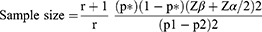Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 17
Association Between Dietary Habits and Type 2 Diabetes Mellitus in Thai Adults: A Case-Control Study
Authors Kalandarova M, Ahmad I , Aung TNN , Moolphate S, Shirayama Y, Okamoto M, Aung MN, Yuasa M
Received 17 October 2023
Accepted for publication 1 February 2024
Published 6 March 2024 Volume 2024:17 Pages 1143—1155
DOI https://doi.org/10.2147/DMSO.S445015
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Juei-Tang Cheng
Makhbuba Kalandarova,1 Ishtiaq Ahmad,1 Thin Nyein Nyein Aung,2 Saiyud Moolphate,3 Yoshihisa Shirayama,1,4 Miyoko Okamoto,1 Myo Nyein Aung,1,4,5 Motoyuki Yuasa1,4
1Department of Global Health Research, Juntendo University Graduate School of Medicine, Tokyo, 113-8421, Japan; 2Department of Family Medicine, Faculty of Medicine, Chiang Mai University, Chiang Mai, 50200, Thailand; 3Department of Public Health, Faculty of Science and Technology, Chiang Mai Rajabhat University, Chiang Mai, 50300, Thailand; 4Faculty of International Liberal Arts, Juntendo University, Tokyo, 113-8421, Japan; 5Juntendo Advanced Research Institute for Health Sciences, Juntendo University, Tokyo, 113-8421, Japan
Correspondence: Ishtiaq Ahmad; Myo Nyein Aung, Department of Global Health Research, Juntendo University Graduate School of Medicine, Bunkyo City, Hongo, 2 Chome-1-1, Tokyo, 113-8421, Japan, Email [email protected]; [email protected]
Background: The prevalence of T2DM is escalating in Thailand affecting over 10% of adults aged 20– 79 years old. It is imperative to identify modifiable risk factors that can potentially help mitigate the risk of developing diabetes.
Objective: This study aimed to investigate the relationship between dietary habits and type 2 diabetes in Chiang Mai, Thailand.
Methods: This case-control study involved 300 individuals aged 25– 74 years residing in Chiang Mai, Thailand including 150 newly diagnosed T2DM patients (cases) and 150 community residents without diabetes (controls). Dietary habits were assessed based on Food Frequency Questionnaire (FFQ). Socio-demographic characteristics and anthropometric information of the participants were collected. Data analysis was performed using the STATA-17.
Results: The case group participants were older and had a higher proportion of males compared to the control group. The case group exhibited a significantly higher consumption of meat, beans, nuts, soft drinks, and topping seasonings (p< 0.001), conversely, a lower intake of vegetables (p< 0.001), fruits (p=0.006), fish, rice (p< 0.001), eggs (p=0.032), milk products, coffee, and tea (p< 0.001) compared to the control group. Furthermore, the case group demonstrated a higher level of certain dietary practices such as a greater frequency of having meals with family, not removing visible fat from food (p< 0.001), and eating snacks between meals compared to controls. Multiple logistic regression analysis showed that after adjusting for potential confounding factors not removing visible fat from food (aOR 5.61, 95% CI: 2.29– 13.7, p< 0.001) and using topping seasonings (aOR 3.52 95% CI: 1.69– 7.32 p=0.001) were significantly associated with the risk of T2DM, whereas daily vegetable intake (aOR 0.32 95% CI: 0.15– 0.68 p=0.003) was inversely associated with T2DM.
Conclusion: The study findings caution against the consumption of food rich in fat and using salty seasonings, while advocating for an increased intake of vegetables to prevent the prevalence of T2DM.
