after endometrial polypectomy. Ann Pal Liat Med. 2021;10(11):11628–11634.
32. de Rijk SR, Steenbergen ME, Nieboer TE, Coppus SF. Atypical endometrial polyps and concurrent endometrial cancer: a systematic review. Obstet Gynecol. 2016;128(3):519–525. PMID: 27500332. doi:10.1097/AOG.0000000000001566
33. Mutlu L, Manavella DD, Gullo G, McNamara B, Santin AD, Patrizio P. Endometrial cancer in reproductive age: fertility-sparing approach and reproductive outcomes. Cancers. 2022;14(21):5187. doi:10.3390/cancers14215187
34. Zaami S, Stark M, Signore F, Gullo G, Marinelli E. Fertility preservation in female cancer sufferers: (only) a moral obligation? Eur J Contracept Reprod Health Care. 2022;27(4):335–340. doi:10.1080/13625187.2022.2045936
35. Gullo G, Cucinella G, Chiantera V, et al. Fertility-sparing strategies for early-stage endometrial cancer: stepping towards precision medicine based on the molecular fingerprint. Int J Mol Sci. 2023;24(1):811. doi:10.3390/ijms24010811
36. Gullo G, Bifulco G, Della Corte L. Fertility-sparing approach in patients with endometrioid endometrial cancer grade 2 stage IA (FIGO): a qualitative systematic review. Biomed Res Int. 2022;2022:4070368. doi:10.1155/2022/4070368
37. Carvalho FM, Aguiar FN, Tomioka R, de Oliveira RM, Frantz N, Ueno J. Functional endometrial polyps in infertile asymptomatic patients: a possible evolution of vascular changes secondary to endometritis. Eur J Obstet Gynecol Reprod Biol. 2013;170(1):152–156. doi:10.1016/j.ejogrb.2013.05.012
38. Indraccolo U, Di Iorio R, Matteo M, Corona G, Greco P, Indraccolo SR. The pathogenesis of endometrial polyps: a systematic semi-quantitative review. Eur J Gynaecol Oncol. 2013;34(1):5–22.
39. Stamatellos I, Apostolides A, Stamatopoulos P, Bontis J. Pregnancy rates after hysteroscopic polypectomy depending on the size or number of the polyps. Arch Gynecol Obstet. 2008;277(5):395–399. doi:10.1007/s00404-007-0460-z
40. Gelardi M, Netti GS, Giancaspro R, et al. Chronic rhinosinusitis with nasal polyposis (CRSwNP): the correlation between expression of Galectin-10 and Clinical-Cytological Grading (CCG). Am J Rhinol Allergy. 2022;36(2):229–237. doi:10.1177/19458924211049867
41. Ashktorab H, Brim H, Hassan S, et al. Inflammatory polyps occur more frequently in inflammatory bowel disease than other colitis patients. BMC Gastroenterol. 2020;20(1):170. doi:10.1186/s12876-020-01279-y
42. Humphrey PA. Polypoid/Papillary cystitis. J Urol. 2013;189(3):1091–1092. doi:10.1016/j.juro.2012.12.010
43. Cicinelli E, Tinelli R, Lepera A, Pinto V, Fucci M, Resta L. Correspondence between hysteroscopic and histologic findings in women with chronic endometritis. Acta Obstet Gynecol Scand. 2010;89(8):1061–1065. doi:10.3109/00016349.2010.498496
44. Wu D, Kimura F, Zheng L, et al. Chronic endometritis modifies decidualization in human endometrial stromal cells. Reprod Biol Endocrinol. 2017;15(1):16. doi:10.1186/s12958-017-0233-x
45. Wang WJ, Zhang H, Chen ZQ, et al. Endometrial TGF-β, IL-10, IL-17 and autophagy are dysregulated in women with recurrent implantation failure with chronic endometritis. Reprod Biol Endocrinol. 2019;17(1):2.
46. Matteo M, Cicinelli E, Greco P, et al. Abnormal pattern of lymphocyte subpopulations in the endometrium of infertile women with chronic endometritis. Am J Reprod Immunol. 2009;61(5):322–329. doi:10.1111/j.1600-0897.2009.00698.x
47. Kitaya K, Tada Y, Hayashi T, et al. Comprehensive endometrial immunoglobulin subclass analysis in infertile women suffering from repeated implantation failure with or without chronic endometritis. Am J Reprod Immunol. 2014;72(4):386–391. doi:10.1111/aji.12277
48. Li Y, Yu S, Huang C, et al. Evaluation of peripheral and uterine immune status of chronic endometritis in patients with recurrent reproductive failure. Fertil Steril. 2020;113(1):187–196.e181. doi:10.1016/j.fertnstert.2019.09.001
49. Gullo G, Basile G, Cucinella G, et al. Fresh vs. frozen embryo transfer in assisted reproductive techniques: a single center retrospective cohort study and ethical-legal implications. Eur Rev Med Pharmacol Sci. 2023;27(14):6809–6823. doi:10.26355/eurrev_202307_33152
50. Gullo G, Perino A, Cucinella G. Open vs. closed vitrification system: which one is safer? Eur Rev Med Pharmacol Sci. 2022;26(4):1065–1067. doi:10.26355/eurrev_202202_28092
51. Resta L, Cicinelli E, Lettini T. Possible inflammatory origin of endometrial polyps. Arch Reprod Med Sex Health. 2018;1:8–16.
52. Sattar S, Jaffar N, Iftikhar A, et al. A retrospective analysis of gynaecological polyps at a tertiary care hospital in Karachi. Ann Jinnah Sindh Med Univ. 2018;4(1):41–45.
53. Guo L, Gu F, Tan J, Luo L, Gao J, Zhou C. Multiple endometrial polyps is associated with higher risk of chronic endometritis in reproductive-aged women. J Obstet Gynaecol Res. 2021;47(1):389–396. doi:10.1111/jog.14541
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

