Back to Journals » International Journal of General Medicine » Volume 17
Association Analysis Between Maternal Neutrophil Ratio and the Risk of Histological Chorioamnionitis in Pregnant Women with Premature Rupture of Membranes in Late Pregnancy
Received 5 February 2024
Accepted for publication 12 April 2024
Published 20 April 2024 Volume 2024:17 Pages 1499—1508
DOI https://doi.org/10.2147/IJGM.S457645
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Woon-Man Kung
Yan Lv, Zheren Huang, Yan Ma
Department of Obstetrics and Gynecology, the Third Affiliated Hospital of Soochow University, Changzhou, 213003, People’s Republic of China
Correspondence: Yan Ma, Department of Obstetrics and Gynecology, the Third Affiliated Hospital of Soochow University, Changzhou, 213003, People’s Republic of China, Tel +86-0-13813693377, Email [email protected]
Background: We aimed to investigate the association between maternal neutrophil ratio and histological chorioamnionitis (HCA) risk in pregnant women with premature rupture of membranes (PROM) in late pregnancy.
Methods: A retrospective analysis was conducted on 95 cases of women with PROM in their late pregnancy between March 2018 and August 2021. These women were divided into two groups based on the presence of HCA. General clinical data and laboratory indicators were compared between the two groups. A generalized additive model was used for curve fitting, and a segmented regression model was used to explain further the non-linear relationship between neutrophil ratio and HCA risk.
Results: After adjusting for confounding factors, the curve fitting showed a “U”-shaped curve relationship between the neutrophil ratio and the risk of HCA. When the neutrophil ratio was < 76.3%, the risk of HCA exhibited a decreasing trend, but the difference was not statistically significant (adjusted OR = 0.884, 95% CI: 0.781– 1.001, P = 0.053). However, when the neutrophil ratio was > 76.3%, the HCA risk was significantly increased (adjusted OR = 1.339, 95% CI: 1.067– 1.680, P = 0.012). Furthermore, we equally divided the neutrophil ratio into three groups. The risk of HCA was significantly increased in the low-ratio group (OR = 4.292, 95% CI: 1.247– 14.706, P = 0.021) compared with the middle-ratio group, which was used as the reference group. Similarly, the HCA risk of the high-ratio group (OR = 13.145, 95% CI: 1.796– 96.233, P = 0.011) was also significantly enhanced. However, there was no significant difference in HCA risk between the high-ratio and low-ratio groups (OR = 1.182, 95% CI: 0.357– 3.909, P = 0.784).
Conclusion: There was a significant “U”-shaped relationship between maternal neutrophil ratio and HCA risk in women with PROM in late pregnancy.
Keywords: premature rupture of membranes, late pregnancy, neutrophil ratio, histological chorioamnionitis, non-linear relationship
Introduction
Premature rupture of membranes (PROM) refers to the spontaneous rupture of membranes before the onset of labor, and it is classified into the term PROM and preterm PROM (PPROM) based on gestational age.1 PROM occurs in 2–4% of singleton pregnancies,2 and histologic chorioamnionitis (HCA) is one of the most significant complications associated with PROM, which affects around 50–60% of PPROM cases.3,4 The potential inflammatory process of HCA may result in prolonged labor, uterine infection, perinatal death, preterm birth, neonatal sepsis, and neurological disorders in newborns.5,6 HCA usually does not present with early symptoms, and its diagnosis is mainly made through pathological examination of the placenta after delivery.7 Inflammatory markers in amniotic fluid obtained through amniocentesis effectively predict HCA in patients with PROM.8–10 However, the use of amniocentesis in clinical practice is limited due to its invasive nature, associated risks, and decreased amniotic fluid volume in patients with PROM.11 Therefore, there is an urgent need for early, accurate, and non-invasive prenatal predictive markers to identify HCA.
