Back to Journals » International Journal of General Medicine » Volume 16
Association Between Chinese Visceral Adipose Index and Albuminuria in Chinese Adults: A Cross-Sectional Study
Authors Yu F, Liu A, Deng Z , Gan S , Zhou Q , Long H
Received 13 March 2023
Accepted for publication 30 May 2023
Published 5 June 2023 Volume 2023:16 Pages 2271—2283
DOI https://doi.org/10.2147/IJGM.S411416
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Fang Yu,1,* Aizhong Liu,2,* Zhiming Deng,1,* Shenglian Gan,1 Quan Zhou,3 Haowen Long4
1Department of Endocrinology, The First People’s Hospital of Changde City, Changde, Hunan, People’s Republic of China; 2Hunan Provincial Key Laboratory of Clinical Epidemiology, Xiangya School of Public Health, Central South University, Changsha, Hunan, People’s Republic of China; 3Department of Science and Education Section, The First People’s Hospital of Changde City, Changde, Hunan, People’s Republic of China; 4Department of Radiology, The First People’s Hospital of Changde City, Changde, Hunan, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Zhiming Deng, Department of Endocrinology, The First People’s Hospital of Changde City, 818 Renmin Road, Changde, Hunan, 415000, People’s Republic of China, Tel +86 736-7788026, Email [email protected]
Purpose: To explore the correlation between Chinese visceral adipose index (CVAI) and urinary microalbumin/creatinine ratio (UACR) and urinary albumin, and whether there is any difference in correlation between Han and Tujia ethnicity.
Methods: This cross-sectional study was conducted in Changde, Hunan, China from May 2021 to December 2021. Biochemical indicators including anthropometric parameters, blood pressure, blood glucose, blood lipids, and UACR of the participants were measured. Univariate analysis, multivariate analyses and multinomial logistic regression analysis were carried out to assess the association between CVAI and albuminuria. In addition, curve fitting and threshold effect analysis were used to explore the nonlinear association between CVAI and albuminuria, and to observe whether there were ethnic differences in this association.
Results: A total of 2026 adult residents were enrolled in this study, 500 of whom had albuminuria. Population-standardized prevalence of albuminuria is 19.06%. In the multivariable model adjusted for confounding factors, the odds ratio (OR) of albuminuria for pre-unit increase of CVAI and pre-SD increase of CVAI were 1.007 (1.003– 1.010) and 1.298 (1.127– 1.496), respectively. Multinomial logistic regression analysis confirmed the robustness and consistency of the results.The generalized additive model showed that CVAI and albuminuria had a nonlinear relationship with inflection point at 97.201 using the threshold effect. Compared with Han ethnic groups, the threshold between CVAI and albuminuria in Tujia people moved backward. The thresholds were 159.785 and 98.527, respectively.
Conclusion: There was a positive nonlinear dose-response relationship between increased CVAI and higher levels of albuminuria. Maintaining appropriate CVAI levels may be important for the prevention of albuminuria.
Keywords: Chinese visceral adipose index, urinary microalbumin to creatinine ratio, albuminuria, obesity
Introduction
The number of people with chronic kidney disease (CKD) is increasing year by year and has become a global public health problem. In 2017, the global prevalence of CKD reached 9.1%, an increase of 29% compared to 1990.1 This has brought enormous economic pressure and an increasingly heavy health burden. Increased urinary protein excretion and progressive decline in glomerular filtration rate have long been recognized as hallmarks of CKD. UACR is widely recognized clinically as a reliable measure to assess the degree of increased urinary protein excretion.2,3 Studies have shown that UACR, like estimated glomerular filtration rate (eGFR) decline, is associated with faster CKD disease progression, higher incidence of cardiovascular outcomes and increased all-cause mortality, especially in the presence of some metabolic abnormalities (eg diabetes mellitus (DM), hypertension and hyperlipidemia) were more significant.4–6 Further studies have shown that even when UACR is in the high normal range, it still increases the risk of cardiovascular and cerebrovascular disease and all-cause mortality in diabetic patients.7,8 Many studies have shown that obese people are more prone to increased UACR, and obesity is closely related to the risk of CKD progressing to end stage kidney disease (ESKD).9,10 Obesity can also participate in the whole process of increased urinary protein excretion, occurrence and progression of CKD to ESKD through the indirect effect of related metabolic abnormalities caused by obesity. There is a causal relationship between obesity and the risk of CKD.11
Chinese visceral adipose index (CVAI) is a composite index composed of anthropometric index and blood metabolic index. The CVAI method is relatively simple to obtain results, and at the same time, it can well reflect abdominal obesity and metabolic health.12 There are many studies showing that CVAI is associated with diabetes-related macrovascular complications.13 However, there are few clinical studies on the correlation between CVAI and UACR and albuminuria.
