Back to Journals » Clinical Epidemiology » Volume 9
Association between coffee or caffeine consumption and fecundity and fertility: a systematic review and dose–response meta-analysis
Authors Lyngsø J , Ramlau-Hansen CH , Bay B, Ingerslev HJ, Hulman A , Kesmodel US
Received 17 July 2017
Accepted for publication 26 September 2017
Published 15 December 2017 Volume 2017:9 Pages 699—719
DOI https://doi.org/10.2147/CLEP.S146496
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Henrik Sørensen
Julie Lyngsø,1 Cecilia Høst Ramlau-Hansen,1 Bjørn Bay,2 Hans Jakob Ingerslev,3 Adam Hulman,1,4 Ulrik Schiøler Kesmodel5
1Department of Public Health, Section for Epidemiology, Aarhus University, Aarhus, 2The Fertility Clinic, Regional Horsens Hospital, Horsens, 3Department of Obstetrics and Gynaecology, Aarhus University Hospital, Aarhus, 4Danish Diabetes Academy, Odense, 5Department of Obstetrics and Gynaecology, Herlev and Gentofte Hospital, Herlev, Denmark
Objective: The aim was to investigate whether coffee or caffeine consumption is associated with reproductive endpoints among women with natural fertility (ie, time to pregnancy [TTP] and spontaneous abortion [SAB]) and among women in fertility treatment (ie, clinical pregnancy rate or live birth rate).
Design: This study was a systematic review and dose–response meta-analysis including data from case–control and cohort studies.
Methods: An extensive literature search was conducted in MEDLINE and Embase, with no time and language restrictions. Also, reference lists were searched manually. Two independent reviewers assessed the manuscript quality using the Newcastle–Ottawa Scale (NOS). A two-stage dose–response meta-analysis was applied to assess a potential association between coffee/caffeine consumption and the outcomes: TTP, SAB, clinical pregnancy, and live birth. Heterogeneity between studies was assessed using Cochrane Q-test and I2 statistics. Publication bias was assessed using Egger’s regression test.
Results: The pooled results showed that coffee/caffeine consumption is associated with a significantly increased risk of SAB for 300 mg caffeine/day (relative risk [RR]: 1.37, 95% confidence interval [95% CI]: 1.19; 1.57) and for 600 mg caffeine/day (RR: 2.32, 95% CI: 1.62; 3.31). No association was found between coffee/caffeine consumption and outcomes of fertility treatment (based on two studies). No clear association was found between exposure to coffee/caffeine and natural fertility as measured by fecundability odds ratio (based on three studies) or waiting TTP (based on two studies).
Conclusion: Results from this meta-analysis support the growing evidence of an association between coffee/caffeine intake and the risk of SAB. However, viewing the reproductive capacity in a broader perspective, there seems to be little, if any, association between coffee/caffeine consumption and fecundity. In general, results from this study are supportive of a precautionary principle advised by health organizations such as European Food Safety Authority (EFSA) and World Health Organization (WHO), although the advised limit of a maximum of two to three cups of coffee/200–300 mg caffeine per day may be too high.
Keywords: coffee, caffeine, fecundity, fertility, spontaneous abortion, assisted reproduction
Plain language summary
Coffee and other caffeinated beverages are widely consumed by women desiring to conceive as well as pregnant women. Whether such consumption reduces the chance of achieving a pregnancy or may have harmful effects on the developing fetus is widely debated. As a contribution to this debate and given the growing concern of subfecundity and infertility in public health, we investigated the risk of coffee or caffeine consumption on the ability to conceive a pregnancy and to carry the pregnancy to term. Results from this study showed an increased risk of experiencing a spontaneous abortion with increasing consumption of coffee/caffeine during early pregnancy. No clear association was found between coffee/caffeine consumption and the chance of achieving a pregnancy among women/couples trying to conceive naturally or by women/couples receiving fertility treatment. Results from this study support the precautionary principle advised by health organizations such as European Food Safety Authority (EFSA) and World Health Organization (WHO), although the advised limit of a maximum of two to three cups of coffee/200–300 mg caffeine per day may be too high.
Introduction
Subfecundity and infertility are multifactorial conditions of growing concern in public health. Worldwide, one in six couples experience fecundity problems during their reproductive lifetime.1–3 While the cause often may be explained by failure of ovulation, damages on the fallopian tubes, or low sperm count, the reduced fecundity is still unexplained in many cases. Thus, investigations of a potential effect of modifiable exposures on longer waiting time to pregnancy (TTP), increased rate of spontaneous abortion (SAB), or reduced live birth rate are important.
Coffee consumption is common among people throughout the world. Worldwide, Finland has the highest consumption (12.0 kg coffee/person/year), with Denmark being the fourth most coffee-consuming country (8.7 kg coffee/person/year). Also, coffee and other caffeine-containing drinks and foods are widely consumed by women desiring to conceive and by pregnant women. On average, a cup of coffee contains ~100 mg of caffeine.4
Even small effects of coffee or caffeine intake on fecundity could have considerable public health consequences, considering the large consumption of these substances.
The European Food Safety Authority (EFSA) recommends women desiring to conceive and pregnant women to keep their daily caffeine consumption below 200 mg,5 while WHO recommends a daily caffeine intake below 300 mg.6 Caffeine is a central ingredient in coffee, while other sources of caffeine include tea, soft drinks, chocolate, and some medications.
The association between caffeine intake and female fecundity has been studied with inconsistent findings, in studies with both retrospectively7–13 and prospectively collected exposure data.14–19 A high level of coffee consumption during pregnancy has been associated with an increased risk of fetal death after 20 weeks of gestation20,21 and stillbirth.22 In meta-analyses, an increased risk of low birth weight and small for gestational age was found,23 but no association was found between caffeine intake during pregnancy and the risk of preterm birth.23,24
Recently, a meta-analysis reported that an increment in intake of 100 mg caffeine per day was associated with a 14% increased risk of SAB.23 However, the authors did not account for the varying quality of the included studies, including varying (or none) adjustment for potential confounders. Studies that fail to adjust for important risk factors associated with SAB may over- or underestimate the risk associated with caffeine consumption. Hence, taking adjustment status into account would considerably improve the quality of a meta-analysis. Moreover, other substances in coffee besides caffeine might play an important role. Finally, applying a broader view on fecundity through inclusion of different reproductive endpoints among both women conceiving naturally and following fertility treatment could extend the use of the results and help general practitioners and fertility clinics provide balanced information to women and couples seeking advice or even fertility treatment. No meta-analysis has previously reported on a possible association between coffee/caffeine intake and TTP or endpoints among couples in fertility treatment.
