Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Associated Outcomes of Different Intravenous Antibiotics Combined with 2% Mupirocin Ointment in the Treatment of Pediatric Patients with Staphylococcal Scalded Skin Syndrome
Authors You C , Wu Z, Liao M, Ye X, Li L , Yang T
Received 18 April 2023
Accepted for publication 8 June 2023
Published 28 June 2023 Volume 2023:16 Pages 1691—1701
DOI https://doi.org/10.2147/CCID.S417764
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Cong You, Zhiwei Wu, Mingyi Liao, Xiaoying Ye, Longnian Li, Tao Yang
Department of Dermatology and Venereology, Candidate Branch of National Clinical Research Centre for Skin and Immune Diseases, The First Affiliated Hospital of Gannan Medical University, Ganzhou, People’s Republic of China
Correspondence: Longnian Li; Tao Yang, Department of Dermatology and Venereology, Candidate Branch of National Clinical Research Centre for Skin and Immune Diseases, The First Affiliated Hospital of Gannan Medical University, Ganzhou, People’s Republic of China, Tel +8614770833995 ; +8618720732351, Email [email protected]; [email protected]
Purpose: To compare treatment duration, influencing factors, and costs among intravenous antibiotic groups combined with 2% mupirocin ointment for treating staphylococcal scalded skin syndrome (SSSS).
Patients and Methods: Sex, age, onset days before admission, febrile status, white blood cell (WBC) count, and C-reactive protein (CRP) level were recorded as baseline characteristics for 253 included patients. The antibiotic sensitivity results were statistically compared by Cochran’s Q test. Kruskal–Wallis tests were used to compare days and the total costs of hospitalization with different intravenous antibiotic applications. Mann–Whitney U-tests or Spearman’s rank correlation tests were used for the univariate analysis. Finally, a multivariate linear regression model was employed to determine the variables with statistical significance.
Results: The sensitivity rates of oxacillin (84.62%), vancomycin (100%), and mupirocin (100%) were significantly higher than those of clindamycin (7.69%) (p< 0.0001). The duration of intravenous ceftriaxone administration was significantly longer than that of amoxicillin-clavulanic acid, cefathiamidine, and cefuroxime (p< 0.01). The total hospitalization costs for cefathiamidine were significantly higher than those for amoxicillin-clavulanic acid and cefuroxime (p< 0.05). According to the multiple linear regression, ages ≥ 60 months old were correlated with shorter treatment duration (β=− 1.48, [95% CI: − 2.29, − 0.66] for amoxicillin-clavulanic acid, and β=− 1.44, [95% CI: − 2.06, − 0.83] for cefathiamidine, and β=− 0.96, [95% CI: − 1.58, − 0.34] for cefuroxime) (all p< 0.01). In multivariate analysis for cefathiamidine, higher WBC count (β=0.05, [95% CI: 0.01, 0.10], p< 0.05) and CRP level (β=1.12, [95% CI: 0.14, 2.10], p< 0.05) were associated with longer treatment course.
Conclusion: Oxacillin resistance was rare, and clindamycin resistance was high in pediatric patients with SSSS in our district. Intravenous amoxicillin-clavulanic acid and cefuroxime combined with topical mupirocin were favorable due to a shorter intravenous treatment course and lower costs. Younger age, elevated WBC count, and CRP levels could indicate a longer course of treatment with intravenous antibiotics.