Keywords: Thailand, T2DM, dietary habits, food frequency questionnaire, diabetes prevention, global health
Introduction
Type 2 Diabetes Mellitus (T2DM) is a common non-communicable disease and its upward trend is one of the major concerns in Global Health. Worldwide, the prevalence of diabetes was approximately 537 million (1 in 10 adults 20–79 years) in 2021 and is expected to increase to 643 million by 2030 and 783 million by 2045 (International Diabetes Federation; IDF).1 The majority of diabetes patients are living in low- and middle-income countries; in 2030, 69% of the incidence of diabetes mellitus will occur in the developing world.2,3 Thailand, an upper-middle-income country in Southeast Asia,4 has an age-adjusted prevalence of diabetes of around 10% of the adult population aged 20–79 years (10.8% in women and 8.9% in men).5 The number of Thai people with diabetes is estimated to reach 5.3 million in 2040 according to the IDF; which means one out of every five people aged over 60 years will be diabetic.6 Despite improvement in the diabetes detection rate and treatment in Thailand, glycaemic control remains sub-optimal and complications due to diabetes are still prevalent.7
Diabetes refers to complex metabolic disorders characterized by hyperglycemia and develops with deficiencies in insulin secretion, and/or insulin action commonly known as insulin resistance.8 The etiology of T2DM is linked to modifiable lifestyle habits such as an energy-dense diet and less physical activity.9 A Cluster Randomized Controlled Trial shows that changes in dietary habits and physical activity can reduce the incidence of T2DM by 50% among people with impaired glucose tolerance.10 Previous studies on the relationship between dietary patterns and T2DM have identified that diets rich in meat, high-fat foods, and refined carbohydrates such as sweets and desserts are linked to the elevated risk of metabolic syndrome and its components including T2DM.11,12 Studies conducted in Korea and Japan have reported the association of carbohydrate-rich food with diabetes.13,14 High intake of red meat has also been associated with an increased risk of diabetes.15 Conversely, adopting certain diets such as the Mediterranean diet which is mainly composed of raw vegetables, fish, whole grains, nuts, and beans has been reported to be associated with a protective effect against diabetes and metabolic syndrome.16,17 Based on the evidence, dietary behaviors such as skipping breakfast, speed eating, and eating alone are associated with obesity and T2DM.18–20
A sedentary lifestyle, low consumption of fruits and vegetables, and high consumption of added sugar are common among Thai people.21 Northern Thailand is known for its distinctive cultures and lifestyles, which encompass unique cooking practices. The staple food is sticky rice and oily noodles consumption is also widespread.22 In recent years, the northern region has been labeled as having the highest percentage of citizens over 60 years and having one of the highest prevalence of diabetes mellitus in Thailand, while its economic status ranks lower compared to the central and southern regions.23
Since dietary behavior is based on individual lifestyle choices, such as food selection, frequency, and intake schedule, establishing further evidence and understanding the mechanism is crucial for designing interventions and programs aimed at improving dietary patterns. Despite the importance of nutritional behaviors and dietary patterns as modifiable risk factors in the prevention and control of diabetes, they have been only marginally evaluated in Thailand. In this study, we aimed to identify the prevailing dietary habits and their relationship with the prevalence of T2DM in adult residents in Chiang Mai, Northern Thailand. The present study will contribute to filling the knowledge gap in the existing literature and its findings can assist policy designs that aim at nutritional behavior change, thereby reducing the social and economic burden of this disease.
Materials and Methods
Study Design and Setting
This observational study applied a case-control design conducted in 2019–2020; recruiting 300 participants (150 cases and 150 controls) aged 25–74 years and residing in Sanpatong district, Chiang Mai province, Thailand.
Eligibility Criteria for Case and Control Groups
Case group definition: T2DM patients who were newly diagnosed within 6 months before data collection and attending the diabetes clinic of Sanpatong District Hospital. According to the Thailand National Guideline for Diabetes, diagnostic criteria for T2DM diagnosis are fasting plasma glucose level ≥126 mg/dL and/or 2 hours Oral Glucose Tolerance Test (OGTT) ≥200 mg/dL.
Control group definition: control group participants were recruited from one of the primary care settings under Sanpatong District Hospital and gave informed consent to become part of the study. Controls were confirmed as non-diabetes participants after obtaining negative results from a plasma glucose test, which is a common screening method for diabetes (Table 1).
 |
Table 1 Inclusion and Exclusion Criteria for Study Participants |
Sample Size
In the current study, the sample size was calculated based on the findings of “Sociodemographic differences affecting insufficient fruit and vegetable intake: a population-based household survey of Thai people”.24 The survey reported that the overall prevalence of insufficient fruit and vegetable intake (FV) was 65.6% among respondents. Therefore, we assumed diabetes patients had fewer FV intake per day and the proportion of exposure to the risk factor was 75% in the case group. Assuming that people without diabetes had a higher rate of FV intake, we set the proportion of exposure for the control group as 55%. The formula for the unmatched case-control study was utilized with a power of 80% and a confidence interval (CI) of 95%.25 The sample size was calculated using the following formula:
Data Collection
A face-to-face interview was conducted by primary health care nurses after obtaining written informed consent from all participants. The standardized questionnaire included demographic characteristics (age, sex, occupation, marital status), anthropometric measurements (height, weight, BMI), and behavioral factors (tobacco use, alcohol consumption, physical activity knowledge) (Tables 2 and 3). Participants were requested to recall their last week’s eating habits to answer the dietary habits questions (Tables 4 and 5). The survey instrument and consent cover letter were translated into Thai according to the WHO guidelines on translation.26 To confirm the accuracy, the translated questionnaire was revised by native experts.