Inflammatory markers in maternal blood are non-invasive and ideal for predicting HCA. Commonly used markers in clinical practice include white blood cell (WBC) count, C-reactive protein (CRP), procalcitonin (PCT), and so on.12–14 However, the relationship between these markers and HCA has not been fully investigated. Pregnancy-induced physiological changes cause an increase in WBC, and its value is limited by the influence of steroid administration.12,15 CRP is an acute-phase protein that lacks specificity for infection16 and is influenced by pregnancy-related physiological changes,17 which has led to controversy regarding its utility13,18 as a prenatal predictive marker for HCA. PCT is a highly sensitive inflammatory marker that increases within 3–6 hours following bacterial infection, and the degree of its serum level increase is directly related to the severity of the disease. Some studies have suggested that PCT is a better diagnostic marker for HCA than CRP and WBC.14,19
Although PROM associated with HCA is increasingly understood, it remains a challenge for obstetricians. It is reported that the prevalence of PROM in my country is higher than in developed countries.20 A prospective study of 15,926 Chinese women found that the incidence of PROM reached 18.7%, of which 11.08% were combined with HCA.21 It can be seen that early identification of PROM women with HCA is extremely important. In our previous study, we developed and validated a multivariable predictive model for assessing the risk of HCA in late preterm and term PROM patients. Based on a range of clinical and laboratory parameters, this model provided a new perspective for understanding and predicting HCA risk.22 In this study, we further analyze the clinical characteristics and laboratory indicators of HCA in patients with late-term PROM. We focused on exploring the relationship between maternal neutrophil ratio and the risk of HCA in patients with PROM, providing valuable guidance for clinicians to manage PROM patients better.
Methods
Clinical Data
We conducted a retrospective analysis of 162 pregnant women with PROM admitted to the Department of Obstetrics at the Third Affiliated Hospital of Soochow University between March 2018 and August 2021. Inclusion criteria were as follows: 1) pregnant women with PROM in late pregnancy(gestational age≥34 weeks); 2) singleton pregnancy; 3) live birth; 4) patients with placental pathological diagnosis results; and 5) pregnant women without severe diseases (such as preeclampsia, intrahepatic cholestasis of pregnancy, placental abruption, cardiovascular disease, autoimmune diseases, cancer, kidney disease, and other inherited diseases). Exclusion criteria were: 1) patients without placental pathological diagnosis results; 2) patients in early or mid-pregnancy; and 3) patients with bloody amniotic fluid. General information about the included patients, such as age, gender, weight, gravida, gestational age, parity, blood pressure, and so on, was recorded. This study was conducted under the principles of the Helsinki Declaration and was approved by the Ethics Committee of our hospital [(ethics number: (2023) KD 091)]. As the data were anonymized, informed consent was not required. Our institutional ethics committee approved this waiver of informed consent. The study flowchart is shown in Figure 1.
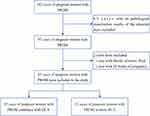 |
Figure 1 Flowchart of patient enrollment. |
Diagnosis
The diagnosis of PROM was made based on the patient’s medical history and physical examination. The diagnostic criteria were as follows:1 before labor, pregnant women complained of vaginal discharge or moistness in the external genitalia; examination with a speculum showed that fluid was flowing out of the cervical opening or there was a fluid pool in the posterior fornix; ultrasound examination displayed a decrease in amniotic fluid volume before membrane rupture; pH test strip underwent a color change (the normal vaginal pH varies between 4.4 and 6.0, while the pH of amniotic fluid is 8.0);23 and test positive for insulin-like growth factor. No patients were treated with antibiotics before admission. Antibiotic treatment will be initiated if membrane rupture persists for more than 12 hours without delivery. In the event of PROM complicated by GBS infection, antibiotics, typically penicillin or cephalosporins, will be promptly administered upon admission. The gold standard for diagnosing HCA24 is a pathological examination of placental and fetal membrane sections, with at least five neutrophils infiltrating each high-power field, according to the diagnostic criteria.
Laboratory Tests
On the day of admission, the patient’s venous blood was collected for analysis. The blood routine test was performed using a Sysmex XN9000 hematology analyzer (Hyogo, Japan). CRP, blood glucose (GLU), and blood lipids were measured using a Beckman Coulter AU5800 (Brea, CA, USA). PCT was measured using a Roche cobas®8000 (Indianapolis, IN, USA). Meconium enters the amniotic fluid during pregnancy, causing meconium-stained amniotic fluid (MSAF). According to the meconium standard grading system, MSAF can be divided into three grades: grade I: light green and thin; grade II: dark green or yellow-green and thick; grade III: yellow-brown and viscous. The mode of delivery, newborn’s gender, birth weight, Apgar scores at 1 minute and 5 minutes, amniotic fluid volume, and characteristics (clear, degree I, degree II, degree III) were documented following delivery. Sampling and examination methods for pathological specimens were as follows. After delivery, a tissue sample of 3 cm x 3 cm was taken from the placental and fetal membrane tissues around the rupture site. The sample was then fixed in 10% formalin, embedded in paraffin per standard protocol, and stored at room temperature. The sample was sent to the pathology department of our hospital to determine the presence of HCA.