The aim of this systematic review and dose-response meta-analysis was to investigate whether consumption of coffee or caffeine is associated with reproductive endpoints among women with natural fertility (ie TTP and SAB) and among women in fertility treatment (ie clinical pregnancy rate and live birth rate).
Methods
A review protocol is available from the PROSPERO database.25 The protocol was registered on June 9, 2015 (registration number: CRD42015020333).
Search strategy and selection
We conducted a comprehensive literature search in MEDLINE and Embase databases with the help of a medical librarian on May 26, 2015. The following keywords and medical subject headings were used to identify relevant articles in the databases: ([“Fertility” or “Infertility, Female” or “Reproduction” or “Preconception Care”) or [“Reproductive Techniques, Assisted” or “Reproductive Techniques” or “fertility treatment”]) AND (“Caffeine/adverse effects” or “Coffee”) AND (“Case-Control Studies” or “Cohort Studies”). To include non-indexed new literature, an additional search was performed using free text terms. No restrictions were made regarding study language or year of publication. Further, the reference lists of retrieved articles and reviews were searched manually for additionally relevant studies. The first author reviewed all retrieved articles for eligibility by title and abstract by consulting with at least one co-author in cases of any doubt about the inclusion of a given study or not. Potentially eligible articles were thoroughly read in full text to check whether they fulfilled the following inclusion criteria:
- Participants had to be either women/couples trying to conceive naturally or women/couples trying to conceive by medically assisted reproduction (MAR) treatment
- Studies that reported a numerical exposure range of either coffee or caffeine consumption
- Studies that investigated at least one of the outcomes of interest: TTP, SAB, clinical pregnancy, and live birth
- Articles that reported data from original studies (ie, no review articles)
- Studies of which the design was a case-control study or a cohort study (ie, no RCT)
Before finalizing the manuscript, the database search was repeated on April 3, 2017.
Data extraction
All included full text articles were read independently by at least two authors. A data extraction form was developed a priori, ensuring a standardized procedure. The following data were extracted: lead author surname, study title, country of origin, year of publication and journal, study design, study population (size and selection), exposure and outcome assessment, covariates adjusted for, main results, study limitations, and assessment of quality scoring.
We assessed the quality of all included studies using the Newcastle–Ottawa Scale (NOS).26 Thus, the selection process and comparability of the study groups as well as the ascertainment of exposure or outcome of interest of the included studies were evaluated for either cohort or case–control studies (please see scoring details in the Supplementary material). In addition to the NOS scheme, we constructed an explanatory form ensuring a standardized scoring among the authors with regard to the specific scope of this review (Supplementary material). When using NOS to evaluate study comparability, one has to choose the two most important covariates. Hence, based on the current knowledge, maternal age was chosen as the most important factor to adjust for. As additionally important adjustment factors, smoking was chosen for studies evaluating TTP and MAR treatment, while maternal alcohol consumption was chosen for those investigating SAB. All studies were allocated a total score between 0 and 9 independently by a minimum of two authors. Any inconsistency was resolved by discussion, leading to a uniform decision. Studies with a total score of ≥7 were considered as “high-quality studies”, and in a sub-analysis, studies were stratified according to this selected cut-off value. Regardless of total NOS score, all studies were included in the review.
This review is reported in accordance with the PRISMA statement27 for reporting of systematic review and meta-analysis. Supplementary material regarding the full search strategy for MEDLINE, elaborated reasons for exclusion, and NOS explanatory form are all available online.
Outcomes
The ability to naturally conceive was investigated by two broad fecundability measures including TTP (ie, the number of months or cycles taken by a couple to conceive, when being sexually active and not using birth control) and fecundability odds ratio (FOR) (ie, the ratio of couple’s probability of conceiving in one menstrual cycle).28 The capability of maintaining a pregnancy after conception was evaluated by the risk of experiencing an SAB (yes/no). Among couples receiving MAR treatment, a possible association was investigated using different endpoints to evaluate a successful fertility treatment (ie, achieving a pregnancy or live birth after fertility treatment; yes/no).
Statistical analysis
Although still included in the review, studies were excluded from the meta-analyses if they did not provide information on the number of cases and total number of subjects within each exposure category and whose corresponding author did not respond to emails asking for additional data to calculate these numbers.7,9,10,14,15,17,29–33 Also, we excluded studies that did not report on three or more quantitative categories of coffee/caffeine consumption,34–37 as these cannot contribute sufficient data when performing a dose–response meta-analysis.38
We applied a two-stage dose–response meta-analysis to assess a potential association between coffee/caffeine consumption and our outcomes.39 For fecundability and MAR treatment, we included only fixed effects in the meta-analysis due to the low number of included studies,40 while for SAB, we conducted a random-effects meta-analysis after considering heterogeneity between studies. As coffee/caffeine consumption was reported on various scales, we converted exposure data into a uniform measurement (mg/day). Studies reporting consumption in cups of coffee were recalculated into an approximate caffeine content, assuming that one cup of coffee in general contains 100 mg caffeine.4,41 As the approximation of caffeine content per cup of coffee might be imprecise, a sensitivity analysis was performed, assuming that one cup of coffee contained 150 mg caffeine. Coffee/caffeine consumption was modeled using restricted cubic splines with three knots at the 10th, 50th, and 90th percentiles of the distribution of doses.38,42,43 For each model, we present an overall p-value testing that the two regression coefficients describing the nonlinear relationship were simultaneously equal to zero. Also, upon finding a statistical significant association, we present a p-value testing the linear versus nonlinear relationship.44 When analyzing risk of SAB, we performed stratified analyses by type of consumption (caffeine vs coffee), adjustment status (crude vs any adjustment), study quality (NOS>7 vs NOS<7), and study design (cohort vs case–control). Similar analyses were not possible for other outcomes due to the low number of studies. Heterogeneity between studies was assessed using the Cochrane Q-test and the I2 statistic. Low heterogeneity was defined by I2 values <25%, while a I2 value >75% was considered an indicator of substantial heterogeneity.45 Publication bias was assessed for SAB studies, using Egger’s regression test.46 As meta-analysis for fecundity and MAR endpoints only included a maximum of three studies, tests of heterogeneity and publication bias were irrelevant for these outcomes.47 All statistical analyses were performed using the “dosresmeta” and “metafor” packages in R version 3.3.1.48,49 p-values <0.05 were considered statistically significant.