 |
Table 2 Socio-Demographic Characteristics of the Participants |
 |
Table 3 Lifestyle-Related Behaviors of the Participants |
 |
Table 4 Comparison of Food Intake by High-Frequency Analysis Between Diabetes and Non-Diabetes Participants |
 |
Table 5 Comparison of Dietary Behaviours by High Frequency Between Diabetes and Non-Diabetes Participants |
Measurements
Dietary Habits Assessment
The Food Frequency Questionnaire (FFQ) was used to gather information on the dietary habits of research subjects.27 This questionnaire has been used widely to estimate the daily food intake frequency over a certain period of time. The food items: rice, noodles, meat, dairy products, beans, eggs, fish, seasonings, dessert, soft drinks, coffee or tea, fruit, and vegetables were selected based on the Association of Southeast Asian Nations (ASEAN) food composition table28 and local common foods (Table 4). Questions asked also included portion sizes for different foods and eating behaviors: having three meals in a day, skipping breakfast, consuming a snack between meals, having meals with family, and eating out (Table 5). To estimate the frequency of dietary habits all respondents were asked to classify their daily food item intake by choosing the different time patterns from “never or rarely” to “every day”.
Sociodemographic and Health Data
Data included sociodemographic characteristics, lifestyle-related questions, and medical test results. Anthropometric measurements were taken by trained investigators using the WHO STEPS protocol.29 Blood samples were obtained from participants after fasting for at least 8 hours. Persons with fasting plasma glucose levels <110 mg/dL were selected as the control group. The surveys were comprised of information about age, gender, education level, employment rate, residing area, marital status, received knowledge related to nutrition, behavioral choices on food consumption, family history of diabetes, hypertension, and current medication use.
Statistical Analysis
Data in categorical variables were assessed by applying the chi-square test. Study participants were classified into two categories by age (<60 and ≥60 years old) and three categories by BMI (<22, 22–25, >25). The monthly household income level was classified into two groups based on the mean of valid responses (<12,842 and ≥12,842 Thailand Baht). The statistical significance level was considered at P<0.05 and a confidence interval (CI) of 95% with 80% power. Food intake frequency and dietary behavior were categorized as dichotomous categorical variables based on high frequency and low frequency. High frequency was defined as daily intake, while low frequency encompassed categories such as completely avoiding or very rarely consuming (1–2 times/week, 3–4 times/week, 5–6 times/week). We used univariate logistic regression analysis to identify factors associated with T2DM. Multivariable logistic regression models were constructed to adjust for potential confounders in the association between dietary habits and T2DM. The dependent variable was diabetes status as binary data and the independent variables were age, sex, BMI, employment status, household monthly income levels, hypertension, family history of diabetes, alcohol consumption, and dietary habits including food intake and eating behavior. All analyses were performed using the STATA version 17.0 (Stata Corp, College Station, TX, USA).
Ethical Approval
The present study was approved by the Ethical Review Committee for Research on Human Participants, Chiang Mai Provincial Health Office, and the Ethical Review Board of the Juntendo University, Tokyo (authorization number 2017141). The present study was conducted according to the guidelines in the Declaration of Helsinki.
Results
In this study, we analyzed the data collected from a sample of 300 participants consisting of 150 T2DM patients and 150 non-diabetes participants in Chiang Mai, Thailand. Table 2 shows the demographic and social characteristics of the participants. From examining the proportion of the age, it was found that the case group has a higher percentage (51.3%) compared to the control group (43.3%) of individuals 60 years old and above. The number of males was greater in cases (n=63, 42.0%) compared to controls (n=41, 27.3%) (P=0.008). In the case group, a larger proportion of participants were employed (72.0%) and had higher monthly income levels (48.0%) (P<0.001). Marital status, level of education, BMI, and HWC did not make a significant difference between the two groups.
The case group included a larger percentage of participants with a family history of diabetes (P<0.001), hypertension (P<0.001), and a lower percentage of participants with alcohol consumption (P=0.001, chi-square test) than the control group. Almost all participants in the case group (98.7%) were taking medicine for T2DM treatment during the data collection period, whereas, no one in the control group was on medication (Table 3).