Statistical Analysis
Continuous variables were expressed as mean ± SD, and categorical variables were defined as N (%). General information and laboratory indicators were compared between the HCA and non-HCA groups. Student’s t-test (normal distribution) or Mann–Whitney U-test (non-normal distribution) was used to compare continuous variables between the two groups, and the chi-square test was used to compare categorical variables. After adjusting for age, a univariate linear regression model was used to evaluate the relationship between general information, laboratory indicators, and neutrophil ratio. A generalized additive model (GAM) was used to observe whether there was a non-linear relationship between the neutrophil ratio in patients with PROM and the risk of HCA after adjusting for confounding factors. Motulsky et al25 have provided detailed instructions on fitting smooth curves. Then, a segmented logistic regression model and likelihood ratio test (LRT) were used to evaluate whether the fitted curve had a threshold effect. All data were analyzed using R software (version 3.4.3, http://www.R-project.org). A P-value less than 0.05 (two-tailed) was considered statistically significant.
Results
The study included 95 PROM pregnant women with a median gestational age of 38 weeks (range: 34+1-41+3), of whom 43 (45.3%) were diagnosed with HCA. The two groups’ general data and laboratory indicators are compared in Table 1. Results showed that there were no significant differences between the two groups in terms of age, body mass index (BMI), gestational diabetes, hypertension, systolic and diastolic pressures, gestational age, preterm birth, rupture-to-delivery interval, amniotic fluid volume, group B streptococcus (GBS), and multiple laboratory indicators [prenatal CRP, prenatal PCT, hemoglobin (HGB), platelet (PLT) count, GLU, cholesterol (TC), and triglycerides (TG)]. However, it was worth noting that there were significant differences between the two groups in terms of parity and gravida (both P<0.05), with the HCA group having lower parity and gravida. There were also significant differences in amniotic fluid characteristics between the two groups (P=0.005), with a lower proportion of clear amniotic fluid in the HCA group (76.7% vs 96.2%) and a higher proportion of grade I–II and III amniotic fluid compared to the non-HCA group (6.98% vs 1.92% and 16.28% vs 1.92%, respectively). As for laboratory indicators, there were significant differences between the two groups in terms of prenatal WBC count (P<0.001) and prenatal neutrophil ratio (P=0.008) (both P<0.01), with the HCA group having higher prenatal WBC count and neutrophil ratio. In addition, the delivery outcomes of the two groups are shown in Supplementary Table S1. There were no significant differences between the two groups in terms of fetal weight, fetal gender, Apgar score (at 1 minute and 5 minutes), neonatal WBC count, and neonatal CRP (all P>0.05). The HCA group had a significantly higher rate of cesarean section (44.2% vs 23.1%, P=0.029) and a higher neutrophil ratio in newborns (61.9% vs 57.0%, P=0.006).
 |
Table 1 Comparison of General Information and Laboratory Indicators Between Pregnant Women with and without HCA in Late-Stage PROM |
After adjusting for age, single-factor linear regression analysis was performed using clinical data and laboratory indicators as independent variables and the prenatal neutrophil ratio as the dependent variable (Y) (Table 2). It was found that gestational age, preterm delivery, amniotic fluid volume, amniotic fluid type III, prenatal WBC count, prenatal CRP, prenatal PCT, GLU, and HCA were significantly correlated with the neutrophil ratio (P <0.05), while the association between other factors and the neutrophil ratio was not statistically significant.
 |
Table 2 Univariate Analysis of Clinical Data, Laboratory Indices, and Neutrophil Ratios (Adjusted for Age) |
Based on the results in Tables 1 and 2, we identified several confounding factors that require adjustment, including age, prenatal WBC count, prenatal CRP, and amniotic fluid characteristics. We further used a GAM to examine the relationship between prenatal neutrophil ratio and the risk of HCA. We observed a “U”-shaped curve between the two variables, with a degree of freedom of 1.842. Figure 2A intuitively illustrates the relationship between the change in neutrophil ratio and the risk of HCA, displaying a segmented linear (or non-linear) trend with a decreasing trend followed by an increasing trend.