Results
Characteristics of the included studies
A total number of 379 articles were identified through the systematic literature search, and an additional 68 articles were identified through a manual search of their references (Figure 1). Of the total 447 articles, 136 were duplicates and thus excluded. The remaining 311 unique articles were screened by title and, if necessary, by abstract, and 195 did not meet the eligibility criteria. Full-texts were assessed for the remaining 116 articles. Of these, 68 articles were excluded due to not meeting the eligibility criteria, and one article was excluded due to insufficient data reporting.
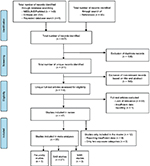  | Figure 1 Flow diagram. Abbreviations: SAB, spontaneous abortion; MAR, medically assisted reproduction. |
Thus, a total of 47 studies were included in the systematic review. Of these, 35 studies had dose–response information (ie, reporting at least three or more quantitative exposure categories) and were included in our meta-analyses. Of these 35 studies, 5 studies reported about the endpoints of fecundity9,10,16,19,50, 27 studies reported about SAB,21,32,34,51–73 and 3 studies reported on the outcomes of MAR.74–76 Results from the remaining 12 studies that did not provide sufficient data to be included in the meta-analysis are included as a narrative description.
All included studies were published between 1988 and 2015. Thirty-one were cohort studies, while 16 were case–control studies. Study characteristics, results, and the assigned total NOS scores are presented in Tables 1–3 (for NOS elaborations, please see the Table S1). The presented results cover a variety of countries with large differences in exposure levels ranging from non-consumers to those who consume over 1,200 mg/day (corresponding to 12 cups of coffee/day). Also, adjustment for potential confounders varied considerably between studies (Tables 1–3).
FOR
In the three cohort studies providing sufficient data to be included in the meta-analysis, we found no indication of an association between caffeine intake up to 400 mg/day and altered FOR (overall p=0.76; Figure 2A). Compared with no caffeine intake, the pooled odds ratios for altered fecundability were 1.03 (95% confidence interval [CI]: 0.83; 1.28) for 100 mg caffeine/day and 1.09 (95% CI: 0.86; 1.37) for 400 mg caffeine/day.
Studies not eligible for meta-analysis
Five other studies have addressed a possible association between coffee/caffeine consumption and fecundability with contradicting findings. Two studies from the US13,15 including a total of 104 and 6,303 women, respectively reported reduced fecundability with higher caffeine consumption in the late 1980s. However, results by three later studies (two European studies and one US study)14,17,30 including 259 and 423, and 470 women, respectively, support the findings of no association between caffeine consumption on fecundability.
TTP
Only two cohort studies provided sufficient data on TTP, to be included in the dose–response meta-analysis. The results did not indicate an association (p=0.43 for TTP >6 months and p=0.98 for TTP >12 months) (Figure 2B and 2C).
Compared with no caffeine intake, the pooled relative risks for TTP >6 months were 0.92 (95% CI: 0.79; 1.07) for 100 mg caffeine/day and 0.76 (95% CI: 0.49; 1.18) for 600 mg caffeine/day. For TTP >12 months, the pooled relative risks were 0.99 (95% CI: 0.88; 1.11) for 100 mg caffeine/day and 0.97 (95% CI: 0.71; 1.33) for 600 mg caffeine/day, compared with no caffeine intake.
Studies not eligible for meta-analysis
Two studies that were not included in the meta-analysis have investigated a possible association between caffeine consumption and TTP. Bolúmar et al7 reported on 3,146 European women and found an increased risk of TTP >9.5 months with a caffeine intake of >500 mg/day when reporting retrospectively on their first pregnancy. However, no association was found when reporting on the most recent waiting time. Another study including 66 American women29 did not find an association between caffeine consumption and TTP.
SAB
We found that higher intake of caffeine was associated with higher risk of spontaneous abortion (overall association p<0.0001; p for non-linearity <0.01), based on 27 studies providing sufficient data to be included in the meta-analysis (Figure 2D). Compared with no caffeine intake, the pooled relative risks for SAB were 1.08 (95% CI: 1.03; 1.13) for 100 mg caffeine/day, 1.37 (95% CI: 1.19; 1.57) for 300 mg caffeine/day, and 2.32 (95% CI: 1.62; 3.31) for 600 mg caffeine/day. There was evidence of significant between-study heterogeneity (I2=73,7%; p<0.0001), and Egger’s regression test provided evidence of substantial publication bias (p<0.0001; Table 2).
We stratified the SAB analyses on type of consumption, adjustment status, study quality, and study design. The analyses revealed that the associations were similar between studies reporting caffeine and coffee consumption (p for heterogeneity=0.87). Also, the associations were similar across strata according to adjustment status (ie, studies with adjusted results compared to unadjusted results) (p for heterogeneity=0.44) and according to study quality (ie, comparing studies with NOS ≥7 with those of NOS <7) (p for heterogeneity=0.22). We found weak evidence of a difference by study design with the associations being more pronounced in cohort studies than in case–control studies (p for heterogeneity=0.04). In a sensitivity analysis, changing the conversion factor of caffeine contained in one cup of coffee from 100 mg to 150 mg did not substantially change the results.