Table 6 and Figure 1 show the knowledge related to nutrition among participants. Table 6 shows that in the case group, significantly more participants (95.3%) had received nutrition knowledge compared to controls (73.3%) (p<0.001, chi-square test). Figure 1 shows the sources of nutrition knowledge received by all participants in both case and control groups. The majority of the individuals had received knowledge at a hospital and health centers. Some participants had obtained knowledge related to nutrition from the television, internet, radio, school, and newspapers.
 |
Table 6 Knowledge Related to Nutrition |
 |
Figure 1 The sources of nutrition knowledge received by participants. Note: Sources are not mutually exclusive. |
Table 4 shows the comparison of food intake frequency analysis, the intake frequency of meat, beans, topping seasonings (such as salt, and soy sauce), and soft drinks was higher in the case group than in the control group. Conversely, the intake frequency of rice, fish, eggs, vegetables, and fruits daily was lower in the case group than in the control group.
Table 5 shows the comparison of the dietary behaviors by high frequency between diabetes and non-diabetes participants. The frequency of having meals with family, not removing visible fat from food (pork, chicken), and eating snacks between meals was higher in the case group than in the control group.
Table 7 shows the relationship between a high food intake frequency and T2DM by univariate and multivariable logistic regression analysis. Statistically significant association of variables such as skipping breakfast, eggs, deep-fried food, and stir-fried food to the outcome remain significant after adjusting with age, sex, and BMI.
 |
Table 7 Factors Associated with Diabetes Through Logistic Regression Analysis |
Table 8 shows results from multiple logistic regression models. Not removing visible fat from food (aOR 5.61, 95% CI 2.29–13.70), and topping seasonings (aOR 3.52, 95% CI 1.69–7.32) were associated with a higher risk of T2DM, whereas daily vegetables (aOR 0.32, 95% CI 0.15–0.68) and fruits (aOR 0.95, 95% CI 0.43–2.11) intake were associated with lower risk of T2DM.
 |
Table 8 Dietary Habits Associated with T2DM Among the Adult Residents of Chiang Mai, Thailand |
Discussion
This study identified dietary habits and assessed the association between T2DM in Northern Thailand. Our results revealed that an increased prevalence of T2DM was linked to dietary practices such as not removing visible fat from food or only partially removing it before eating (Table 8). Moreover, using seasonings on a daily basis was significantly associated with T2DM risk, and daily vegetable and fruit intake was found to have a protective effect against T2DM (Table 8). The northern region of Thailand has been reported to have one of the highest prevalence of diabetes mellitus among other regions where the culinary practice is popular with the consumption of sticky rice and oily foods.23
A descriptive analysis of socio-demographic characteristics and lifestyle behaviors showed the case group consisted of more male participants and had higher household income compared to controls (Table 2). These findings are consistent with previous studies reported that T2DM is more frequently diagnosed in men.30 Regarding the risk factors of diabetes, the case group had a higher proportion of family history of diabetes (36.7%) and elevated blood pressure (77%) (Table 3). A family history of diabetes and hypertension has long been recognized as a potent risk factor for the development of T2DM.31,32 In terms of alcohol consumption, the case group participants reported a lower percentage of alcohol drinking which might be the result of the active promotion of education related to health risk factors among diabetes patients (Table 3). Both case and control groups received physical activity and nutrition knowledge with the dominance of “cases” (Tables 3 and 6). The dietary habits of the participants revealed the statistically significant difference between diabetes and non-diabetes groups by intake frequency of rice, eggs, deep-fried food, stir-fried food, vegetables, fruits, seasonings, and coffee/tea (Table 4) as well as dietary practice eg skipping breakfast and not removing visible fat from food (Table 5).