To sum up the above, this study plans to analyze the correlation between CVAI and UACR in 2026 residents of Changde, Hunan, China, and try to study whether the correlation between CVAI and UACR varies among different ethnic groups.
Methods
Study Design and Population
The research for this study is a population-based cross-sectional survey conducted between May 2021 and December 2021 in Changde City, China. The study data were derived from part of the ongoing epidemiological study “Epidemiological survey on the prevalence of diabetes and diabetes complications among adults in Changde City”. The epidemiological survey plan randomly selected 10 Sub-districts/Towns in Changde as the survey area. Our research comes from 4 Sub-districts/Towns in Moshi Town, Erdu Sub-district, Longyang Sub-district and Danzhou Town, where samples and information collection have been completed. A random sample of 630 adult residents with a continuous residence of at least 6 months will be selected from each selected Sub-districts/Towns. They will be invited to participate in the study via telephone or home visit. Participants who met the following conditions were excluded: 1. Recent drug use that affected renal function; 2. Currently in acute infection, trauma or other state of stress; 3. Pregnant women; 4. Suffering from severe mental illness, unable to cooperate with the scene investigation. We invited a total of 2520 residents over 18 years old from the above 4 Sub-districts/Towns to participate in this study. Of these, 2229 residents agreed to participate in this study, with a participation rate of 88.45%. After excluding subjects with missing data regarding demographic information (n=101) or body measurement information (n=20) or venous blood/urine sample (n=51) and with missing data both venous blood/urine sample and body measurement indicators (n=31), 2026 participants were included in the final analysis (Figure 1).
 |
Figure 1 A flow chart. |
The present study was approved by the Ethics Committee of the First People’s Hospital of Changde City, China and performed in accordance with the principles of the Declaration of Helsinki. A written informed consent was obtained from all participants.
Study Variables
Trained physicians used home-made standardized questionnaires to collect demographic information and past medical history of hypertension and diabetes. Relevant measurements such as weight, height, waist circumference (WC), hip circumference (HC) and blood pressure were also collected.
Participants without or with a clear history of diabetes underwent 75g oral glucose tolerance test (OGTT) or 100g steamed‐bread meal test, separately. And all they were instructed to fast for more than 8 hours before performing the above trials. Pretest venous blood and 2 hour posttest venous blood samples were collected for fasting blood glucose (FPG) and postprandial blood glucose (PPG) by glucose oxidase method. In addition, we measured Triglycerides (TG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), alanine aminotransferase (ALT), aspartate aminotransferase (AST), serum albumin (SA), serum creatinine (SCR), blood urea nitrogen (BUN) and blood uric acid (UA) in participants’ fasting venous blood samples using an automated biochemical analyzer. Glycated hemoglobin (HbA1c) was measured in fasting venous blood samples by high performance liquid chromatography. Morning fasting urine samples were taken from all participants. All blood and urine samples were transported to the central laboratory of the First People’s Hospital of Changde City for testing on the day of collection. Glycated hemoglobin (HbA1c) was measured using high performance liquid chromatography.
Definition of Concepts and Related Index
The calculation formulas of CVAI, BMI, TyG and eGFR were calculated as follows: CVAI(male) = −267.93 + 0.68 × age + 0.03 × BMI(kg/m^2) + 4.00 × WC(cm) + 22.00 × LgTG(mmol/L) - 16.32 × HDL-C(mmol/L); CVAI(female) = −187.32 + 1.71 × age + 4.23 × BMI(kg/m^2) + 1.12 × WC(cm) + 39.76 × LgTG(mmol/L) - 11.66 × HDL-C(mmol/L);12 BMI = weight(kg)/height^2(m); TyG = ln [TG (mmol/L) × FPG (mmol/L) × 0.5×159.37];14 The calculation of eGFR with improvement of diet in renal disease (MDRD) equation.15
UACR was calculated through dividing the urinary albumin by the urinary creatinine. Albuminuria was defined as UACR ≥ 30mg/g in clinical practice. Microalbuminuria was defined as UACR ≥ 30mg/g and < 300mg/g. Macroalbuminuria was defined as UACR > 300mg/g.16
Statistical Analysis
We expressed the distribution of continuous variables as mean ± standard deviation and median (interquartile range). Student-t-test or Kruskal–Wallis H-test was used for between-group differences in continuous variables. Categorical variables were expressed as frequencies and percentages (%), and differences between groups were expressed using chi-square or Fisher’s exact test. We used univariate analysis and multivariate analysis to evaluate the association between CVAI and the level of UACR and to identify possible confounding factors. The association between CVAI and albuminuria was further evaluated after adjusting for confounding factors. In addition, we used the multinomial logistic regression analysis to investigate the correlation between CVAI and different degrees of albuminuria. We did mediation analysis using the product of coefficients method and calculating the indirect effect of CVAI on albuminuria mediated through TyG compared with the total effect of CVAI on albuminuria.Then, the nonlinear relationship between CVAI and the probability of participants developing albuminuria was studied by generalized additive model and smooth curve fitting. If nonlinearity was detected, the further threshold effect analysis was used to investigate whether there was a threshold saturation effect between the two and to find relevant threshold points. As a final step, stratified analysis was performed according to Tujia and Han ethnic groups to explore whether there were differences in the correlation between CVAI and albuminuria. Statistical software packages R (http://www.R-project.org, The R Foundation, Vienna, Austria) and Empower Stats (http://www.empowerstats.com, X&Y Solutions, Inc, Boston, MA, USA) were used for all statistical analyses in this study. A p-value <0.05 was considered statistically significant.