Keywords: staphylococcal scalded skin syndrome, Staphylococcus aureus, amoxicillin-clavulanic acid, cephalosporins, mupirocin, intravenous antibiotic treatment course
Introduction
Staphylococcal Scalded Skin Syndrome (SSSS) is a desquamative disease caused by the production of Exfoliative Toxin A (ETA) and Exfoliative Toxin B (ETB) by Staphylococcus aureus (S. aureus).1 It primarily affects newborns and children under the age of five with very rare cases reported in adults.2 The susceptibility to SSSS is related to the inability to metabolize ETA and ETB in the kidney and low titers of antibodies against ETA or ETB in children.3 Recent epidemiological studies have reported an increase in the morbidity of SSSS, with estimates suggesting that the incidence of SSSS in children is approximately 7.67 per million every year in the US4 and 696.4 per million in China.5
Children with SSSS typically present with several skin signs, including disseminated erythema, baggy blister-like lesions, and epidermal detachment.6 Consequently, a series of complications occur, including deprivation of body fluids, electrolyte abnormalities, and hypothermic and secondary infections such as septicemia and ethmyphitis, etc.6,7 It is important to use intravenous antibiotics as quickly as possible to treat SSSS.8 Current recommendations for the treatment of SSSS remain controversial. As clindamycin can inhibit the synthesis of toxins that mediate the lysis of the granular layer in the skin, it is recommended by some researchers for the treatment of SSSS.9,10 However, some studies have demonstrated that clindamycin does not improve clinical outcomes.2,11,12 For example, some experts have found that clindamycin monotherapy does not significantly reduce hospital length for patients with SSSS.2,12 Another retrospective analysis by Gray et al revealed that clindamycin does not improve patient prognosis.1 Penicillinase-resistant penicillins and cephalosporins are recommended for the empiric management of SSSS. Anti-methicillin-resistant S. aureus (MRSA) was considered if the prevalence of MRSA was high in the communities or if the patients failed to improve after empiric administration of cephalosporins or penicillinase-resistant penicillins.11 Mupirocin ointment is one of the most effective topical antibiotics used to clear nasal S. aureus strains, including methicillin-susceptible S. aureus (MSSA) and MRSA. Mupirocin prevents protein and RNA synthesis by inhibiting isoleucyl-tRNA synthetase, which ultimately leads to bacterial death.13 Mupirocin has also been reported to eliminate MRSA.14 The number of cases of MRSA skin infections treated with mupirocin has increased over the past decade.15 Mupirocin can also be applied externally to treat SSSS.16
With China’s aging population presenting significant challenges to its healthcare system, the government aims to maximize health outcomes while minimizing medical costs. To reduce the disease burden, total medical costs are strictly controlled.17 Therefore, selecting effective low-cost antibiotics for treating SSSS is crucial. To the best of our knowledge, there is a lack of data on the outcomes of pediatric patients with SSSS treated with anti-MSSA intravenous antibiotics along with topical mupirocin. Here, we compared the days of the treatment duration and costs among different intravenous antibiotic groups in combination with topical 2% mupirocin ointment. Furthermore, the antimicrobial susceptibility of S. aureus and the factors influencing treatment duration for different intravenous antibiotics were investigated.
Patients and Methods
Study Population
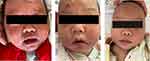
We conducted a retrospective study using data from pediatric patients with SSSS who were admitted to the Department of Dermatology, The First Affiliated Hospital of Gannan Medical University, between January 1, 2012, and December 31, 2022. This study adhered to the principles of the Declaration of Helsinki of the World Medical Association and ethics approval was granted by the Ethics Committee of the First Affiliated Hospital of Gannan Medical University (No. 2019100). All legal guardians provided written informed consent for the publication of their data. The SSSS cases were identified by reviewing electronic medical records using the International Classification of Diseases, ninth revision code 695.81, or tenth revision code L00.x00. A total of 400 patients were identified. We have included 253 patients meeting diagnostic criteria based on clinical manifestations. Excluded were 147 patients with comorbidities or complications (n=69), transferred to pediatrics (n=31), or not administered antibiotics in the present study (n=47) (Figure 1). The diagnostic criteria were based on the following clinical manifestations: erythema, bullae, and scaling on the skin with a positive Nikolsky sign and tenderness; radial fissuring in the periorificial area was considered a characteristic sign of SSSS, and the mucosal area was not involved (Figure 2A).18,19
 |
Figure 1 Study flowchart of patients and methods. Abbreviations: SSSS, Staphylococcal Scalded Skin Syndrome; S. aureus, Staphylococcus aureus. |
The exclusion criteria were as follows: patients whose caregivers refused intravenous antibiotics; patients with comorbidities such as bacterial infection that could influence the treatment course of intravenous antibiotics; patients with severe complications such as secondary infection, sepsis, and severe dehydration; patients with diseases related to immune deficiency or impaired kidney function, and patients who were transferred to other departments. Baseline characteristics of 253 patients with SSSS were collected, including sex, age, onset days before admission, febrile status, white blood cell (WBC) count, and C-reactive protein (CRP) level. The normal range of CRP levels is 0–6 mg/L. The baseline characteristics of patients with SSSS are presented in Table 1.