Our findings indicate a significant association between the habit of not removing visible fat from food and an increased risk of T2DM (Tables 7 and 8 Crude: 5.13 CI 2.69–10.1; Model 1: aOR 5.18 CI 2.76–9.70, Model 2: aOR 5.35 CI 2.36–12.1, Model 3: aOR 5.61 CI 2.29–13.7). These results are consistent with previous research demonstrating that excessive fat intake, particularly when combined with other lifestyle factors, can contribute to the development of T2DM.33 The association between visible fat intake and T2DM risk can be understood through several mechanisms. Firstly, high-fat diets, including those containing visible fat, have been linked to weight gain and obesity, which are well-established risk factors for T2DM.33 Excess body weight, especially visceral adiposity, contributes to insulin resistance, a key pathophysiological feature of T2DM.34
The role of dietary fat in T2DM is still being studied, and the evidence is not entirely conclusive. Some studies have suggested a positive association between a higher intake of saturated fats, typically found in animal products and certain tropical oils, and an increased risk of T2DM.35 However, other studies found no significant association between them.36 It is worth noting that the impact of visible fat intake on T2DM risk should be considered within the context of overall diet quality. The consumption of visible fat alone may not fully explain the association with T2DM. Diets high in processed foods, sugary beverages, and unhealthy fats are consistently associated with an increased risk of T2DM, while diets rich in whole foods, fruits, vegetables, lean proteins, and healthy fats have been shown to lower the risk.37 Therefore, the overall dietary pattern should be taken into account when assessing the relationship between visible fat intake and diabetes risk. Individual variations also play a role in the association between visible fat intake and T2DM. Genetic, environmental, and lifestyle factors influence an individual`s response to dietary fat intake.38 Moreover, the impact of visible fat consumption on T2DM risk may vary depending on an individual`s metabolic health, physical activity levels, and other contextual factors.35 Regular physical activity plays a crucial role in the prevention of T2DM by enhancing metabolic health and reducing the overall risk associated with dietary habits.39
We found a significant positive association between salt intake, particularly in the form of salty seasonings like salt and different types of sauces, and the risk of T2DM (Tables 7 and 8 Crude: 2.32 CI 1.42–3.79; Model 1: aOR 2.25 CI 1.40–3.61, Model 2: aOR 2.19 CI 1.15–4.16, Model 3: aOR 3.52 CI 1.69–7.32). Our findings align with previous research conducted in different countries, advocating the notion that excessive sodium consumption may contribute to an increased risk of T2DM.40,41 Several mechanisms may explain the association between excessive salt intake on the development of T2DM, including the effects of high salt consumption on blood pressure, insulin resistance, and pancreatic function.42,43 High dietary salt intake has long been recognized as a significant contributor to hypertension.43 Hypertension is a known risk factor for T2DM; evidence suggests that salt intake may play a role in the development of insulin resistance is a key underlying factor in the pathogenesis of T2DM.42
Furthermore, a recent meta-analysis showed that patients with T2DM had higher levels of sodium intake when compared to non-diabetic controls.44 This suggests that excessive sodium consumption may play a role in the development risk of T2DM. According to a study result examining the association between dietary habits and T2DM conducted by our research team in Myanmar, it was observed that the consumption of salty seasonings with daily meals is significantly linked to a higher prevalence of T2DM.45 Rasouli et al confirmed the notable association between high sodium intake and the elevated risk of T2DM through the findings of their population-based study conducted in Sweden.46
The intake of vegetables (Tables 7 and 8 Crude: 0.40 CI 0.24–0.65; Model 1: aOR 0.42 CI 0.26–0.67, Model 2: aOR 0.31 CI 0.16–0.62, Model 3: aOR 0.32 CI 0.15–0.68) and fruits (Tables 7 and 8 Crude: 0.50 CI 0.29–0.84; Model 1: aOR 0.56 CI 0.33–0.95, Model 2: aOR 0.70 CI 0.35–1.41, Model 3: aOR 0.95 CI 0.43–2.11) has a significant inverse association with the prevalence of T2DM in our study. Daily consumption of vegetables has a consistently significant inverse association with diabetes prevalence in multivariate analysis, while daily intake of fruits has a significant inverse association in the first two models of multivariate analysis (Table 8). Similar results have been observed in studies exploring associations of incidence of T2DM with a higher intake of fruits and vegetables.47,48 High fruit and vegetable consumption was found to be linked to a lower risk of T2DM, hypertension, and dyslipidemia in Korean adults regardless of their gender.49 The risk of T2DM decreased by 9–10% with increasing intake of vegetables and fruits up to 200–300 gr/day according to the meta-analysis comparing high versus low intake.15 The observed protective effect of fruits and vegetables on the reduction of type 2 diabetes risk can potentially be attributed to their rich composition of dietary fibers, antioxidants, vitamins, and various phytochemicals. Additionally, the intake of fruits and vegetables might contribute to reducing adiposity and controlling weight gain, eventually decreasing the risk of T2DM over the long term.50
T2DM not only leads to increased morbidity and mortality rates but also affects both society and the national economy.51 Therefore, prioritizing preventive measures is paramount to mitigate the far-reaching consequences of T2DM.