Results
Characteristics of Participants
A total of 2026 participants participated in this study, 500 of whom had albuminuria. The prevalence of albuminuria was 24.67%. According to the age structure of the sixth national census in China in 2010, the standardized prevalence of abnormal proteinuria was calculated to be 19.06%. In this study, the mean CVAI of the participants was 94.928, and the median UACR was 13.351 mg/g. There was no significant difference in LDL-C, HDL-C and gender between the normal albuminuria group and the albuminuria group. The eGFR was lower in albuminuria group. Participants with albuminuria group had higher values in Age, BMI, WC, HC, blood pressure, blood glucose, HbA1c, TG, TC, UA, BUN, SA, ALT, AST, CVAI,TyG and consisted of more preexisting hypertension and preexisting DM than those of the other groups. The proportion of Tujia ethnicity in albuminuria group was significantly lower than that in the normal albuminuria group (Table 1).
 |
Table 1 Participant Characteristics |
Association Between CVAI and Albuminuria
Univariate analysis showed that there was no significant linear correlation between UACR and LDL-C and HDL-C, but UACR was linearly correlated with other indicators. Further multivariate analysis with potential related factors of UACR showed that after adjusting for the confounding effect of other factors, SBP, FPG, TC, LDL-C, BUN, Tujia ethnicity, preexisting hypertension, preexisting DM and CVAI were independently associated with UACR. Considering that Age, BMI, WC, TG and HDL-C are the component parts of CVAI, they are not included in the multivariate analysis model (Table 2).
 |
Table 2 Univariate and Multivariate Analysis of Factors Associated with UACR |
The logistic regression analysis model between CVAI and albuminuria showed that CVAI was associated with the probability of albuminuria after adjusting for age, gender, ethnicity, HC, SBP, FPG, TC, LDL-C, BUN, eGFR, hypertension and DM. The associated trend was a 0.7% increase in the probability of concomitant albuminuria for pre unit increase in CVAI. For pre SD increase in CVAI, the OR for albuminuria was 1.298 (1.127–1.496) in the multivariable model adjusted for confounding factors (Table 3).
 |
Table 3 Multivariate Logistic Analysis for the Association Between CVAI and Albuminuria |
A multinomial logistic regression model of factors associated with albuminuria severity showed that CVAI, SBP, TC and LDL-C were strongly associated with the level of albuminuria. The results showed that with the increase of CVAI, SBP, TC level and the decrease of LDL-C level, the likelihood of concomitant more severe albuminuria increased. The probability of microalbuminuria increased by 0.8%, 2.03%, 39% and 32.3% with the increase of CVAI, SBP and TC level and the decrease of LDL-C level by 1 unit. The probability of combined macroalbuminuria increased by 1.1%, 3.48%, 78.66% and 54.18%, respectively. In addition, increased eGFR was associated with a higher probability of concomitant macroalbuminuria, while there was no statistically significant relationship with microalbuminuria. Participants with preexisting diabetes were 276.27% more likely to have macroalbuminuria than those without preexisting diabetes. Participants with preexisting hypertension were 82.85% more likely to have microalbuminuria than those without preexisting hypertension (Table 4).
 |
Table 4 Multinomial Logistic Regression Model of Factors Influencing the Degree of Albuminuria (Normal Albuminuria, Microalbuminuria and Macroalbuminuria) |
In addition, Mediation analyses showed that 24.95% of the observational association of CVAI with probability of concomitant albuminuria was mediated through TyG (Supplementary Table 1).