 |
Table 1 Baseline Characteristics of Pediatric Patients with SSSS (n=253) |
Antibiotic Regimen Groups and Laboratory Tests
Amoxicillin–clavulanic acid (Huabei Pharmaceutical Co., Ltd. Shijiazhuang, China), or cefathiamidine (Luoxin Pharmaceutical Co., Ltd. Linyi, China) or cefuroxime (Huidisen Pharmaceutical Co., Ltd. Hanzhou, China) or ceftriaxone (Kelun Pharmaceutical Co., Ltd. Chengdu, China) were intravenously administered in 48, 82, 65, and 58 patients, respectively. The dosages of the intravenous antibiotics were according to their instructions. The doses of amoxicillin-clavulanic acid and ceftriaxone were 30mg per kilogram of body weight every 8 hours and 20mg per kilogram of body weight once a day, respectively. Cefathiamidine and cefuroxime at a dose of 20mg per kilogram of body weight every 8 hours were prescribed. Twice daily, all patients were administered 2% mupirocin ointment (China-USA SmithKline Pharmaceutical Co., Ltd. Tianjin, China) on their skin lesions. Thirteen S. aureus clinical strains were successfully cultured, and antimicrobial susceptibility tests were performed. S. aureus drug sensitivity tests of levofloxacin, erythromycin, tetracycline, clindamycin, oxacillin, penicillin G, vancomycin, and mupirocin were performed using the microdilution method. Minimum inhibitory concentration results were interpreted in accordance with Clinical and Laboratory Standards Institute guidelines and the previous publication.20,21 The intravenous and topical application of antibiotics was withdrawn if all the erythema and bullae resolved with a few desquamations and without scarring.
Statistical Analysis
SPSS v.23.0 (IBM Corp., Armonk, NY, USA) and GraphPad Prism 6.0 (Insightful Science Company, San Diego, CA, USA) were used for statistical analyses and graphing data, respectively. Data are presented as frequencies for categorical variables or (25th and 75th percentiles) for continuous variables. Pearson’s chi-square test or the Kruskal–Wallis test was used to analyze the differences in baseline characteristics among the different groups. Mann–Whitney U-test was used to compare the treatment duration of intravenous antibiotics between different age groups. Differences in the resistance rates of the eight antibiotics were compared using Cochran’s Q test. Kruskal–Wallis tests were used to compare the days of intravenous antibiotic application and total hospitalization costs among the different antibiotic groups. Univariate analysis with the Mann–Whitney U-test or Spearman’s rank correlation test was used for simple factor analysis. Covariates were adjusted using a multivariate linear regression model to analyze factors affecting the course of treatment with different intravenous antibiotics, including amoxicillin-clavulanic acid, cefathiamidine, and cefuroxime. P <0.05 was considered statistically significant.
Results
A total of 253 pediatric patients, including 106 females and 147 males were included in the present study, most of whom were aged below 5 years (84.58%). Four types of antibiotics were administered intravenously: amoxicillin-clavulanic acid (18.97%), cefathiamidine (32.41%), cefuroxime (25.69%), and ceftriaxone (22.92%). The WBC count and CRP levels in most patients were normal. There were no significant differences in baseline characteristics among the different antibiotic groups (all p>0.05). The treatment course of intravenous amoxicillin-clavulanic acid, cefathiamidine and cefuroxime in the age groups below 60 months were significantly longer than those in the age groups equal to or above 60 months (p<0.01, Supplementary Table 1). Meanwhile, the treatment duration of the intravenous antibiotics were comparable between the other age groups (p>0.05, Supplementary Table 1). Skin manifestations at admission, three days post-treatment, and at the endpoint of antibiotic administration are presented in Figure 2A–C, respectively. Gradual resolution of skin lesions can be observed in these images. All the patients achieved full recovery by the end of the study period.
The antibiotic sensitivity results of 13 S. aureus strains are shown in Table 2 and Supplementary Table 2. There were significant differences in sensitivity rates among eight antibiotics, including levofloxacin, erythromycin, tetracycline, clindamycin, oxacillin, penicillin G, vancomycin, and mupirocin. The pairwise comparisons showed that the sensitivity rates of oxacillin (84.62%), vancomycin (100%), and mupirocin (100%) were significantly higher than those of erythromycin (0), penicillin G (0), and clindamycin (7.69%) (p<0.0001), and the sensitivity rates of oxacillin (84.62%) was comparable to those of vancomycin (100%) and mupirocin (100%) (p>0.05). Two S. aureus strains which were resistant to oxacillin were cultured from two SSSS patients who were successfully treated with intravenous amoxicillin-clavulanic acid combined with 2% mupirocin ointment. The medians of days of intravenous amoxicillin-clavulanic acid, cefathiamidine, cefuroxime, and ceftriaxone application were 7, 7, 7, and 8 days, respectively. According to the Kruskal–Wallis test, the duration of intravenous ceftriaxone application was significantly longer than amoxicillin-clavulanic acid, cefathiamidine, and cefuroxime (p<0.01, Figure 3A). There were no significant differences in the duration among patients treated with amoxicillin-clavulanic acid, cefathiamidine, or cefuroxime (p>0.05, Figure 3A). The medians of total hospitalization costs for patients using amoxicillin-clavulanic acid, cefathiamidine, and cefuroxime were 449.7, 502.8, and 429.9 US dollars, respectively. The total hospitalization costs for patients using cefathiamidine were significantly higher than those using amoxicillin-clavulanic acid and cefuroxime (p<0.05, Figure 3B). There were no significant differences in total hospitalization costs between the patients using amoxicillin-clavulanic acid and those treated with cefuroxime (p>0.05, Figure 3B).