Strengths and Limitations of This Study
The notable strength is we used a case-control design with well-defined eligibility criteria for the selection of both groups, cases were selected from a hospital setting, while controls were chosen from the community site to maintain the distribution of parameters within the target population. Furthermore, the use of multiple logistic analysis enhancedthe precision of the association between dietary habits and T2DM, providing a more comprehensive assessment of the research findings by adjusting potential confounding factors, including age, gender, BMI, employment status, household monthly income level, and health conditions: hypertension, family history of diabetes, also lifestyle behaviors such as alcohol consumption.
Despite the robustness of our findings, there are some limitations to be considered. The use of self-reported data may be subject to recall bias as participants responded to the questions based on their dietary habits in the previous seven days. Secondly, during the six-month period following the initial consultation, it is possible that certain participants in the case group may have made lifestyle behavior changes, including adjustments to their dietary habits. This could be attributed to the information they received during the consultation regarding lifestyle modifications.
Conclusion
The study indicates that the traditional dietary practice in Thailand, which involves consuming food rich in fat and adding salty seasonings to meals is associated with an increased risk of T2DM. The study findings highlight the adverse effects of these seasonings and “unhealthy” fats on T2DM and promote the consumption of vegetables to prevent the accelerating prevalence of diabetes. Study findings contribute recommendations and opportunities for the primary prevention of T2DM in Thailand.
Acknowledgments
We would like to acknowledge Dr.Satomi Ueno and Dr. Hira Taimur from the Department of Global Health Research for contribution and support to this work.
Funding
This study was supported by JSPS: Grant No. 18K10110.
Disclosure
The authors report no conflicts of interest in this work.
References
1. IDF diabetes atlas 10th edition international diabetes federation. Available from: https://diabetesatlas.org/atlas/tenth-edition/.
2. Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabet Res Clin Pract. 2014;103(2):137–149. doi:10.1016/j.diabres.2013.11.002
3. Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabet Res Clin Pract. 2010;87(1):4–14. doi:10.1016/j.diabres.2009.10.007
4. Sodeno M, Aung MN, Yuasa M, et al. Association between physical activity and type 2 diabetes using the international physical activity questionnaires: a case-control study at a health promoting hospital in Chiang Mai, Northern Thailand. Diabetes Metab Syndr Obes. 2022;15:3655–3667. doi:10.2147/dmso.S382528
5. Phoosuwan N, Ongarj P, Hjelm K. Knowledge on diabetes and its related factors among the people with type 2 diabetes in Thailand: a cross-sectional study. BMC Public Health. 2022;22(1):2365. doi:10.1186/s12889-022-14831-0
6. Clin HE. Emeritus Piyasakol Sakolsatayadorn MDMoPH, Thailand. Partnering to innovate diabetes care in Thailand. Novo Nordisk; 2017.
7. Reutrakul S, Deerochanawong C. Diabetes in Thailand: status and policy. Curr Diab Rep. 2016;16(3):28. doi:10.1007/s11892-016-0725-7
8. Association AD. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2012;36(Supplement_1):S67–S74. doi:10.2337/dc13-S067
9. Kolb H, Martin S. Environmental/lifestyle factors in the pathogenesis and prevention of type 2 diabetes. BMC Med. 2017;15(1):131. doi:10.1186/s12916-017-0901-x
10. Aekplakorn W, Tantayotai V, Numsangkul S, Tatsato N, Luckanajantachote P, Himathongkam T. Evaluation of a community-based diabetes prevention program in Thailand: a cluster randomized controlled trial. J Prim Care Community Health. 2019;10:2150132719847374. doi:10.1177/2150132719847374