We further evaluated whether there was a threshold effect on this fitted curve using a segmented logistic regression model. The logarithmic LRT showed a statistically significant threshold at 76.3% for the neutrophil ratio (P=0.003). A segmented regression model was selected (Figure 2A and Table 3). When the neutrophil ratio was less than 76.3%, the risk of HCA decreased with increasing it, but it was not significant after adjustment (adjusted OR = 0.884, 95% CI: 0.781–1.001, P = 0.053). When the neutrophil ratio was greater than 76.3%, an increase in the neutrophil ratio significantly increased the risk of HCA (adjusted OR = 1.339, 95% CI: 1.067–1.680, P = 0.012).
 |
Table 3 Non-Linear Relationship Between Neutrophil Ratio and Combined Risk of Late-Stage Pregnancy PROM and HCA |
Taking into account the uneven distribution of neutrophil ratios in pregnant women with PROM in late pregnancy(less at both ends, concentrated in the middle), we divided the neutrophil ratio into three equal parts (low 69.2 ± 4.4 (52.0–74.0), medium 76.2 ± 1.2 (74.1–77.9), and high 83.4 ± 4.6 (78.1–93.4)), with 32, 30, and 33 individuals in each group, respectively. We used GAM analysis to fit the relationship between different levels of neutrophil ratio and HCA risk (Figure 2B). Using the medium-ratio group as the reference, we found that the low-ratio group had a significantly increased risk of HCA (OR = 4.292, 95% CI: 1.247–14.706, P = 0.021). Similarly, the high-ratio group also had a significantly increased risk of HCA (OR = 13.145, 95% CI: 1.796–96.233, P = 0.011). However, there was no significant difference in HCA risk between the high- and low-ratio groups (OR = 1.182, 95% CI: 0.357–3.909, P = 0.784). This finding suggested that low and high prenatal neutrophil ratios significantly increased the risk of HCA.
Discussion
Early detection of HCA in pregnant women with PROM is crucial, as it holds significant clinical value in preventing maternal and neonatal complications. Our study showed that late-stage PROM pregnant women with HCA had significantly higher prenatal WBC count and neutrophil ratios than those without HCA. Furthermore, we observed a non-linear relationship between the neutrophil ratio and HCA risk, with both low and high neutrophil ratios significantly increasing the risk of HCA.
The occurrence of PROM is related to various factors, such as fetal malposition, uneven stress on the fetal membrane, high amniotic cavity pressure, uterine overexpansion, ischemia, bleeding, and, most importantly, infection.26,27 HCA is a frequently occurring complication of PROM, and in pregnant women with PROM who also have HCA, the risk of neonatal asphyxia, respiratory distress, and even fetal death in utero is significantly increased.28 Therefore, early identification of HCA in pregnant women with PROM is particularly important.
Evaluating biomarkers in maternal blood is a quick and non-invasive method commonly used to identify HCA. The CRP concentration in maternal serum is one of the clinically used parameters. CRP is a plasma protein synthesized by liver cells and is produced and released after infection and tissue damage.29 In the early stages of HCA, infection and inflammation are limited to the chorionic membrane and amniotic membrane, and interleukin-6 (IL-6) is released from the membrane into the maternal circulation, promoting maternal secretion of CRP, which can be used as a screening tool for HCA.30 Numerous studies have demonstrated that CRP is the most reliable maternal serum biomarker for predicting HCA.13,17,31 When using 8 mg/L as the cutoff, CRP has been shown to have a higher predictive value for HCA at this threshold.32 However, some studies have suggested that CRP alone cannot diagnose HCA.18,33 Our study also found no difference in CRP results between the HCA and non-HCA groups. Oludag et al34 have suggested that maternal PCT is a suitable indicator for predicting HCA and is a better predictor than CRP. PCT is considered a more specific indicator of bacterial infection. However, maternal PCT levels increase only after systemic maternal infection and inflammation occur. In clinical practice, HCA usually presents as asymptomatic, with local infection at this stage. Therefore, PCT levels may not have increased yet.14 Our study confirmed this view, as we found no difference in PCT levels between the two groups.