 |
Table 2 Antibiotic Sensitivity Test Results of S. aureus Isolated from Patients with SSSS (n=13) |
According to the univariate analysis, age below 60 months was associated with more days of intravenous antibiotic application of amoxicillin-clavulanic acid, cefathiamidine, and cefuroxime compared with age equal to or above 60 months (p<0.01, Table 3); WBC count was positively correlated with the days of intravenous cefathiamidine application (p<0.05, Table 3). In the multivariate linear regression model for amoxicillin-clavulanic acid, cefathiamidine, and cefuroxime, the associations between the days of antibiotic application and patient age remained significant (β=−1.48, [95% CI: −2.29, −0.66] for amoxicillin-clavulanic acid, and β=−1.44, [95% CI: −2.06, −0.83] for cefathiamidine, and β=−0.96, [95% CI: −1.58, −0.34] for cefuroxime) (all p<0.01, Table 4). In multivariate analysis for cefathiamidine, the days of intravenous cefathiamidine application were significantly associated with WBC count (β=0.05, [95% CI: 0.01, 0.10], p<0.05) and CRP level (β=1.12, [95% CI: 0.14, 2.10], p<0.05) (Table 4).
 |
Table 3 Univariate Analysis of Variables Affecting the Treatment Course of Different Intravenous Antibiotics in Patients with SSSS |
 |
Table 4 Multivariate Analysis of Factors Affecting the Number of Days of Intravenous Antibiotic Application in Patients with SSSS |
Discussion
To our knowledge, our study is the first and the largest retrospective study to compare the days of treatment course and costs among different intravenous anti-MSSA antibiotic applications in combination with topical use of 2% mupirocin ointment and to investigate the factors affecting the intravenous antibiotic treatment course in pediatric patients with SSSS. Consistent with previous studies, most patients (84.58%) were below five years of age, and the number of male patients was nearly equal to the female patients at a ratio of 1.39:1.6,22–24 Similar to the results previously described in some studies, 96.05% of patients had their CRP levels within the normal range.16,25,26 The features of these patients were consistent with the typical characteristics of patients with SSSS.
Some studies have found that S. aureus strains isolated from patients with SSSS display higher rates of clindamycin resistance compared with overall S. aureus strains.11,27,28 We found a similarly high resistance rate (92.31%) to clindamycin in S. aureus strains in our study. Therefore, we consider that the combined use of clindamycin in SSSS cases is unnecessary, and clindamycin monotherapy in patients with SSSS in our district should be avoided. Previous epidemiological studies have shown that the predominant isolates in children with SSSS are MSSA.3,27,29 Several studies in China and Japan have reported that more than 95% of S. aureus isolates are methicillin-sensitive.5,11,29 In line with previous studies, MSSA was the predominant pathogen identified in culture, with 84.62% of the clinical strains sensitive to oxacillin in our study.27,28 In our study, we used amoxicillin-clavulanic acid, cefathiamidine, cefuroxime, and ceftriaxone combined with an external application of 2% mupirocin ointment for the treatment of SSSS without treatment failure. Amoxicillin-clavulanic acid is an anti-β lactamase penicillin that is commonly used to treat skin infections with MSSA.30,31 Cefathiamidine is a first-generation cephalosporin approved by the China Food and Drug Administration for the treatment of infections caused by MSSA.32,33 Cefuroxime is a second-generation cephalosporin antibiotic that effectively eliminates penicillinase-producing staphylococci.34 Cefuroxime and β-lactamase-resistant penicillin such as amoxicillin-clavulanic acid are recommended for prompt empiric treatment of SSSS.6 Ceftriaxone is also considered a treatment option for MSSA infection.35,36 However, we found that the treatment course of intravenous ceftriaxone for SSSS was longer than amoxicillin-clavulanic acid, cefathiamidine, and cefuroxime. Previous studies have shown that it is not suitable to use a routine dose of ceftriaxone to treat MSSA infections.37 Another retrospective study comparing the efficacy of cefazolin and ceftriaxone in the treatment of MSSA bacteremia revealed a higher rate of treatment failure with ceftriaxone.38 We speculate that lower serum concentrations of free ceftriaxone, as a result of higher protein binding, could contribute to unsatisfactory treatment outcomes. Therefore, we do not recommend the use of intravenous ceftriaxone as an empirical regimen to treat SSSS. However, further studies are needed to confirm this hypothesis.