Studies not eligible for meta-analysis
Five other studies, not included in the meta-analysis, have investigated a possible association between coffee/caffeine consumption and SAB with consistent findings in line with our results. A Swedish cohort study including 654 women31 reported an increased risk of SAB with a daily coffee consumption greater than three cups, while a case–control study among 452 Saudi-Arabian women37 showed an association at caffeine consumption levels >150 mg/day. Also, the association of an increased risk of SAB with caffeine consumption was found among a selected group of 191 American women with type-1 diabetes.35 In an Italian case–control study including 312 women,33 a dose–response association was reported between caffeine intake and risk of recurrent SAB. Also, Parazzini et al36 found an association with any coffee consumption among women with recurrent pregnancy loss in a case–control study including 260 women; however, results were not statistically significant.
MAR treatment
Only two cohort studies provided sufficient data on live birth rate among couples undergoing in vitro fertilization (IVF) treatment74,76 to be included in the dose–response meta-analysis. The results did not indicate an association between caffeine intake and the live birth rate among women in fertility treatment (p= 0.94; Figure 2E). The pooled relative risks for achieving a live birth were 0.99 (95% CI: 0.89; 1.10) for 100 mg caffeine/day and 1.00 (95% CI: 0.80; 1.24) for 300 mg caffeine/day, compared with no caffeine intake.
Study not eligible for meta-analysis
Only one study by Al-Saleh et al75 reported on the pregnancy rate among couples undergoing fertility treatment. The study showed no association between coffee/tea consumption or serum caffeine concentration and the success rate of pregnancy among 619 Saudi-Arabian women undergoing their first IVF-treatment cycle. However, the number of eggs was lower at higher levels of serum caffeine.
Discussion
This systematic review and meta-analysis showed no clear association between exposure to coffee/caffeine and natural fertility as measured by FOR or waiting TTP. However, due to the limited number of studies included, no firm conclusions can be drawn for these endpoints. Our pooled results indicate that coffee/caffeine consumption is associated with a significantly increased risk of SAB, as expected from previous reviews and meta-analyses.23,78,79 Further, in addition to previous studies, we intended to capture nonlinear associations using novel statistical methods but found no evidence of a “threshold effect”. With regard to the potential effect of coffee or caffeine consumption on the outcomes of fertility treatment, our sparse results on MAR treatment as measured by the pregnancy and live birth rate in connection with IVF treatment did not show an association. Yet, the data available enable us to conclude little about the potential dose–response relationship.
Various hypotheses exist concerning the possible mechanisms of the potential effect of caffeine on different fecundity and pregnancy outcomes, but the eventual mechanisms are still unclear. Within the human body, caffeine acts as a non-selective adenosine antagonist;80 it increases the intracellular concentration of cyclic AMP81 and alters the blood levels of catecholamines.82 Coffee and caffeine consumption may affect the reproductive health by affecting endogenous hormone levels through changes in ovarian function or alterations in hormone metabolism: numerous studies have reported an inverse association between coffee/caffeine intake and levels of free estradiol83–86 in line with the increased levels of sex hormone–binding globulin reported by others.87–89 However, contradicting results of increased levels76,90 or no effects on estradiol levels are also reported.87,88,91,92 Caffeine and estradiol are both metabolized by the hepatic enzyme CYP1A293,94, so a possible pathway for caffeine to interfere with estradiol levels is through a common metabolism. Also, besides caffeine, coffee contains numerous other bioactive substances including lignans and isoflavonoids, both belonging to the phytoestrogen family with great affinity for the estrogen receptor.4 As a consequence, changes in hormonal levels might impact on the menstrual cycle, and a daily caffeine consumption >300 mg has been associated with shorter but not with longer menstrual cycle length.95 Moreover, caffeine intake was not associated with the number of oocytes retrieved, the fertilization rate, or implantation rate among women receiving fertility treatment.76 The conflicting results on coffee’s potential effect on the hormonal pathways are in line with our results suggesting no clear association with fecundity endpoints. In pregnant women, caffeine readily crosses the placental barrier and as a result is distributed to all fetal tissue, making the fetus exposed to the same caffeine levels as the mother.96 Also, coffee intake has been associated with decreased levels of both estrogen and hCG,97 and caffeine consumption during pregnancy is shown to increase levels of catecholamines and cyclic adenosine monophosphate that might impact on placental blood flow.98–100 Indeed, these pathways could have an influence on the increased risk of SAB as observed in this meta-analysis.
This review and meta-analysis has several strengths, including the broader investigation of possible routes of interfering with the ability to conceive and carry a pregnancy to term. Another strength is the dose–response analysis, allowing us to include all information on exposure outcome by including information on intermediate categories, thus making the analysis more effective than the traditional highest versus lowest approach. Using this method, we were able to provide a detailed and more flexible description of the risk of outcome throughout the observed range of exposure. For the SAB analysis, we were able to include a relatively large number of studies providing higher statistical power and allowing us to perform stratified analyses. Hence, we were able to consider potential sources of heterogeneity, including taking into account the varying quality of the included studies.
However, this systematic review and dose–response meta-analysis also has several potential limitations. Though a majority of the included studies adjusted for potential confounders, the presence of residual or unmeasured confounding cannot be excluded due to the observational nature of the included studies. We observed marked between-study heterogeneity, which can be caused by methodological or actual differences between the included studies. Consequently, data on SAB were analyzed using a random-effects model. In addition, possible sources of heterogeneity in SAB studies were addressed in stratified analysis, and only study design was found of significance.
Based on the current knowledge, we chose alcohol instead of smoking to be the second most important factor to adjust for in studies reporting on SAB. However, had smoking been selected instead, the NOS would have differed only by one point in three of the 33 evaluated studies as 18 adjusted for both alcohol consumption and smoking, while 12 did not adjust for either of the two. A priori, we determined the threshold for depicting a high-quality study as an NOS ≥7. However, changing the threshold in a sensitivity analysis to NOS ≥8 did not noticeably alter the association in the two strata (p for heterogeneity=0.28).
We found evidence of publication bias for studies reporting on SAB, indicating that small studies not reporting any association are less likely to be published. Thus, results from SAB analysis should be interpreted bearing this in mind. Due to the limited number of studies reporting sufficient data on fecundity or MAR endpoints, assessment of publication bias for these could not be done.