WBC in the blood is one of the most commonly used methods for evaluating systemic inflammation and its intensity. Several studies have assessed the association between maternal WBC and HCA risk.35–37 Cho et al37 have suggested that maternal WBC count is independently associated with HCA risk, and our results also found that maternal WBC count and neutrophil ratio were higher in PROM patients with HCA. However, Asadi et al14 have reported that maternal WBC count cannot be used as a screening tool to identify HCA in pregnant women with PROM. Pregnancy is considered a physiological inflammatory process, and an increase in WBC can occur even in the absence of infection, limiting the value of evaluating maternal WBC.38 Therefore, different maternal WBC count cutoff values have been proposed to predict HCA, and a study has determined the cutoff value to be 14.0×109/L, indicating a good negative predictive value.35 A report on reference ranges for blood cell counts in pregnant women suggests that the reference range for neutrophil ratio in Chinese women in the late stages of pregnancy is between 61.7% and 91.9%.39
The increase in WBC count during pregnancy is largely due to an increase in neutrophil count, which may be a physiological stress response resulting from the redistribution of WBCs induced by pregnancy status between the marginal and circulating pools.40 Shi et al36 found that the neutrophil count in the HCA group was significantly higher than that in the non-HCA group, and the neutrophil-lymphocyte ratio accurately predicted early diagnosis of HCA. A retrospective analysis41 also showed that the neutrophil ratio was a reliable indicator for diagnosing PPROM patients with CA, and the diagnostic cutoff value was 75.286%. Our study identified a threshold effect between maternal neutrophil ratio and the risk of HCA in patients with PROM. When the neutrophil ratio was lower than 76.3%, an increase in the neutrophil ratio corresponded to a lower risk of HCA in pregnant women with PROM. However, when the neutrophil ratio was higher than 76.3%, an increase in the neutrophil ratio also significantly increased the risk of HCA in patients with PROM. This threshold was almost in the middle of the recommended neutrophil ratio range mentioned earlier. Thus, both low and high prenatal neutrophil ratios significantly increased the HCA risk. When PROM occurs, the immune system of pregnant women is affected to varying degrees, and the immune tolerance state between the fetus and mother may change, leading to an imbalance in immune regulation and an increased risk of infection.42 Neutrophils are the first line of defense against infection and, upon detection of a pathogen, are attracted to the site of infection by chemotactic factors to initiate an inflammatory response.43,44 A decrease in neutrophils in the blood leads to a decline in immune function, increasing the risk of HCA. High levels of neutrophils can inhibit the occurrence and development of inflammation,45 but a sustained increase in neutrophil levels means a long-term, severe, and uncontrolled immune response that can lead to excessive inflammation, disease deterioration, and adverse outcomes.46 Therefore, understanding the complex relationship between neutrophils and HCA risk provides a valuable reference for clinical physicians to use in early diagnosis and treatment.
This study has some limitations: 1) It is a retrospective study and may have selection bias. A prospective study in the future may provide more accurate information. 2) The sample size is relatively small, which may affect the statistical significance and generalizability of the results. Increasing the sample size may help better understand the impact of HCA in pregnant women with PROM. 3) As all pregnant women in this study were in the late stage of PROM, the results may not be generalizable to early-stage PROM. 4) The study only explored some potential clinical features and laboratory indicators but did not consider all possible factors affecting the neutrophil ratio and HCA risk. Future studies may need to evaluate more risk factors and biological mechanisms. 5) The study did not establish a relationship between HCA and neonatal outcomes. Although some differences were found between the two groups, it is uncertain whether these differences have clinical significance. Future research can further investigate the potential effects of HCA on the health of newborns.
Conclusions
In summary, the neutrophil ratio during pregnancy could be a non-invasive predictor of HCA risk in pregnant women with PROM in late pregnancy. Both low and high neutrophil ratios significantly increased the risk of HCA. Therefore, we recommend that clinical doctors closely monitor maternal neutrophil ratios in managing late-stage PROM. This can help identify high-risk groups promptly and enable the implementation of corresponding intervention measures to reduce the risk of HCA and its related complications. Meanwhile, future research can explore other potential inflammatory indicators or establish prediction models to improve the assessment and prediction of HCA risk in late-stage PROM.
Abbreviations
HCA, histological chorioamnionitis; PROM, premature rupture of membranes; WBC, white blood cell; CRP, C-reactive protein; PCT, procalcitonin; GLU, blood glucose; GAM, generalized additive model; LRT, likelihood ratio test; BMI, body mass index; GBS, group B streptococcus; HGB, hemoglobin; PLT, platelet; TC, cholesterol; TG, triglycerides.
Data Sharing Statement
The datasets used and/or analysed during the current study are available from the corresponding author upon reasonable request.
Ethical Approval and Informed Consent
This study adhered to the principles of the Helsinki Declaration and was approved by the ethics committee of the Third Affiliated Hospital of Soochow University [(ethics number: (2023) KD 091)]. Since the data were anonymized to protect the privacy and confidentiality of the participants, informed consent was not required. Our institutional ethics committee approved this waiver of informed consent.