Recent reports have found cases of SSSS due to MRSA infection.39,40 Therefore, some experts recommend the use of vancomycin for the empirical treatment of SSSS in districts where MRSA is the predominant strain.19 In our study, we found only two patients with SSSS infected with MRSA who were successfully treated with intravenous amoxicillin-clavulanic acid and topical mupirocin. Several studies have reported similar results showing that clinical improvement can be achieved regardless of the susceptibility of S. aureus strains.11,26 We inferred that antibodies against exfoliative toxins might play a role in the improvement of SSSS caused by MRSA. Additionally, according to a systematic review, the prevalence of clinical S. aureus resistant to mupirocin was low, which could help eliminate MRSA in patients with SSSS.41 Moreover, the culture results of the clinical specimens could only represent the colonization of S. aureus rather than the causative organism. Further studies on the classification of S. aureus using the method of multilocus sequence typing are warranted to confirm whether there are molecular strains such as sequence types 15, 121, 2126, and 2993 that cause SSSS.28
In our study, we found that the cost of cefathiamidine was higher than that of amoxicillin-clavulanic acid and cefuroxime with comparable treatment courses. We reduced the types of ancillary tests used in our study because they did not improve diagnostic precision.1 Thus, we inferred that antibiotic costs may be the largest driver of total hospitalization cost differences among the different groups. Indeed, the price of cefathiamidine is higher than amoxicillin-clavulanic acid and cefuroxime in our country. Based on these data, amoxicillin-clavulanic acid and cefuroxime are the preferred antibiotics for the treatment of patients with SSSS.
In the final multiple linear regression model, younger age correlated with a longer duration of intravenous antibiotic treatment in the amoxicillin-clavulanate, cefathiamidine, and cefuroxime treatment groups. Low titers of antibodies against exfoliative toxins and limited capacity for renal clearance of the toxin at a young age may lead to a relatively high incidence of SSSS at a younger age.6,23,42 Therefore, we speculate that the increased antibody titers and renal clearance capacity in older children might be favorable in the reduction of the length of intravenous antibiotic treatment course. Larger studies investigating the clinical responses of SSSS patients of different age groups are necessary.
The total WBC count is commonly used to assess the risk of serious bacterial infections. Studies have explored the role of the WBC count in evaluating the severity of infectious diseases, with some researchers reporting that treatment failure and prolonged hospital stay in patients with skin and soft tissue infections are significantly associated with a high WBC count.43,44 Higher WBC counts on admission are also associated with a higher risk of mortality in patients with S. aureus induced infective endocarditis.45 CRP is an acute-phase protein that is widely measured in infectious diseases, and a valuable marker of systemic inflammation, as well as a good clinical indicator of severe infections.46 A few studies have reported that in infectious diseases, increased CRP levels are associated with an increased length of hospital stay.47,48 CRP levels are important predictors of fatal clinical outcomes in S. aureus bacteremia.49 The studies above demonstrated that a higher WBC count and CRP level could indicate a more severe infection, resulting in a longer duration of intravenous cefathiamidine application, which is consistent with the findings of the present study and similar with findings of our previous study.16
It is worth noting that the association between elevated WBC and CRP levels and prolonged duration was only found in the intravenous cefathiamidine group, but not in the amoxicillin-clavulanic acid and cefuroxime groups. This could be because of the small sample size of each group in our study, or it could be because some covariates, such as differential titers of antibodies against exfoliative toxins and renal clearance capacity, which could affect the treatment course, were not examined.
Our study had a few limitations. First, ours is a single-center, retrospective study with a small sample size. Second, we did not measure the anti-toxin antibiotics titers or the toxin-clearing renal clearance capacity of patients to confirm their influence on the improvement of SSSS. Third, multi-locus sequence typing on the S. aureus clinical strains were not performed. Future prospective and randomized trials are needed to evaluate the performance of the laboratory tests mentioned above.
Conclusions
Oxacillin resistance is rare, whereas the clindamycin resistance rate is high in pediatric patients with SSSS in our district. The administration of intravenous amoxicillin-clavulanic acid and cefuroxime combined with topical mupirocin was favorable in treating SSSS because of their relatively shorter intravenous treatment course compared with ceftriaxone and lower costs compared with cefathiamidine. Younger age, as well as elevated WBC count and CRP levels, may indicate that a longer treatment course with intravenous anti-MSSA antibiotics is required.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Informed Consent
The study adhered to the principles of the World Medical Association’s Declaration of Helsinki, and ethics approval for this study was granted by the Ethics Committee of The First Affiliated Hospital of Gannan Medical University (No. 2019100). Before undergoing the treatment, all patients’ legal guardians provided written informed consent for their data to be published in the article.