Nonlinear Correlation Between CVAI and Albuminuria
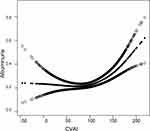
In this study, the curve fitting between CVAI and albuminuria using a generalized additive model showed that there was a non-linear relationship between CVAI and albuminuria (Figure 2). The threshold effect analysis was further performed with piece-wise binary logistic regression models. After adjusting for age, gender, ethnicity, HC, SBP, FPG, TC, LDL-C, BUN, eGFR, hypertension and DM, it was calculated that the inflection point is 97.201. When CVAI > 97.201, the probability of albuminuria increased by 1.4% with the increase of 1 unit of CVAI; however, the correlation was not significant when CVAI was less than 97.201 (Table 5).
 |
Table 5 Threshold Effect of the Association Between CVAI and Albuminuria in Han and Tujia Ethnicity |
The Difference Between Han and Tujia Ethnicity
We examined threshold effects with piece-wise binary logistic regression models in Han and Tujia ethnic group. Compared with Han ethnic groups, the threshold between CVAI and albuminuria in Tujia people moved backward (Figure 3). After adjusting for relevant factors, when CVAI was greater than 159.785, the probability of albuminuria increased by 9.8% for every 1 unit increase in CVAI in the Tujia population; however, the correlation was not significant when CVAI was less than 159.785. In Han population, when CVAI > 98.527, the probability of albuminuria increased by 1.1% with the increase of 1 unit of CVAI; but the correlation was not significant when CVAI < 98.527 (Table 5).
Multinomial logistic regression model of factors influencing the degree of albuminuria among Tujia and Han ethnicity showed differences in the correlation between CVAI and different degrees of albuminuria. The correlation between CVAI and albuminuria was significant between microalbumin and normal albuminuria, but not significant at large albuminuria levels. We also found ethnic differences in the correlation between TC, LDL-C and albuminuria (Supplementary Table 2 and Supplementary Figure 1).
Discussion
The prevalence of standardized abnormal albuminuria observed in this study was 19.06% among adult residents of Changde city, which was significantly higher than that observed in a recent national CKD survey in China and a healthy population study in the southeastern coastal area of China (9.50% and 5.35% respectively).17,18 However, it is lower than the prevalence of 25.98% observed in the elderly population study in Tianjin, China.19 Some previous studies have shown that increasing age is a risk factor for proteinuria and CKD.20 The mean age of the population in this study was 58 years, and the mean age of the population studied in the national CKD survey in China was 42.4 years. We speculate that this situation may be due to factors such as economic development and population outflow, which has resulted in the average age of resident population in Changde, located in the northwest region of Hunan Province, China, being higher than the national average.
Our study demonstrated that individuals with albuminuria had significantly higher levels of CVAI, BMI, WC, blood lipids, blood pressure, and blood glucose compared to those with normal albuminuria. Univariate analysis further showed that abnormal glucose metabolism, hypertension, obesity, and dyslipidemia were significantly associated with increased UACR. Consistent with our findings, an epidemiological survey of CKD risk factors in central China revealed that age, sex, obesity, diabetes, hypertension, dyslipidemia, and hyperuricemia were all significant risk factors for albuminuria.21 In another larger epidemiological survey of the rural population in Henan Province, China, the aforementioned factors were closely associated with CKD or DKD.22 Our study also found that the above-mentioned population had a higher degree of urinary protein excretion and a higher probability of concomitant albuminuria than those who did not have the above risk factors. Albuminuria is not only related to BMI, but also closely related to WC and other indicators reflecting abdominal obesity in our study. A series of studies have shown that obesity is associated with increased urinary protein excretion. The studies from Qingdao, China and from the United States both found that obesity and dyslipidemia were independently associated with elevated UACR and had a higher risk of albuminuria.23,24 An Australian Aboriginal study further showed a strong link between abdominal obesity and proteinuria.25 A subgroup study of kidney disease in the China Stroke Primary Prevention Trial study (CSPPT study) further demonstrated that BMI and WC were independently strongly associated with albuminuria.26 A similar situation is observed in our study. The Chinese Diabetes Cancer Risk Cohort Study (REACTION study) also found that obesity was significantly associated with a higher risk of elevated UACR, but unlike this study, the association was not significant in simple abdominal obesity people or simple systemic obesity people.10 Given that the REACTION study excluded people with a diagnosis of CKD, and the REACTION study was based on the ratio of UACR to the median UACR in each sub-center population not directly study the level of UACR, we still believe that abdominal obesity is associated with increased UACR.