As data on caffeine were self-reported and obtained by various questionnaires or interview, exposure misclassification of coffee/caffeine intake is unavoidable. Especially studies with retrospectively collected exposure data are prone to recall bias. Further, due to the numerous types of questionnaires used to assess exposure, the accuracy and data quality are expected to differ accordingly.
Yet, validation studies support that coffee consumption can be assessed with fairly high validity.101 Due to the various sources of exposure, it is difficult to estimate the total caffeine consumption accurately. Further, coffee contains a series of different chemical substances, making it difficult to segregate potential biological effects from caffeine from those of other bioactive compounds. Also, content of the different substances in coffee varies by type of bean, brewing method, and cup size.102 Thus, a possible association might be caused by other compounds or by a cocktail effect due to simultaneous exposure to multiple bioactive substances. However, most studies have focused on exposure to caffeine and thus only to some extent on exposure to coffee. In general, exposure assessment was reported as the average daily intake, and consequently we were not able to take the consumption patterns of coffee/caffeine into account. Also, different conversion factors were used in studies when translating coffee consumption into caffeine. In the meta-analysis, we tried to quantify the amount of caffeine intake when exposure was only reported as cups of coffee, but by doing so, we were unable to integrate the pronounced variation in caffeine content by different types of coffee consumed. However, changing the conversion factor up to 150 mg caffeine per cup of coffee in a sensitivity analyses did not change the risk of SAB markedly.
The actual level of caffeine exposure might vary greatly due to individual differences in the CYP1A2 gene activity.70,103 Hence, polymorphism of CYP1A2 has been linked to both variability in the clearance rate of caffeine104 and variability in serum estradiol concentrations.105 As a consequence, the half-life of caffeine varies between 1.5 hours for fast metabolizers and up to 10 hours for slow metabolizers,4 making it difficult to settle on a relevant window of exposure.
In the context of SAB, reverse causation has to be considered; a viable pregnancy might produce more pronounced pregnancy symptoms, resulting in greater aversion to coffee and thus a decreased caffeine intake. However, a clinically verified SAB may be diagnosed weeks after the actual fetal demise and thus the yet unrecognized pregnancy loss might be the cause of an increased caffeine consumption rather than the opposite – an increased consumption causing adverse reproductive outcomes. In contrast to previous meta-analyses78,79 that presented pooled endpoints of SAB with still birth/fetal death, we only included SAB, as the underlying etiology indeed are different.
Six cohort studies out of the included 32 studies on SAB verified the presence of a pregnancy at the time of reporting exposure, thereby reducing the risk of reversed causation. Among these studies, an association between caffeine intake and SAB was indicated, albeit only statistically significant in three studies.
Due to the great differences in caffeine metabolism, future studies may benefit from actually evaluating circulating caffeine levels or its metabolites (eg, serum paraxanthine, the main metabolite) in linkage with genotyping to heighten the information of the actual exposure level. Thus, future studies could consider Mendelian randomization. Also, to possibly segregate effects of caffeine from those of other bioactive substances in coffee, future studies could consider evaluating the consumption of decaffeinated coffee.
Conclusion
In conclusion, results from this dose–response meta-analysis support the evidence of an association between coffee/caffeine intake and the risk of SAB. However, results from the SAB analyses have to be interpreted with caution due to the significant heterogeneity and risk of bias detected, not least the considerable risk of publication bias. As coffee is the main source of caffeine consumption, it is still unclear whether the association between coffee/caffeine consumption and SAB is related to the potential harmful effects of caffeine or to some of the other bioactive components of coffee. Viewing the reproductive capacity in a broader perspective, there seems to be little, if any, association between coffee/caffeine consumption and fecundity. Even so, there is still a need for further studies to investigate the fecundity and MAR endpoints on a larger scale than previously done, using better study designs and including improved reporting combined with relevant adjustment strategies.
Acknowledgments
JL is supported by a fully financed PhD-scholarship from Aarhus University. AH is supported by the Danish Diabetes Academy. The Danish Diabetes Academy is funded by the Novo Nordisk Foundation. The funding sources had no involvement in the conduct of this article.
Author contributions
All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Juul S, Karmaus W, Olsen J. Regional differences in waiting time to pregnancy: pregnancy-based surveys from Denmark, France, Germany, Italy and Sweden. The European Infertility and Subfecundity Study Group. Hum Reprod. 1999;14(5):1250–1254. | ||
Schmidt L. Infertility and assisted reproduction in Denmark. Epidemiology and psychosocial consequences. Dan Med Bull. 2006;53:390–417. | ||