Consent for Publication
Consent for publication was included in the informed consent obtained from each participant’s legal guardians.
Acknowledgments
We would like to thank Editage (www.editage.cn) for English language editing. We thank the patients for their written consent to use their data.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work. Longnian Li and Tao Yang are co-correspondence authors.
Funding
This work was funded by the National Natural Science Foundation of China (Grant No.: 81960566). The study sponsor was not involved in performing the research or in writing the paper.
Disclosure
Authors declare no competing interests for this study.
References
1. Gray L, Olson J, Brintz BJ, Cipriano SD. Staphylococcal scalded skin syndrome: clinical features, ancillary testing, and patient management. Pediatr Dermatol. 2022;39(6):908–913. doi:10.1111/pde.15102
2. Liy-Wong C, Pope E, Weinstein M, Lara-Corrales I. Staphylococcal scalded skin syndrome: an epidemiological and clinical review of 84 cases. Pediatr Dermatol. 2021;38(1):149–153. doi:10.1111/pde.14470
3. Lamand V, Dauwalder O, Tristan A, et al. Epidemiological data of staphylococcal scalded skin syndrome in France from 1997 to 2007 and microbiological characteristics of Staphylococcus aureus associated strains. Clin Microbiol Infect. 2012;18(12):E514–E521. doi:10.1111/1469-0691.12053
4. Arnold JD, Hoek SN, Kirkorian AY. Epidemiology of staphylococcal scalded skin syndrome in the United States: a cross-sectional study, 2010–2014. J Am Acad Dermatol. 2018;78(2):404–406. doi:10.1016/j.jaad.2017.09.023
5. Li MY, Hua Y, Wei GH, Qiu L. Staphylococcal scalded skin syndrome in neonates: an 8-year retrospective study in a single institution. Pediatr Dermatol. 2014;31(1):43–47. doi:10.1111/pde.12114
6. Handler MZ, Schwartz RA. Staphylococcal scalded skin syndrome: diagnosis and management in children and adults. J Eur Acad Dermatol Venereol. 2014;28(11):1418–1423. doi:10.1111/jdv.12541
7. Aydin D, Alsbjørn B. Severe case of staphylococcal scalded skin syndrome in a 5-year-old child - case report. Clin Case Rep. 2016;4(4):416–419. doi:10.1002/ccr3.535
8. Blyth M, Estela C, Young AE. Severe staphylococcal scalded skin syndrome in children. Burns. 2008;34(1):98–103. doi:10.1016/j.burns.2007.02.006
9. Berk DR, Bayliss SJ. MRSA, staphylococcal scalded skin syndrome, and other cutaneous bacterial emergencies. Pediatr Ann. 2010;39(10):627–633. doi:10.3928/00904481-20100922-02
10. Hodille E, Rose W, Diep BA, et al. The role of antibiotics in modulating virulence in Staphylococcus aureus. Clin Microbiol Rev. 2017;30(4):887–917. doi:10.1128/CMR.00120-16
11. Wang Z, Feig JL, Mannschreck DB, Cohen BA. Antibiotic sensitivity and clinical outcomes in staphylococcal scalded skin syndrome. Pediatr Dermatol. 2020;37(1):222–223. doi:10.1111/pde.14014
12. Vernali S, Blasiak RC, Morrell DS. Demographic characteristics, clinical features, and optimal management of hospitalized patients with staphylococcal scalded skin syndrome. Pediatr Dermatol. 2021;38(4):825–830. doi:10.1111/pde.14629
13. Poovelikunnel T, Gethin G, Humphreys H. Mupirocin resistance: clinical implications and potential alternatives for the eradication of MRSA. J Antimicrob Chemother. 2015;70(10):2681–2692. doi:10.1093/jac/dkv169
14. Huang SS, Singh R, McKinnell JA, et al. Decolonization to reduce postdischarge infection risk among MRSA carriers. N Engl J Med. 2019;380(7):638–650. doi:10.1056/NEJMoa1716771
15. Doudoulakakis A, Spiliopoulou I, Spyridis N, et al. Emergence of a Staphylococcus aureus clone resistant to mupirocin and fusidic acid carrying exotoxin genes and causing mainly skin infections. J Clin Microbiol. 2017;55(8):2529–2537. doi:10.1128/JCM.00406-17
16. Yang T, Wang J, Cao J, et al. Antibiotic-resistant profile and the factors affecting the intravenous antibiotic treatment course of generalized Staphylococcal Scalded Skin Syndrome: a retrospective study. Ital J Pediatr. 2021;47(1):169. doi:10.1186/s13052-021-01120-6
17. Miao Y, Yuan X, Gu J, et al. Constructing a value-based healthcare system for hypertensive patients through changing payment mode: evidence from a comparative study in rural China. J Med Econ. 2019;22(3):245–251. doi:10.1080/13696998.2018.1558864
18. Bolognia JL, Jorizzo JL, Schaffer JV. Dermatology. In: Millett CR, Halpern AV, Reboli AC, Heymann WR, editors. Bacterial Diseases.