11. Aekplakorn W, Satheannoppakao W, Putwatana P, et al. Dietary pattern and metabolic syndrome in Thai adults. J Nutr Metab. 2015;2015:468759. doi:10.1155/2015/468759
12. Cai J, Nuli R, Zhang Y, et al. Association of dietary patterns with type 2 diabetes mellitus among middle-aged adults in Uygur population of Xinjiang Region. J Nutr Sci Vitaminol. 2019;65(4):362–374. doi:10.3177/jnsv.65.362
13. Kim K, Yun SH, Choi BY, Kim MK. Cross-sectional relationship between dietary carbohydrate, glycaemic index, glycaemic load and risk of the metabolic syndrome in a Korean population. Br J Nutr. 2008;100(3):576–584. doi:10.1017/s0007114508904372
14. Murakami K, Sasaki S, Takahashi Y, et al. Dietary glycemic index and load in relation to metabolic risk factors in Japanese female farmers with traditional dietary habits. Am J Clin Nutr. 2006;83(5):1161–1169. doi:10.1093/ajcn/83.5.1161
15. Schwingshackl L, Hoffmann G, Lampousi AM, et al. Food groups and risk of type 2 diabetes mellitus: a systematic review and meta-analysis of prospective studies. Eur J Epidemiol. 2017;32(5):363–375. doi:10.1007/s10654-017-0246-y
16. Vitale M, Masulli M, Calabrese I, et al. Impact of a mediterranean dietary pattern and its components on cardiovascular risk factors, glucose control, and body weight in people with type 2 diabetes: a real-life study. Nutrients. 2018;10(8):1067. doi:10.3390/nu10081067
17. Ceriello A, Esposito K, La Sala L, et al. The protective effect of the Mediterranean diet on endothelial resistance to GLP-1 in type 2 diabetes: a preliminary report. Cardiovasc Diabetol. 2014;13(1):140. doi:10.1186/s12933-014-0140-9
18. Ballon A, Neuenschwander M, Schlesinger S. Breakfast skipping is associated with increased risk of type 2 diabetes among adults: a systematic review and meta-analysis of prospective cohort studies. J Nutr. 2019;149(1):106–113. doi:10.1093/jn/nxy194
19. Fujii H, Funakoshi S, Maeda T, et al. Eating speed and incidence of diabetes in a Japanese general population: ISSA-CKD. J Clin Med. 2021;10(9):1949. doi:10.3390/jcm10091949
20. Mahmood L, González-Gil EM, Schwarz P, et al. Frequency of family meals and food consumption in families at high risk of type 2 diabetes: the Feel4Diabetes-study. Eur J Pediatr. 2022;181(6):2523–2534. doi:10.1007/s00431-022-04445-4
21. Chavasit V, Kriengsinyos W, Photi J, Tontisirin K. Trends of increases in potential risk factors and prevalence rates of diabetes mellitus in Thailand. Eur J Clin Nutr. 2017;71(7):839–843. doi:10.1038/ejcn.2017.52
22. Chanpiwat P, Kim KW. Arsenic health risk assessment related to rice consumption behaviors in adults living in Northern Thailand. Environ Monit Assess. 2019;191(11):674. doi:10.1007/s10661-019-7836-y
23. Yeemard F, Srichan P, Apidechkul T, Luerueang N, Tamornpark R, Utsaha S. Prevalence and predictors of suboptimal glycemic control among patients with type 2 diabetes mellitus in northern Thailand: a hospital-based cross-sectional control study. PLoS One. 2022;17(1):e0262714. doi:10.1371/journal.pone.0262714
24. Phunkerd S. Sociodemographic differences affecting insufficient fruit and vegetable intake: a population-based household survey of Thai people. J Health Res. 2019. doi:10.1108/JHR-07-2019-0150
25. Charan J, Biswas T. How to calculate sample size for different study designs in medical research? Indian J Psychol Med. 2013;35(2):121–126. doi:10.4103/0253-7176.116232
26. Process of translation and adaptation of instruments; 2020. Available from: https://www.who.int/substance_abuse/research_tools/translation/en/.
27. Cade JE, Burley VJ, Warm DL, Thompson RL, Margetts BM. Food-frequency questionnaires: a review of their design, validation and utilisation. Nutr Res Rev. 2004;17(1):5–22. doi:10.1079/nrr200370
28. ASEAN food composition database. Available from: http://www.inmu.mahidol.ac.th/aseanfoods/composition_data.html.
29. World Health Organization. STEPS manual. Available from: https://cdn.who.int/media/docs/default-source/ncds/ncd-surveillance/steps/part3-section5.pdf?sfvrsn=a46653c7_2.