Acknowledgments
We extend our special thanks to Scientific Writing Solutions USA for the English language editing services provided for our paper. Their professional team played a significant role in enhancing the language quality of our manuscript, ensuring clarity, grammatical accuracy, and readability to meet the high standards of international scientific communication.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors of this manuscript declare that there is no conflict of interest related to this study.
References
1. Kuba K, Bernstein PS. ACOG Practice Bulletin No. 188: prelabor Rupture of Membranes. Obstetrics Gynecol. 2018;131(1):e1–e14. doi:10.1097/AOG.0000000000002455
2. Bakar RZ, Koroglu N, Turkgeldi LS, Tola EN, Cetin BA, Gedikbasi A. Maternal serum procalcitonin levels in prediction of chorioamnionitis in women with preterm premature rupture of membranes. Arch Med Sci. 2021;17(3):694–699. doi:10.5114/aoms.2019.86191
3. Menon R, Richardson LS. Preterm prelabor rupture of the membranes: a disease of the fetal membranes. Semin Perinatol. 2017;41(7):409–419. doi:10.1053/j.semperi.2017.07.012
4. Aviram A, Quaglietta P, Warshafsky C, et al. Utility of ultrasound assessment in management of pregnancies with preterm prelabor rupture of membranes. Ultrasound Obstet Gynecol. 2020;55(6):806–814. doi:10.1002/uog.20403
5. Etyang AK, Omuse G, Mukaindo AM, Temmerman M. Maternal inflammatory markers for chorioamnionitis in preterm prelabour rupture of membranes: a systematic review and meta-analysis of diagnostic test accuracy studies. Syst Rev. 2020;9(1):141. doi:10.1186/s13643-020-01389-4
6. Jung EY, Park KH, Han BR, Cho SH, Yoo HN, Lee J. Amniotic Fluid Infection, Cytokine Levels, and Mortality and Adverse Pulmonary, Intestinal, and Neurologic Outcomes in Infants at 32 Weeks’ Gestation or Less. J Korean Med Sci. 2017;32(3):480–487. doi:10.3346/jkms.2017.32.3.480
7. Kim CJ, Romero R, Chaemsaithong P, Chaiyasit N, Yoon BH, Kim YM. Acute chorioamnionitis and funisitis: definition, pathologic features, and clinical significance. Am J Obstet Gynecol. 2015;213(4 Suppl):56.
8. Park JW, Park KH, Jung EY. Clinical significance of histologic chorioamnionitis with a negative amniotic fluid culture in patients with preterm labor and premature membrane rupture. PLoS One. 2017;12(3):e0173312. doi:10.1371/journal.pone.0173312
9. Oh KJ, Park KH, Kim SN, Jeong EH, Lee SY, Yoon HY. Predictive value of intra-amniotic and serum markers for inflammatory lesions of preterm placenta. Placenta. 2011;32(10):732–736. doi:10.1016/j.placenta.2011.07.080
10. Cobo T, Kacerovsky M, Palacio M, et al. A prediction model of histological chorioamnionitis and funisitis in preterm prelabor rupture of membranes: analyses of multiple proteins in the amniotic fluid. J Matern Fetal Neonatal Med. 2012;25(10):1995–2001. doi:10.3109/14767058.2012.666592
11. Lee JE, Dan K, Kim HJ, Kim YM, Park KH. Plasma proteomic analysis to identify potential biomarkers of histologic chorioamnionitis in women with preterm premature rupture of membranes. PLoS One. 2022;17(7):e0270884. doi:10.1371/journal.pone.0270884
12. Tita AT, Andrews WW. Diagnosis and management of clinical chorioamnionitis. Clin Perinatol. 2010;37(2):339–354. doi:10.1016/j.clp.2010.02.003
13. Caloone J, Rabilloud M, Boutitie F, et al. Accuracy of several maternal seric markers for predicting histological chorioamnionitis after preterm premature rupture of membranes: a prospective and multicentric study. Eur J Obstet Gynecol Reprod Biol. 2016;205:133–140. doi:10.1016/j.ejogrb.2016.08.022
14. Asadi N, Faraji A, Keshavarzi A, Akbarzadeh-Jahromi M, Yoosefi S. Predictive value of procalcitonin, C-reactive protein, and white blood cells for chorioamnionitis among women with preterm premature rupture of membranes. Int J Gynaecol Obstet. 2019;147(1):83–88. doi:10.1002/ijgo.12907