19. Leung AKC, Barankin B, Leong KF. Staphylococcal-scalded skin syndrome: evaluation, diagnosis, and management. World J Pediatr. 2018;14(2):116–120. doi:10.1007/s12519-018-0150-x
20. Clinical and Laboratory Standards Institute [CLSI]. M100–S32. Performance Standards for Antimicrobial Susceptibility Testing: 32th Informational Supplement. Wayne, PA: Clinical and Laboratory Standards Institute; 2022.
21. Shivanna V, Dasegowda V. Comparison of disk diffusion and agar dilution method for the detection of mupirocin resistance in staphylococcal isolates from skin and soft tissue infections. J Lab Physicians. 2023. doi:10.1055/s-0042-1760672
22. Nishifuji K, Shimizu A, Ishiko A, Iwasaki T, Amagai M. Removal of amino-terminal extracellular domains of desmoglein 1 by staphylococcal exfoliative toxin is sufficient to initiate epidermal blister formation. J Dermatol Sci. 2010;59(3):184–191. doi:10.1016/j.jdermsci.2010.07.010
23. Mishra AK, Yadav P, Mishra A. A systemic review on staphylococcal scalded skin syndrome (SSSS): a rare and critical disease of neonates. Open Microbiol J. 2016;10(1):150–159. doi:10.2174/1874285801610010150
24. Oliveira AR, Aires S, Faria C, Santos E. Staphylococcal scalded skin syndrome. BMJ Case Rep. 2013;2013:bcr2013009478. doi:10.1136/bcr-2013-009478
25. Neubauer HC, Hall M, Wallace SS, et al. Variation in diagnostic test use and associated outcomes in Staphylococcal scalded skin syndrome at children’s hospitals. Hosp Pediatr. 2018;8(9):530–537. doi:10.1542/hpeds.2018-0032
26. Choi JH, Lee H, Choi EH. Antimicrobial resistance and molecular analysis of Staphylococcus aureus in Staphylococcal Scalded Skin Syndrome among Children in Korea. J Korean Med Sci. 2021;36(3):e22. doi:10.3346/jkms.2021.36.e22
27. Braunstein I, Wanat KA, Abuabara K, et al. Antibiotic sensitivity and resistance patterns in pediatric staphylococcal scalded skin syndrome. Pediatr Dermatol. 2014;31(3):305–308. doi:10.1111/pde.12195
28. Hultén KG, Kok M, King KE, Lamberth LB, Kaplan SL. Increasing numbers of Staphylococcal Scalded Skin Syndrome cases caused by ST121 in Houston, Texas. Pediatr Infect Dis J. 2020;39(1):30–34. doi:10.1097/INF.0000000000002499
29. Yamaguchi T, Yokota Y, Terajima J, et al. Clonal association of Staphylococcus aureus causing bullous impetigo and the emergence of new methicillin-resistant clonal groups in Kansai district in Japan. J Infect Dis. 2002;185(10):1511–1516. doi:10.1086/340212
30. Brogden RN, Carmine A, Heel RC, et al. Amoxycillin/clavulanic acid: a review of its antibacterial activity, pharmacokinetics and therapeutic use. Drugs. 1981;22(5):337–362. doi:10.2165/00003495-198122050-00001
31. Benninger MS. Amoxicillin/clavulanate potassium extended release tablets: a new antimicrobial for the treatment of acute bacterial sinusitis and community-acquired pneumonia. Expert Opin Pharmacother. 2003;4(10):1839–1846. doi:10.1517/14656566.4.10.1839
32. National pediatric multi-center cooperative group of cefathiamidine observation. Tou bao liu mi zhi liao xiao er ji xing xi jun gan ran xing ji bing liao xiao guan cha [Effects of cefathiamidine on the treatment of children with acute bacterial infectious disease]. Zhonghua Er Ke Za Zhi. 2003;41(1):54–55. Chinese.
33. Tze-ying T, Fu W, Chih-lin C, et al. Clinical and laboratory studies of a new cephalosporin derivative--cefathiamidine. Chin Med J. 1979;92(1):26–36.