30. Ciarambino T, Crispino P, Leto G, Mastrolorenzo E, Para O, Giordano M. Influence of gender in diabetes mellitus and its complication. Int J Mol Sci. 2022;23(16):8850. doi:10.3390/ijms23168850
31. Petrie JR, Guzik TJ, Touyz RM. Diabetes, hypertension, and cardiovascular disease: clinical insights and vascular mechanisms. Can J Cardiol. 2018;34(5):575–584. doi:10.1016/j.cjca.2017.12.005
32. Scott RA, Langenberg C, Sharp SJ, et al. The link between family history and risk of type 2 diabetes is not explained by anthropometric, lifestyle or genetic risk factors: the EPIC-InterAct study. Diabetologia. 2013;56(1):60–69. doi:10.1007/s00125-012-2715-x
33. Blüher M. Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol. 2019;15(5):288–298. doi:10.1038/s41574-019-0176-8
34. Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444(7121):840–846. doi:10.1038/nature05482
35. Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med. 2011;364(25):2392–2404. doi:10.1056/NEJMoa1014296
36. Imamura F, Micha R, Wu JH, et al. Effects of saturated fat, polyunsaturated fat, monounsaturated fat, and carbohydrate on glucose-insulin homeostasis: a systematic review and meta-analysis of randomised controlled feeding trials. PLoS Med. 2016;13(7):e1002087. doi:10.1371/journal.pmed.1002087
37. Ley SH, Hamdy O, Mohan V, Hu FB. Prevention and management of type 2 diabetes: dietary components and nutritional strategies. Lancet. 2014;383(9933):1999–2007. doi:10.1016/s0140-6736(14)60613-9
38. Qi Q, Chu AY, Kang JH, et al. Sugar-sweetened beverages and genetic risk of obesity. N Engl J Med. 2012;367(15):1387–1396. doi:10.1056/NEJMoa1203039
39. Ahmad I, Aung MN, Ueno S, et al. Physical activity of type 2 diabetes mellitus patients and non-diabetes participants in Yangon, Myanmar: a case-control study applying the International Physical Activity Questionnaires (IPAQ-S). Diabetes Metab Syndr Obes. 2021;14:1729–1739. doi:10.2147/dmso.S291468
40. Radzeviciene L, Ostrauskas R. Adding salt to meals as a risk factor of type 2 diabetes mellitus: a Case-Control Study. Nutrients. 2017;9(1):67. doi:10.3390/nu9010067
41. Itoh N, Tsuya A, Togashi H, et al. Increased salt intake is associated with diabetes and characteristic dietary habits: a community-based cross-sectional study in Japan. J Clin Biochem Nutr. 2022;71(2):143–150. doi:10.3164/jcbn.21-153
42. He FJ, Li J, Macgregor GA. Effect of longer term modest salt reduction on blood pressure: Cochrane systematic review and meta-analysis of randomised trials. BMJ. 2013;346(apr03 3):f1325. doi:10.1136/bmj.f1325
43. Cogswell ME, Zhang Z, Carriquiry AL, et al. Sodium and potassium intakes among US adults: NHANES 2003–2008. Am J Clin Nutr. 2012;96(3):647–657. doi:10.3945/ajcn.112.034413
44. Kolahdouz-Mohammadi R, Soltani S, Clayton ZS, Salehi-Abargouei A. Sodium status is associated with type 2 diabetes mellitus: a systematic review and meta-analysis of observational studies. Eur J Nutr. 2021;60(7):3543–3565. doi:10.1007/s00394-021-02595-z
45. Ueno S, Aung MN, Yuasa M, et al. Association between dietary habits and type 2 diabetes mellitus in Yangon, Myanmar: a Case-Control Study. Int J Environ Res Public Health. 2021;18(21):11056. doi:10.3390/ijerph182111056
46. Rasouli B, Ahlqvist E, Andersson T, et al. Sodium intake and the risk of type 2 diabetes and Latent Autoimmune Diabetes in Adults (LADA); 2017.
47. Cooper AJ, Sharp SJ, Lentjes MAH, et al. A prospective study of the association between quantity and variety of fruit and vegetable intake and incident type 2 diabetes. Diabetes Care. 2012;35(6):1293–1300. doi:10.2337/dc11-2388
48. Ford ES, Mokdad AH. Fruit and vegetable consumption and diabetes mellitus incidence among U.S. adults. Preventive Med. 2001;32(1):33–39. doi:10.1006/pmed.2000.0772
49. Nguyen HD, Oh H, Kim MS. Higher intakes of nutrients are linked with a lower risk of cardiovascular diseases, type 2 diabetes mellitus, arthritis, and depression among Korean adults. Nutr Res. 2022;100:19–32. doi:10.1016/j.nutres.2021.11.003
50. Halvorsen RE, Elvestad M, Molin M, Aune D. Fruit and vegetable consumption and the risk of type 2 diabetes: a systematic review and dose-response meta-analysis of prospective studies. BMJ Nutr Prev Health. 2021;4(2):519–531. doi:10.1136/bmjnph-2020-000218
51. Soares Andrade CA, Shahin B, Dede O, et al. The burden of type 2 diabetes mellitus in states of the European Union and United Kingdom at the national and subnational levels: a systematic review. Obes Rev. 2023;24(9):e13593. doi:10.1111/obr.13593
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.