15. Chang J, Streitman D. Physiologic adaptations to pregnancy. Neurol Clin. 2012;30(3):781–789. doi:10.1016/j.ncl.2012.05.001
16. Simon L, Gauvin F, Amre DK, Saint-Louis P, Lacroix J. Serum procalcitonin and C-reactive protein levels as markers of bacterial infection: a systematic review and meta-analysis. Clin Infectious Dis. 2004;39(2):206–217. doi:10.1086/421997
17. Musilova I, Kacerovsky M, Stepan M, et al. Maternal serum C-reactive protein concentration and intra-amniotic inflammation in women with preterm prelabor rupture of membranes. PLoS One. 2017;12(8):e0182731. doi:10.1371/journal.pone.0182731
18. Maul H, Kunze M, Berger R. Current approach in preterm prelabor rupture of membranes: new definitions? Is CRP determination useful? Are alternatives in sight? Gynakologe. 2021;54(3):186–194. doi:10.1007/s00129-021-04750-3
19. Aggarwal A, Pahwa S. Evaluation of the role of CRP as an early predictor of chorioamnionitis in PPROM. Int J Reprod Contracept Obstet Gynecol. 2018;7(4):1351–1356. doi:10.18203/2320-1770.ijrcog20181037
20. Liu J, Feng ZC, Wu J. The incidence rate of premature rupture of membranes and its influence on fetal-neonatal health: a report from mainland China. J Trop Pediatr. 2010;56(1):36–42. doi:10.1093/tropej/fmp051
21. Zhuang L, Li ZK, Zhu YF, et al. The correlation between prelabour rupture of the membranes and neonatal infectious diseases, and the evaluation of guideline implementation in China: a multi-centre prospective cohort study. Lancet Reg Health West Pac. 2020;3:100029. doi:10.1016/j.lanwpc.2020.100029
22. Wang X, Huang Z, Ma Y. Development and Validation of a Multivariable Predictive Model for the Risk of Histologic Chorioamnionitis in Patients with Premature Rupture of Membranes in the Late Preterm and Term. Int J Gen Med. 2024;17:141–152. doi:10.2147/IJGM.S445374
23. Adama van Scheltema PN, In’t Anker PS, Vereecken A, Vandenbussche FP, Deprest JA, Devlieger R. Biochemical composition of fluids for amnioinfusion during fetoscopy. Gynecol Obstetric Investigation. 2008;66(4):227–230. doi:10.1159/000147168
24. Czikk MJ, McCarthy FP, Murphy KE. Chorioamnionitis: from pathogenesis to treatment. Clin Microbiol Infect. 2011;17(9):1304–1311. doi:10.1111/j.1469-0691.2011.03574.x
25. Motulsky H, Christopoulos A. Fitting Models to Biological Data Using Linear and Nonlinear Regression: A Practical Guide to Curve Fitting. Oxford University Press; 2004.
26. Blumenfeld YJ, Lee HC, Gould JB, Langen ES, Jafari A, El-Sayed YY. The effect of preterm premature rupture of membranes on neonatal mortality rates. Obstet Gynecol. 2010;116(6):1381–1386. doi:10.1097/AOG.0b013e3181fe3d28
27. Kuba K, Bernstein PS. ACOG Practice Bulletin No. 188: prelabor Rupture of Membranes. Obstet Gynecol. 2018;131(6):1163–1164. doi:10.1097/AOG.0000000000002663
28. Metcalfe A, Lisonkova S, Sabr Y, Stritzke A, Joseph KS. Neonatal respiratory morbidity following exposure to chorioamnionitis. BMC Pediatr. 2017;17(1):128. doi:10.1186/s12887-017-0878-9
29. Ansar W, Ghosh S. C-reactive protein and the biology of disease. Immunol Res. 2013;56(1):131–142. doi:10.1007/s12026-013-8384-0
30. Thompson D, Pepys MB, Wood SP. The physiological structure of human C-reactive protein and its complex with phosphocholine. Structure. 1999;7(2):169–177. doi:10.1016/S0969-2126(99)80023-9
31. Kim SA, Park KH, Lee SM. Non-Invasive Prediction of Histologic Chorioamnionitis in Women with Preterm Premature Rupture of Membranes. Yonsei Med J. 2016;57(2):461–468. doi:10.3349/ymj.2016.57.2.461