34. O’Callaghan CH, Sykes RB, Griffiths A, Thornton JE. Cefuroxime, a new cephalosporin antibiotic: activity in vitro. Antimicrob Agents Chemother. 1976;9(3):511–519. doi:10.1128/AAC.9.3.511
35. Mohamed A, Bennett N, Ploetz J, Aragon L, Kennedy K, Boyd S. Ceftriaxone versus cefazolin for the treatment of methicillin-susceptible Staphylococcus aureus bacteraemia. Int J Antimicrob Agents. 2022;60(3):106632. doi:10.1016/j.ijantimicag.2022.106632
36. Yetmar ZA, Razi S, Nayfeh T, et al. Ceftriaxone versus antistaphylococcal antibiotics for definitive treatment of methicillin-susceptible Staphylococcus aureus infections: a systematic review and meta-analysis. Int J Antimicrob Agents. 2022;59(1):106486. doi:10.1016/j.ijantimicag.2021.106486
37. Heffernan AJ, Sime FB, Lim SMS, et al. Pharmacodynamics of ceftriaxone for the treatment of methicillin-susceptible Staphylococcus aureus: is it a viable treatment option? Int J Antimicrob Agents. 2022;59(3):106537. doi:10.1016/j.ijantimicag.2022.106537
38. Carr DR, Stiefel U, Bonomo RA, et al. A comparison of cefazolin versus ceftriaxone for the treatment of methicillin-susceptible Staphylococcus aureus bacteremia in a tertiary care VA Medical Center. Open Forum Infect Dis. 2018;5(5):ofy089. doi:10.1093/ofid/ofy089
39. Hörner A, Hörner R, Salla A, et al. Staphylococcal scalded skin syndrome in a premature newborn caused by methicillin-resistant Staphylococcus aureus: case report. Sao Paulo Med J. 2015;133(5):450–453. doi:10.1590/1516-3180.2013.79400715
40. Lamanna O, Bongiorno D, Bertoncello L, et al. Rapid containment of nosocomial transmission of a rare community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA) clone, responsible for the Staphylococcal Scalded Skin Syndrome (SSSS). Ital J Pediatr. 2017;43(1):5. doi:10.1186/s13052-016-0323-y
41. Dadashi M, Hajikhani B, Darban-Sarokhalil D, et al. Mupirocin resistance in Staphylococcus aureus: a systematic review and meta-analysis. J Glob Antimicrob Resist. 2020;20:238–247. doi:10.1016/j.jgar.2019.07.032
42. Jeyakumari D, Gopal R, Eswaran M, MaheshKumar C. Staphylococcal scalded skin syndrome in a newborn. J Glob Infect Dis. 2009;1(1):45–47. doi:10.4103/0974-777X.52981
43. Abetz JW, Adams NG, Mitra B. Skin and soft tissue infection management failure in the emergency department observation unit: a systematic review. Emerg Med J. 2018;35(1):56–61. doi:10.1136/emermed-2016-205950
44. Schrock JW, Laskey S, Cydulka RK. Predicting observation unit treatment failures in patients with skin and soft tissue infections. Int J Emerg Med. 2008;1(2):85–90. doi:10.1007/s12245-008-0029-z
45. Tascini C, Aimo A, Arzilli C, et al. Procalcitonin, white blood cell count and C-reactive protein as predictors of S. aureus infection and mortality in infective endocarditis. Int J Cardiol. 2020;301:190–194. doi:10.1016/j.ijcard.2019.08.013
46. Lobo SM, Lobo FR, Bota DP, et al. C-reactive protein levels correlate with mortality and organ failure in critically ill patients. Chest. 2003;123(6):2043–2049. doi:10.1378/chest.123.6.2043
47. Bègue L, Schlund M, Raoul G, et al. Biological factors predicting the length of hospital stay in odontogenic cellulitis. J Stomatol Oral Maxillofac Surg. 2022;123(3):303–308. doi:10.1016/j.jormas.2021.07.007
48. Williams DJ, Hall M, Auger KA, et al. Association of white blood cell count and C-reactive protein with outcomes in children hospitalized for community-acquired pneumonia. Pediatr Infect Dis J. 2015;34(7):792–793. doi:10.1097/INF.0000000000000724
49. Mölkänen T, Ruotsalainen E, Rintala EM, Järvinen A, Póvoa P. Predictive value of C-Reactive Protein (CRP) in identifying fatal outcome and deep infections in Staphylococcus aureus bacteremia. PLoS One. 2016;11(5):e0155644. doi:10.1371/journal.pone.0155644
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.