Jacob MC, McQuillan J, Greil AL. Psychological distress by type of fertility barrier. Hum Reprod. 2007;22(3):885–894. | ||
Hermansen K, Bech BH, Dragsted LO, et al. “Kaffe, sundhed og sygdom”, Vidensråd for Forebyggelse. [Coffee, health and disease]. 2015 Available from: http://www.vidensraad.dk/sites/default/files/vidensrad_for_forebyggelse_kaffe_sundhed_og_sygdom_2015.pdf. Accessed November 13, 2017. Danish. | ||
EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the safety of caffeine. EFSA J. 2015;13(5):4102. | ||
WHO. Recommendations on Antenatal Care for a Positive Pregnancy Experience. Geneva: WHO; 2016:152. | ||
Bolumar F, Olsen J, Rebagliato M, Bisanti L. Caffeine intake and delayed conception: a European multicenter study on infertility and subfecundity. European Study Group on Infertility Subfecundity. Am J Epidemiol. 1997;145(4):324–334. | ||
Hatch EE, Bracken MB. Association of delayed conception with caffeine consumption. Am J Epidemiol. 1993;138(12):1082–1092. | ||
Olsen J. Cigarette smoking, tea and coffee drinking, and subfecundity. Am J Epidemiol. 1991;133(7):734–739. | ||
Alderete E, Eskenazi B, Sholtz R. Effect of cigarette smoking and coffee drinking on time to conception. Epidemiology. 1995;6(4):403–408. | ||
Joesoef MR, Beral V, Rolfs RT, Aral SO, Cramer DW. Are caffeinated beverages risk factors for delayed conception? Lancet. 1990;335(8682):136–137. | ||
Curtis KM, Savitz DA, Arbuckle TE. Effects of cigarette smoking, caffeine consumption, and alcohol intake on fecundability. 1997;146:32–41. | ||
Christianson RE, Oechsli FW, Berg BJ. Caffeinated beverages and decreased fertility. Lancet. 1989;1(8634):378. | ||
Jensen TK, Henriksen TB, Hjollund NH, et al. Caffeine intake and fecundability: a follow-up study among 430 Danish couples planning their first pregnancy. Reprod Toxicol. 1998;12(3):289–295. | ||
Wilcox A, Weinberg C, Baird D. Caffeinated beverages and decreased fertility. Lancet. 1988;2(8626–8627):1453–1456. | ||
Caan B, Quesenberry CP, Jr, Coates AO. Differences in fertility associated with caffeinated beverage consumption. Am J Public Health. 1998;88(2):270–274. | ||
Florack EI, Zielhuis GA, Rolland R. Cigarette smoking, alcohol consumption, and caffeine intake and fecundability. Prev Med. 1994;23(2):175–180. | ||
Spinelli A, Figa-Talamanca I, Osborn J. Time to pregnancy and occupation in a group of Italian women. Int J Epidemiol. 1997;26(3):601–609. | ||
Hakim RB, Gray RH, Zacur H. Alcohol and caffeine consumption and decreased fertility. Fertil Steril. 1998;70:632–637. | ||
Matijasevich A, Barros FC, Santos IS, Yemini A. Maternal caffeine consumption and fetal death: a case-control study in Uruguay. Paediatr Perinat Epidemiol. 2006;20(2):100–109. | ||
Bech BH, Nohr EA, Vaeth M, Henriksen TB, Olsen J. Coffee and fetal death: a cohort study with prospective data. Am J Epidemiol. 2005;162(10):983–990. | ||
Wisborg K, Kesmodel U, Bech BH, Hedegaard M, Henriksen TB. Maternal consumption of coffee during pregnancy and stillbirth and infant death in first year of life: prospective study. BMJ. 2003;326(7386):420. | ||
Greenwood DC, Thatcher NJ, Ye J, et al. Caffeine intake during pregnancy and adverse birth outcomes: a systematic review and dose-response meta-analysis. Eur J Epidemiol. 2014;29(10):725–734. | ||
Maslova E, Bhattacharya S, Lin SW, Michels KB. Caffeine consumption during pregnancy and risk of preterm birth: a meta-analysis. Am J Clin Nutr. 2010;92(5):1120–1132. | ||
PROSPERO. International prospective register of systematic reviews. 2017. Available from: https://www.crd.york.ac.uk/PROSPERO/. Accessed November 13, 2017. | ||
Wells GA, Shea B, O’Connell D, et alNewcastle-Ottawa Scale (NOS); 2017. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed May 8, 2017. | ||
Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151(4):W65–W94. | ||
Wilcox A. Fertility and Pregnancy. New York, NY, Oxford University Press; 2010. | ||
Pollack AZ, Buck Louis GM, Sundaram R, Lum KJ. Caffeine consumption and miscarriage: a prospective cohort study. Fertil Steril. 2010;93(1):304–306. | ||
Taylor KC, Small CM, Dominguez CE, et al. Alcohol, smoking, and caffeine in relation to fecundability, with effect modification by NAT2. Ann Epidemiol. 2011;21(11):864–872. | ||
Axelsson G, Rylander R. Outcome of pregnancy in women engaged in laboratory work at a petrochemical plant. Am J Ind Med. 1989;16(5):539–545. | ||
Cnattingius S, Signorello LB, Anneren G, et al. Caffeine intake and the risk of first-trimester spontaneous abortion. N Engl J Med. 2000;343(25):1839–1845. | ||
Stefanidou EM, Caramellino L, Patriarca A, Menato G. Maternal caffeine consumption and sine causa recurrent miscarriage. Eur J Obstet Gynecol Reprod Biol. 2011;158(2):220–224. | ||
Srisuphan WF, Bracken MB. Caffeine consumption during pregnancy and association with late spontaneous abortion. 1986;154(1):14–20. | ||
Khoury JC, Miodovnik M, Buncher CR, et al. Consequences of smoking and caffeine consumption during pregnancy in women with type 1 diabetes. J Matern Fetal Neonatal Med. 2004;15(1):44–50. | ||
Parazzini F, Bocciolone L, Fedele L, Negri E, La Vecchia C, Acaia B. Risk factors for spontaneous abortion. Int J Epidemiol. 1991;20(1):157–161. | ||
Lubna A, Al-Ansary ZAB. Risk factors for spontaneous abortion: a preliminary study on Saudi women. J Roy Soc Health. 1994;114:188–193. | ||
Orsini N, Li R, Wolk A, Khudyakov P, Spiegelman D. Meta-analysis for linear and nonlinear dose-response relations: examples, an evaluation of approximations, and software. Am J Epidemiol. 2012;175(1):66–73. | ||