Obesity with or without metabolic abnormalities was associated with a higher risk of CKD in a large observational study of a UK population.27 The researchers of this UK study defined metabolic abnormalities by whether they were combined with hypertension, dyslipidemia, and diabetes, and measured the degree of metabolic abnormalities by the number of the above diseases. More importantly, this study also found that obesity with metabolic abnormalities had a higher risk of developing CKD than metabolically healthy obese people, and this association showed a graded increase with the degree of metabolic abnormalities. Similar results were observed in the research data from the NHNESK.28 Our study found that HC, CVAI and other indicators reflecting abdominal obesity were closely related to those reflecting urinary protein excretion. After further analysis, we found that the correlation trend between CVAI and UACR still existed, after excluding the effects from blood glucose, blood pressure, LDL-C and TC. We generally traditionally believe that obesity, hyperglycemia, dyslipidemia, and hypertension are widely regarded as high-risk factors for increased urinary protein excretion and CKD. However, this traditional cognition underestimated the mediating effect of diabetes, blood pressure, blood lipids and their related factors on albuminuria. Hypertension, insulin resistance and abnormal glucose metabolism are all directly related to impaired renal tubular endothelial function, renal blood perfusion and renal hemodynamics. The above mechanisms further lead to impairment of podocyte function and filtration barrier, ultimately leading to increased urinary protein excretion.29,30 Obesity and dyslipidemia also can promote the occurrence and progression of proteinuria through lipid deposition in renal tubules and renal vascular atherosclerosis.31 The above research and our research indicates that the etiology of proteinuria can be quite complex and holistic. Therefore, it seems that we should take hypertension, abnormal glucose metabolism, dyslipidemia, obesity and other metabolic diseases together to consider the correlation between these factors and proteinuria and even CKD.
In recent years, more and more studies have been conducted to investigate the relationship between albuminuria and some indexes that can comprehensively evaluate visceral obesity and overall metabolic health. Wan Heng et al studied the relationship between abdominal obesity indicators and DKD in residents with DM in Shanghai, China, and found a strong relationship between CVAI and the risk of CVD and DKD.32 Zhiyuan Wu et al also found that CVAI was independently associated with DKD in patients with diabetes in Beijing, China.33 The study population of these studies was diabetic patients, and all only studied the risk of DKD in the DM population. Our study shows that the increase of CVAI was positively correlated with the increase of UACR and the severity of albuminuria in the general population and further found a non-linear relationship between CVAI and the probability of having albuminuria. At the same time, we found that there is a threshold point of CVAI=97.201. When the CVAI exceeds 97.201, the probability of concomitant albuminuria will increase significantly. This means that it is not necessary to reduce the CVAI too low, but should control the CVAI at appropriate level, which can not only maintain the risk of low level of albuminuria, but also avoid the possible potential adverse effects of excessive reduction of CVAI, although there is a lack of research data on the risk of low CVAI.
Interestingly, in this study, LDL-C, a blood lipid index, was found to have an independent negative correlation with UACR, which is in conflict with our traditional understanding. This may be due to the fact that people with elevated UACR may be complicated with CVD, hyperlipidemia, diabetes and other diseases, which often require the use of statins for lipid-regulating drugs. Moreover, the LDL-C control target of DM patients in Chinese diabetes prevention guidelines is lower than that of people with normal blood glucose. So these people have lower levels of LDL-C but may have greater variability in LDL-C. A recent study found that increased variability of LDL-C can increase the occurrence of DKD and the risk of DKD progression to ESKD in DM patients.34 Another study found that increased variability in LDL-C and overemphasis on LDL-C reduction at the expense of other markers of lipid metabolism increased the risk of CVD and all-cause mortality in DM patients.35 Currently, it is thought that high LDL-C variability may disrupt the stability of renal vascular atherosclerotic plaques, impair vascular endothelial function and promote lipid outflow from plaques, aggravating renal atherosclerotic lesions and leading to increased urinary protein excretion. In a clinical trial in China, fenofibrate lipid-lowering therapy was added to DM patients with hypertriglyceridemia who had been treated with hypoglycemic, hypertensive and statins for lipid regulation. After 180 days of treatment, UACR in the treatment group was significantly higher than that in the non-treatment group. Moreover, the decrease of UACR was significantly correlated with the decrease of TG.36 This suggests that we should think more about obesity and metabolism together. Attaching importance to the control of obesity, abdominal obesity and even overall metabolic health may be more important therapeutic measures to reduce urinary protein excretion and delay the progression of CKD.