32. Kwak DW, Cho HY, Kwon JY, Park YW, Kim YH. Usefulness of maternal serum C-reactive protein with vaginal Ureaplasma urealyticum as a marker for prediction of imminent preterm delivery and chorioamnionitis in patients with preterm labor or preterm premature rupture of membranes. J Perinat Med. 2015;43(4):409–415. doi:10.1515/jpm-2014-0142
33. Trochez-Martinez RD, Smith P, Lamont RF. Use of C-reactive protein as a predictor of chorioamnionitis in preterm prelabour rupture of membranes: a systematic review. BJOG. 2007;114(7):796–801. doi:10.1111/j.1471-0528.2007.01385.x
34. Oludag T, Gode F, Caglayan E, Saatli B, Okyay RE, Altunyurt S. Value of maternal procalcitonin levels for predicting subclinical intra-amniotic infection in preterm premature rupture of membranes. J Obstet Gynaecol Res. 2014;40(4):954–960. doi:10.1111/jog.12273
35. Musilova I, Pliskova L, Gerychova R, et al. Maternal white blood cell count cannot identify the presence of microbial invasion of the amniotic cavity or intra-amniotic inflammation in women with preterm prelabor rupture of membranes. PLoS One. 2017;12(12):e0189394. doi:10.1371/journal.pone.0189394
36. Shi H, Sun L, Wang Z, et al. Non-invasive prediction of histologic chorioamnionitis using maternal serum markers in women with preterm prelabour rupture of membranes. Am J Reprod Immunol. 2022;88(3):e13594. doi:10.1111/aji.13594
37. Cho I, Lee KN, Joo E, Kim YM, Kim TE, Park KH. Plasma E-selectin and kallistatin as predictive markers of histologic chorioamnionitis in women with preterm premature rupture of membranes. Am J Reprod Immunol. 2022;88(3):e13584. doi:10.1111/aji.13584
38. Zhang J, Shynlova O, Sabra S, Bang A, Briollais L, Lye SJ. Immunophenotyping and activation status of maternal peripheral blood leukocytes during pregnancy and labour, both term and preterm. J Cell Mol Med. 2017;21(10):2386–2402. doi:10.1111/jcmm.13160
39. Li A, Yang S, Zhang J, Qiao R. Establishment of reference intervals for complete blood count parameters during normal pregnancy in Beijing. J Clin Lab Anal. 2017;31(6). doi:10.1002/jcla.22150
40. Chandra S, Tripathi AK, Mishra S, Amzarul M, Vaish AK. Physiological changes in hematological parameters during pregnancy. Indian J Hematol Blood Transfus. 2012;28(3):144–146. doi:10.1007/s12288-012-0175-6
41. Kong X, Jiang L, Zhang B, Sun L, Liu K. Predicting chorioamnionitis in patients with preterm premature rupture of membranes using inflammatory indexes: a retrospective study. Taiwan J Obstet Gynecol. 2023;62(1):112–118. doi:10.1016/j.tjog.2022.11.006
42. Cornish EF, Filipovic I, Asenius F, Williams DJ, McDonnell T. Innate Immune Responses to Acute Viral Infection During Pregnancy. Front Immunol. 2020;11:572567. doi:10.3389/fimmu.2020.572567
43. Lee SM, Park KH, Joo E, et al. High-throughput analysis of amniotic fluid proteins associated with histological chorioamnionitis in preterm premature rupture of membranes using an antibody-based microarray. Am J Reprod Immunol. 2022;88(3):e13595. doi:10.1111/aji.13595
44. Helmig BR, Romero R, Espinoza J, et al. Neutrophil elastase and secretory leukocyte protease inhibitor in prelabor rupture of membranes, parturition and intra-amniotic infection. J Matern Fetal Neonatal Med. 2002;12(4):237–246. doi:10.1080/jmf.12.4.237.246
45. Balciuniene G, Kvederaite-Budre G, Gulbiniene V, et al. Neutrophil-lymphocyte ratio for the prediction of histological chorioamnionitis in cases of preterm premature rupture of membranes: a case-control study. BMC Pregnancy Childbirth. 2021;21(1):656. doi:10.1186/s12884-021-04101-z
46. Curbelo J, Luquero Bueno S, Galvan-Roman JM, et al. Inflammation biomarkers in blood as mortality predictors in community-acquired pneumonia admitted patients: importance of comparison with neutrophil count percentage or neutrophil-lymphocyte ratio. PLoS One. 2017;12(3):e0173947. doi:10.1371/journal.pone.0173947
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