Crippa AO, N. Dose-response meta-analysis of differences in means. BMC Med Res Methodol. 2016;16:91. | ||
Chen DG, Fang D, Wilson JR. Meta-analysis of two studies with random effects? J Minim Invasive Gynecol. 2017;24(5):689–690. | ||
McCusker RR, Goldberger BA, Cone EJ. Caffeine content of specialty coffees. J Anal Toxicol. 2003;27(7):520–522. | ||
Greenland S. Dose-response and trend analysis in epidemiology: alternatives to categorical analysis. Epidemiology. 1995;6(4):356–365. | ||
Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8(5):551–561. | ||
Desquilbet L, Mariotti F. Dose-response analyses using restricted cubic spline functions in public health research. Stat Med. 2010;29(9):1037–1057. | ||
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. | ||
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. | ||
Green JPHaS. Cochrane Handbook for Systematic Reviews of Interventions; 2017. Available from: http://handbook.cochrane.org/chapter_10/10_4_3_1_recommendations_on_testing_for_funnel_plot_asymmetry.htm. Accessed June 2, 2017. | ||
R Core Team. R: A language and environment for statistical computing; 2009. Available from: https://www.r-project.org. Accessed November 13, 2017. | ||
Viechtbauer W. Conducting meta-analyses in R with metafor package. J Stat Softw. 2010;36(3):48. | ||
Hatch EE, Wise LA, Mikkelsen EM, et al. Caffeinated beverage and soda consumption and time to pregnancy. Epidemiology. 2012;23(3):393–401. | ||
Fenster L, Eskenazi B, Windham GC, Swan SH. Caffeine consumption during pregnancy and spontaneous abortion. Epidemiology. 1991;2(3):168–174. | ||
Fenster L, Hubbard AE, Swan SH, et al. Caffeinated beverages, decaffeinated coffee, and spontaneous abortion. Epidemiology. 1997;8(5):515–523. | ||
Armstrong BG, McDonald AD, Sloan M. Cigarette, alcohol, and coffee consumption and spontaneous abortion. Am J Public Health. 1992;82(1):85–87. | ||
Dominguez-Rojas V, de Juanes-Pardo R, Astasio-Arbiza P, Ortega-Molina P, Gordillo-Florencio E. Spontaneous abortion in a hospital population: are tobacco and coffee intake risk factors? Eur J Epidemiol. 1994;10(6):665–668. | ||
Dlugosz L, Belanger K, Hellenbrand K, Holford TR, Leaderer B, Bracken MB. Maternal caffeine consumption and spontaneous abortion: a prospective cohort study. Epidemiology. 1996;7(3):250–255. | ||
Agnesi R, Valentini F, Fedeli U, et al. Maternal exposures and risk of spontaneous abortion before and after a community oriented health education campaign. Eur J Public Health. 2010;21(3):282–285. | ||
Agnesi R, Valentini F, Mastrangelo G. Risk of spontaneous abortion and maternal exposure to organic solvents in the shoe industry. Int Arch Occup Environ Health. 1997;69(5):311–316. | ||
Giannelli M, Doyle P, Roman E, Pelerin M, Hermon C. The effect of caffeine consumption and nausea on the risk of miscarriage. Paediatr Perinat Epidemiol. 2003;17(4):316–323. | ||
George L, Granath F, Johansson AL, Olander B, Cnattingius S. Risks of repeated miscarriage. Paediatr Perinat Epidemiol. 2006;20(2):119–126. | ||
Kline J, Levin B, Silverman J, et al. Caffeine and spontaneous abortion of known karyotype. Epidemiology. 1991;2(6):409–417. | ||
Infante-Rivard C, Fernandez A, Gauthier R, David M, Rivard, GE. Fetal loss associated with caffeine intake before and during pregnancy. JAMA. 1993;270(24);2940–2943. | ||
Mills JL, Holmes LB, Aarons JH, et al. Moderate caffeine use and the risk of spontaneous-abortion and intrauterine growth-retardation. JAMA. 1993;269(5):593–597. | ||
Parazzini F, Chatenoud L, Di Cintio E, et al. Coffee consumption and risk of hospitalized miscarriage before 12 weeks of gestation. Hum Reprod. 1998;13(8):2286–2291. | ||
Maconochie N, Doyle P, Prior S, Simmons R. Risk factors for first trimester miscarriage – results from a UK-population-based case-control study. BJOG. 2007;114(2):170–186. | ||
Greenwood DC, Alwan N, Boylan S, et al. Caffeine intake during pregnancy, late miscarriage and stillbirth. Eur J Epidemiol. 2010;25(4):275–280. | ||
Wilcox AJ, Weinberg CR, Baird DD. Risk factors for early pregnancy loss. Epidemiology. 1990;1(5):382–385. | ||
Zhang H, Bracken MB. Tree-based, two-stage risk factor analysis for spontaneous abortion. Am J Epidemiol. 1996;144(10):989–996. | ||
Wen W, Shu XO, Jacobs DR, Jr, Brown JE. The associations of maternal caffeine consumption and nausea with spontaneous abortion. Epidemiology. 2001;12(1):38–42. | ||
Tolstrup JS, Munk C, Madsen LB, Ottesen B, Bergholt T, Gronbaek M. Does caffeine and alcohol intake before pregnancy predict the occurrence of spontaneous abortion? Hum Reprod. 2003;18(12):2704–2710. | ||
Sata F, Yamada H, Suzuki K, et al. Caffeine intake, CYP1A2 polymorphism and the risk of recurrent pregnancy loss. Mol Hum Reprod. 2005;11(5):357–360. | ||
Savitz DA, Chan RL, Herring AH, Howards PP, Hartmann KE. Caffeine and miscarriage risk. Epidemiology. 2008;19(1):55–62. | ||
Weng X, Odouli R, Li DK. Maternal caffeine consumption during pregnancy and the risk of miscarriage: a prospective cohort study. Am J Obstet Gynecol. 2008;198(3):279.e1–8. | ||
Hahn KA, Wise LA, Rothman KJ, et al. Caffeine and caffeinated beverage consumption and risk of spontaneous abortion. Hum Reprod. 2015;30(5):1246–1255. | ||
Klonoff-Cohen H, Bleha J, Lam-Kruglick P. A prospective study of the effects of female and male caffeine consumption on the reproductive endpoints of IVF and gamete intra-Fallopian transfer. Hum Reprod. 2002;17(7):1746–1754. | ||