So far, Some studies have showed differences in the prevalence of proteinuria and CKD among different ethnic groups.An American study showed that Asian Americans had a higher risk of albuminuria than European Americans.37 Another study from Northern California also showed differences in the risk of albuminuria between different ethnic groups.38 Further studies have shown that genetic and genetic factors can explain the differences in kidney disease in different ethnic groups, such as APOL1 genotype variation.39,40 At the same time, some studies have shown that a considerable part of the racial differences in proteinuria can be explained by non-genetic factors such as living environment, dietary habits, exercise habits and socioeconomics.41 There were differences in the probability of concomitant albuminuria between tujia ethnicity and other ethnicities in our study. We further found differences in the correlation between different metabolic abnormalities (such as blood glucose, blood lipids, blood pressure and obesity) and albuminuria between Tujia and Han. The association between CVAI and albuminuria may also be influenced by differences in basal metabolic rate, exercise habits, and prevalence of chronic diseases such as obesity and hypertension. Therefore, we speculated that differences in diet, exercise and other living habits between Han and Tujia might lead to differences in the correlation between CVAI and albuminuria in different ethnic groups. Previous studies have shown that different diets and habits can lead to differences in metabolism between individuals.42 Due to the different living habits and living environment between Han and Tujia ethnicity, the association between CVAI and albuminuria may be affected. At present, there are few related clinical studies on tujia ethnicity and proteinuria. Therefore, further research is needed to clarify the possible mechanism of this difference and whether there are genetic factors involved.
The advantage of this study is that the sample in this study is from an epidemiological survey population, which can theoretically exclude the problem of population heterogeneity. This allowed us to observe the association between CVAI and UACR, albuminuria in the general population, not only in patients with hypertension or diabetes. The main limitations of our study are: (1) This is a cross-sectional study, which can only clarify the correlation between CVAI and albuminuria, but cannot determine whether there is a causal relationship, which requires further prospective cohort studies and further mechanism studies to verify. (2) Although we did our best to avoid drugs when we collected the data, our study was not able to collect detailed data on antihypertensive drug use.And although studies have shown that TYG index can reflect early changes in insulin resistance. A limitation of our study is that we did not collect data on participants’ insulin levels.They are the limitations of our study. In the follow-up study, we will refine participants’ specific drug use and further improve the collection of relevant indicators. (3) This study needs to further expand the sample size and increase the survey area to verify the correlation between CVAI and albuminuria in a larger population. (4) Considering that hypertension, glucose metabolism, obesity and proteinuria are closely related, corresponding improvement of CVAI may still be needed to further improve its clinical application value.
Conclusion
There was a positive nonlinear dose-response relationship between increased CVAI and higher levels of albuminuria. And this relationship is different between han and tujia ethnic groups. Maintaining appropriate CVAI levels may be important for the prevention of albuminuria.
Data Sharing Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
The authors would like to acknowledge the helpful suggestions concerning this study received from their colleagues and express their gratitude to all the participants in this study. In addition, we have thanked Shiwu Wen (School of Epidemiology, Public Health and Preventive Medicine, University of Ottawa, Ottawa, Ontario, Canada) for giving us some guidance during the writing and revision of the manuscript.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The research is financially supported by Hunan Provincial Key Laboratory of Clinical Epidemiology (2021ZNDXLCL001) and The Science and Technology Innovation Special Program of Changde, Hunan Province (No. [2021]67).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Neuen BL, Chadban SJ, Demaio AR, Johnson DW, Perkovic V. Chronic kidney disease and the global NCDs agenda. BMJ Glob Health. 2017;2(2):e000380. doi:10.1136/bmjgh-2017-000380
2. Dobrowolski P, Januszewicz A, Gumprecht J, et al. Why albuminuria should be assessed more frequently in everyday clinical practice? A position statement. Pol Arch Intern Med. 2021;131(4):396–406. doi:10.20452/pamw.15874
3. Ohkuma T, Jun M, Chalmers J, et al. Combination of changes in estimated GFR and albuminuria and the risk of major clinical outcomes. Clin J Am Soc Nephrol. 2019;14(6):862–872. doi:10.2215/CJN.13391118
4. Lees JS, Welsh CE, Celis-Morales CA, et al. Glomerular filtration rate by differing measures, albuminuria and prediction of cardiovascular disease, mortality and end-stage kidney disease. Nat Med. 2019;25(11):1753–1760. doi:10.1038/s41591-019-0627-8
5. Kühn A, van der Giet M, Kuhlmann MK, et al. Kidney function as risk factor and predictor of cardiovascular outcomes and mortality among older adults. Am J Kidney Dis. 2021;77(3):386–396.e1. doi:10.1053/j.ajkd.2020.09.015
6. Xie X, Peng Z, Li H, et al. Association of urine albumin/creatinine ratio below 30 mg/g and left ventricular hypertrophy in patients with type 2 diabetes. Biomed Res Int. 2020;2020:5240153. doi:10.1155/2020/5240153
7. Zhang A, Li M, Qiu J, et al. The relationship between urinary albumin to creatinine ratio and all-cause mortality in the elderly population in the Chinese community: a 10-year follow-up study. BMC Nephrol. 2022;23(1):16. doi:10.1186/s12882-021-02644-z
8. Coresh J, Heerspink HJL, Sang Y, et al. Change in albuminuria and subsequent risk of end-stage kidney disease: an individual participant-level consortium meta-analysis of observational studies. Lancet Diabetes Endocrinol. 2019;7(2):115–127. doi:10.1016/S2213-8587(18)30313-9
9. Martin WP, Bauer J, Coleman J, et al. Obesity is common in chronic kidney disease and associates with greater antihypertensive usage and proteinuria: evidence from a cross-sectional study in a tertiary nephrology centre. Clin Obes. 2020;10(6):e12402. doi:10.1111/cob.12402
10. Qin S, Wang A, Gu S, et al. Association between obesity and urinary albumin-creatinine ratio in the middle-aged and elderly population of Southern and Northern China: a cross-sectional study. BMJ Open. 2021;11(1):e040214. doi:10.1136/bmjopen-2020-040214
11. Xu X, Eales JM, Jiang X, et al. Contributions of obesity to kidney health and disease: insights from Mendelian randomization and the human kidney transcriptomics. Cardiovasc Res. 2022;118(15):3151–3161. doi:10.1093/cvr/cvab357
12. Xia MF, Chen Y, Lin HD, et al. A indicator of visceral adipose dysfunction to evaluate metabolic health in adult Chinese. Sci Rep. 2016;6:38214. doi:10.1038/srep38214
13. Bi H, Zhang Y, Qin P, et al. Association of Chinese visceral adiposity index and its dynamic change with risk of carotid plaque in a large cohort in China. J Am Heart Assoc. 2022;11(1):e022633. doi:10.1161/JAHA.121.022633
14. Sánchez-íñigo L, Navarro-González D, Fernández-Montero A, Pastrana-Delgado J, Martínez JA. The TyG index may predict the development of cardiovascular events. Eur J Clin Invest. 2016;46(2):189–197. doi:10.1111/eci.12583
15. Ma YC, Zuo L, Chen JH, et al. Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol. 2006;17(10):2937–2944. doi:10.1681/ASN.2006040368
16. Chinese Diabetes Society. Guidelines for the prevention and treatment of type 2 diabetes in China (2020 edition). Chin J Diabetes. 2021;13(4):315–409.
17. Wang F, He K, Wang J, et al. Prevalence and risk factors for CKD: a comparison between the adult populations in China and the United States. Kidney Int Rep. 2018;3(5):1135–1143. doi:10.1016/j.ekir.2018.05.011
18. Lin CA, Liu YP, Chen YC, et al. Gender-specific and age-specific associations of the homoeostasis model assessment for IR (HOMA-IR) with albuminuria and renal function impairment: a retrospective cross-sectional study in Southeast China. BMJ Open. 2021;11(12):e053649. doi:10.1136/bmjopen-2021-053649
19. Cao Y, Sun G, Liu R, et al. Plasma triglyceride levels and central obesity predict the development of kidney injury in Chinese community older adults. Ren Fail. 2019;41(1):946–953. doi:10.1080/0886022X.2019.1655451
20. Polkinghorne KR, Wolfe R, Jachno KM, et al. Prevalence of chronic kidney disease in the elderly using the ASPirin in Reducing Events in the Elderly study cohort. Nephrology. 2019;24(12):1248–1256. doi:10.1111/nep.13565
21. Duan JY, Duan GC, Wang CJ, et al. Prevalence and risk factors of chronic kidney disease and diabetic kidney disease in a central Chinese urban population: a cross-sectional survey. BMC Nephrol. 2020;21(1):115. doi:10.1186/s12882-020-01761-5