Al-Saleh I, El-Doush I, Grisellhi B, Coskun S. The effect of caffeine consumption on the success rate of pregnancy as well various performance parameters of in-vitro fertilization treatment. Med Sci Monit. 2010;16(12):CR598–CR605. | ||
Choi JH, Ryan LM, Cramer DW, Hornstein MD, Missmer SA. Effects of caffeine consumption by women and men on the outcome of in vitro fertilization. J Caffeine Res. 2011;1(1):29–34. | ||
Rasch V. Cigarette, alcohol, and caffeine consumption: risk factors for spontaneous abortion. Acta Obstet Gynecol Scand. 2003;82(2):182–188. | ||
Li J, Zhao H, Song JM, Zhang J, Tang YL, Xin CM. A meta-analysis of risk of pregnancy loss and caffeine and coffee consumption during pregnancy. Int J Gynaecol Obstet. 2015;130(2):116–122. | ||
Chen LW, Wu Y, Neelakantan N, Chong MF, Pan A, van Dam RM. Maternal caffeine intake during pregnancy and risk of pregnancy loss: a categorical and dose-response meta-analysis of prospective studies. Public Health Nutr. 2016;19(7):1233–1244. | ||
Fredholm BB. On the mechanism of action of theophylline and caffeine. Acta Med Scand. 1985;217(2):149–153. | ||
Soyka LF. Effects of methylxanthines on the fetus. Clin Perinatol. 1979;6(1):37–51. | ||
Bellet S, Roman L, DeCastro O, Kim KE, Kershbaum A. Effect of coffee ingestion on catecholamine release. Metabolism. 1969;18(4):288–291. | ||
London S, Willett W, Longcope C, McKinlay S. Alcohol and other dietary factors in relation to serum hormone concentrations in women at climacteric. Am J Clin Nutr. 1991;53(1):166–171. | ||
Petridou E, Katsouyanni K, Spanos E, Skalkidis Y, Panagiotopoulou K, Trichopoulos D. Pregnancy estrogens in relation to coffee and alcohol intake. Ann Epidemiol. 1992;2(3):241–247. | ||
Kotsopoulos J, Eliassen AH, Missmer SA, Hankinson SE, Tworoger SS. Relationship between caffeine intake and plasma sex hormone concentrations in premenopausal and postmenopausal women. Cancer. 2009;115(12):2765–2774. | ||
Schliep KC, Schisterman EF, Mumford SL, et al. Caffeinated beverage intake and reproductive hormones among premenopausal women in the BioCycle Study. Am J Clin Nutr. 2012;95(2):488–497. | ||
Goto A, Song Y, Chen BH, Manson JE, Buring JE, Liu S. Coffee and caffeine consumption in relation to sex hormone-binding globulin and risk of type 2 diabetes in postmenopausal women. Diabetes. 2011;60(1):269–275. | ||
Nagata C, Kabuto M, Shimizu H. Association of coffee, green tea, and caffeine intakes with serum concentrations of estradiol and sex hormone-binding globulin in premenopausal Japanese women. Nutr Cancer. 1998;30(1):21–24. | ||
Ferrini RL, Barrett-Connor E. Caffeine intake and endogenous sex steroid levels in postmenopausal women. The Rancho Bernardo Study. Am J Epidemiol. 1996;144(7):642–644. | ||
Lucero J, Harlow BL, Barbieri RL, Sluss P, Cramer DW. Early follicular phase hormone levels in relation to patterns of alcohol, tobacco, and coffee use. Fertil Steril. 2001;76(4):723–729. | ||
Kinney A, Kline J, Kelly A, Reuss ML, Levin B. Smoking, alcohol and caffeine in relation to ovarian age during the reproductive years. Hum Reprod. 2007;22(4):1175–1185. | ||
Cooper C, Atkinson EJ, Wahner HW, et al. Is caffeine consumption a risk factor for osteoporosis? J Bone Miner Res. 1992;7(4):465–471. | ||
Butler MA, Iwasaki M, Guengerich FP, Kadlubar FF. Human cytochrome P-450PA (P-450IA2), the phenacetin O-deethylase, is primarily responsible for the hepatic 3-demethylation of caffeine and N-oxidation of carcinogenic arylamines. Proc Natl Acad Sci U S A. 1989;86(20):7696–7700. | ||
Yamazaki H, Shaw PM, Guengerich FP, Shimada T. Roles of cytochromes P450 1A2 and 3A4 in the oxidation of estradiol and estrone in human liver microsomes. Chem Res Toxicol. 1998;11(6):659–665. | ||
Fenster L, Quale C, Waller K, et al. Caffeine consumption and menstrual function. Am J Epidemiol. 1999;149(6):550–557. | ||
Aldridge A, Bailey J, Neims AH. The disposition of caffeine during and after pregnancy. Semin Perinatol. 1981;5(4):310–314. | ||
Lawson CC, LeMasters GK, Levin LS, Liu JH. Pregnancy hormone metabolite patterns, pregnancy symptoms, and coffee consumption. Am J Epidemiol. 2002;156(5):428–437. | ||
Kirkinen P, Jouppila P, Koivula A, Vuori J, Puukka M. The effect of caffeine on placental and fetal blood flow in human pregnancy. Am J Obstet Gynecol. 1983;147(8):939–942. | ||
Weathersbee PS, Lodge JR. Caffeine: its direct and indirect influence on reproduction. J Reprod Med. 1977;19(2):55–63. | ||
Morris MB, Weinstein L. Caffeine and the fetus: is trouble brewing? Am J Obstet Gynecol. 1981;140(6):607–610. | ||
Ferraroni M, Tavani A, Decarli A, et al. Reproducibility and validity of coffee and tea consumption in Italy. Eur J Clin Nutr. 2004;58(4):674–680. | ||
Bracken MB, Triche E, Grosso L, Hellenbrand K, Belanger K, Leaderer BP. Heterogeneity in assessing self-reports of caffeine exposure: implications for studies of health effects. Epidemiology. 2002;13(2):165–171. | ||
Grosso LM, Bracken MB. Caffeine metabolism, genetics, and perinatal outcomes: a review of exposure assessment considerations during pregnancy. Ann Epidemiol. 2005;15(6):460–466. | ||
Sachse C, Brockmoller J, Bauer S, Roots I. Functional significance of a C-->A polymorphism in intron 1 of the cytochrome P450 CYP1A2 gene tested with caffeine. Br J Clin Pharmacol. 1999;47(4):445–449. | ||
Lurie G, Maskarinec G, Kaaks R, Stanczyk FZ, Le Marchand L. Association of genetic polymorphisms with serum estrogens measured multiple times during a 2-year period in premenopausal women. Cancer Epidemiol Biomarkers Prev. 2005;14(6):1521–1527. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