22. Duan J, Wang C, Liu D, et al. Prevalence and risk factors of chronic kidney disease and diabetic kidney disease in Chinese rural residents: a cross-sectional survey. Sci Rep. 2019;9(1):10408. doi:10.1038/s41598-019-46857-7
23. Ji A, Pan C, Wang H, et al. Prevalence and associated risk factors of chronic kidney disease in an elderly population from Eastern China. Int J Environ Res Public Health. 2019;16(22):4383. doi:10.3390/ijerph16224383
24. Rosenstock JL, Pommier M, Stoffels G, Patel S, Michelis MF. Prevalence of proteinuria and albuminuria in an obese population and associated risk factors. Front Med. 2018;5:122. doi:10.3389/fmed.2018.00122
25. Hughes JT, Maple-Brown LJ, Thomas M, et al. Cross-sectional associations of albuminuria among Aboriginal and Torres Strait Islander adults: the eGFR Study. Nephrology. 2018;23(1):37–45. doi:10.1111/nep.12956
26. Herman-Edelstein M, Weinstein T, Chagnac A. Obesity-related glomerulopathy: clinical management. Semin Nephrol. 2021;41(4):358–370. doi:10.1016/j.semnephrol.2021.06.007
27. Wang J, Niratharakumar K, Gokhale K, et al. Obesity without metabolic abnormality and incident CKD: a population-based British cohort study. Am J Kidney Dis. 2022;79(1):24–35.e1. doi:10.1053/j.ajkd.2021.05.008
28. Choi I, Moon H, Kang SY, Ko H, Shin J, Lee J. The risk of microalbuminuria by obesity phenotypes according to metabolic health and obesity: the Korean National Health And Nutrition Examination Survey 2011–2014. Korean J Fam Med. 2018;39(3):168–173. doi:10.4082/kjfm.2018.39.3.168
29. Tsuda A, Ishimura E, Uedono H, et al. Association of albuminuria with intraglomerular hydrostatic pressure and insulin resistance in subjects with impaired fasting glucose and/or impaired glucose tolerance. Diabetes Care. 2018;41(11):2414–2420. doi:10.2337/dc18-0718
30. Hadi Alijanvand M, Aminorroaya A, Kazemi I, et al. Cross-sectional and longitudinal assessments of risk factors associated with hypertension and moderately increased albuminuria comorbidity in patients with type 2 diabetes: a 9-year open cohort study. Diabetes Metab Syndr Obes. 2019;12:1123–1139. doi:10.2147/DMSO.S189726
31. Castro BBA, Foresto-Neto O, Saraiva-Camara NO, Sanders-Pinheiro H. Renal lipotoxicity: insights from experimental models. Clin Exp Pharmacol Physiol. 2021;48(12):1579–1588. doi:10.1111/1440-1681.13556
32. Wan H, Wang Y, Xiang Q, et al. Associations between abdominal obesity indices and diabetic complications: Chinese visceral adiposity index and neck circumference. Cardiovasc Diabetol. 2020;19(1):118. doi:10.1186/s12933-020-01095-4
33. Wu Z, Yu S, Kang X, et al. Association of visceral adiposity index with incident nephropathy and retinopathy: a cohort study in the diabetic population. Cardiovasc Diabetol. 2022;21(1):32. doi:10.1186/s12933-022-01464-1
34. Wan EYF, Eyt Y, Chin WY, et al. Greater variability in lipid measurements associated with kidney diseases in patients with type 2 diabetes mellitus in a 10-year diabetes cohort study. Sci Rep. 2021;11(1):8047. doi:10.1038/s41598-021-87067-4
35. Hsu W-H, Lai C-W, Chen S-C, et al. Greater low-density lipoprotein cholesterol variability increases the risk of cardiovascular events in patients with type 2 diabetes mellitus. Endocr Pract. 2019;25(9):918–925. doi:10.4158/EP-2019-0002
36. Sun X, Liu J, Wang G. Fenofibrate decreased microalbuminuria in the type 2 diabetes patients with hypertriglyceridemia. Lipids Health Dis. 2020;19(1):103. doi:10.1186/s12944-020-01254-2
37. Kataoka-Yahiro M, Davis J, Gandhi K, Rhee CM, Page V. Asian Americans & chronic kidney disease in a nationally representative cohort. BMC Nephrol. 2019;20(1):10. doi:10.1186/s12882-018-1145-5
38. Choi AI, Karter AJ, Liu JY, Young BA, Go AS, Schillinger D. Ethnic differences in the development of albuminuria: the DISTANCE study. Am J Manag Care. 2011;17(11):737–745.
39. Peralta CA, Bibbins-Domingo K, Vittinghoff E, et al. APOL1 genotype and race differences in incident albuminuria and renal function decline. J Am Soc Nephrol. 2016;27(3):887–893. doi:10.1681/ASN.2015020124
40. Teumer A, Li Y, Ghasemi S, et al. Genome-wide association meta-analyses and fine-mapping elucidate pathways influencing albuminuria. Nat Commun. 2019;10(1):4130. doi:10.1038/s41467-019-11576-0
41. Paluch AE, Pool LR, Isakova T, et al. Association of fitness with racial differences in chronic kidney disease. Am J Prev Med. 2019;57(1):68–76. doi:10.1016/j.amepre.2019.02.016
42. Liang Z, Wang L, Liu H, et al. Genetic susceptibility, lifestyle intervention and glycemic changes among women with prior gestational diabetes. Clin Nutr. 2020;39(7):2144–2150. doi:10.1016/j.clnu.2019.08.032
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.