Back to Journals » Psoriasis: Targets and Therapy » Volume 12
Assessment of Vitamin D Level in Patients with Psoriasis and Its Correlation with Disease Severity: A Case–Control Study
Authors Pokharel R , Agrawal S, Pandey P, Lamsal M
Received 4 June 2022
Accepted for publication 4 August 2022
Published 13 September 2022 Volume 2022:12 Pages 251—258
DOI https://doi.org/10.2147/PTT.S369426
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Uwe Wollina
Ranju Pokharel,1 Sudha Agrawal,1 Prajwal Pandey,1 Madhab Lamsal2
1Department of Dermatology and Venereology, B.P Koirala Institute of Health Sciences, Dharan, Nepal; 2Department of Biochemistry, B.P Koirala Institute of Health Sciences, Dharan, Nepal
Correspondence: Ranju Pokharel, Junior Resident, Department of Dermatology and Venereology, B.P. Koirala Institute of Health Sciences, Dharan, Nepal, Tel +9779860660852, Email [email protected]
Background: Chronic plaque psoriasis is a chronic inflammatory skin disorder. Vitamin D has been shown to have effects on keratinocyte differentiation as well as immune regulation in the skin.
Objective: The main objective of this study was to assess the 25hydroxyvitamin D [25 (OH) D] level in patients with psoriasis in comparison with healthy control subjects.
Materials and Methods: This case–control study included 180 persons (120 cases and 60 age- and sex-matched control subjects) from outpatient department of BPKIHS, a tertiary care hospital in eastern Nepal. Severity of psoriatic skin lesions was assessed using psoriasis area severity index (PASI) scoring. Serum vitamin D level was assessed by chemiluminescent immunoassay.
Results: The mean serum 25(OH) D levels in psoriatic patients and controls were 19.57 ± 6.85 ng/mL and 23.63 ± 6.40 ng/mL, respectively. The difference was statistically significant even after adjusting for confounding factors in a multivariate analysis (aOR 2.929, 95% CI 1.376– 6.230). Low serum 25(OH) D levels were negatively associated with the severity of disease (r= − 0.628, P= 0.01).
Conclusion: Serum 25(OH) D levels are significantly lower in psoriatic patients than in healthy control subjects. Deficiency of serum 25(OH) D was associated with severity of disease with an inverse relationship with PASI score.
Keywords: psoriasis, psoriasis area severity index, 25hydroxyvitamin vitamin D
Introduction
Psoriasis is a chronic inflammatory and proliferative skin condition that results from complex interaction of the innate and acquired immunologic system and is characterized clinically by sharply defined erythematous plaques with silvery white scales predominantly on extensor surfaces and scalp with chronic and relapsing course.1 The presence of vitamin D receptor in keratinocytes was first established from in vitro study of cultured keratinocytes in 1988 and afterward many in vitro and clinical studies have well-documented the role of vitamin D in proliferation and differentiation of keratinocytes.2 Vitamin D inhibits keratinocytes’ proliferation and induces their differentiation. Thus, these two important findings of vitamin D in keratinocytes have led to the discovery of the role of vitamin D in the pathogenesis of psoriasis, ie, a decrease in vitamin D causes an increase in proliferation of keratinocytes and cutaneous inflammation.3
The first meta-analysis was done in July 2018 to assess serum 25-hydroxyvitamin D levels in adults with psoriasis. Of 107 studies, only 10 studies were included for quantitative meta-analysis. Six studies showed statistically significant relationship of low 25(OH) D levels with psoriasis while other studies failed to replicate similar findings. The pooled mean difference in serum 25(OH) D level was −6.13ng/mL (95% Confidence interval −10.93 to 1.32 ng/m, p=0.001) and favored a significant relationship between hypovitaminosis and psoriasis, however, the causal relationship could not be established. Moreover these studies were limited by small sample size and clinical and methodological heterogenecity among the study groups.4 Further, among case-control studies published between 2019 and 2021, few of them showed significant association between 25(OH) D levels and psoriasis,5–9 while other remaining studies did not show a significant association between level of 25(OH) D with psoriasis.10–14 Hence in conclusion from the literature review of clinical studies available to date, the association between psoriasis and vitamin D is still controversial.
Therefore, we conducted this study to evaluate serum 25(OH) D level in psoriasis patients and its association with severity and duration of disease in a larger cohort of samples, concomitantly taking into account the confounding factors to assess the final outcome in our eastern part of Nepal. Also, the finding of this research may further aid in recommending oral supplementation of vitamin D to improve the final outcome of psoriasis patients.
Methods
Patients and Control Subjects
This case–control study included one hundred and eighty outpatients. One hundred and twenty patients with psoriasis were selected consecutively from the dermatology outpatient department. Sixty age- and sex-matched control subjects were selected among healthy volunteers who were the attendants of patients other than psoriasis. All patients were enrolled between January and August 2021 and were from different parts of eastern Nepal.
The diagnosis of plaque psoriasis was made clinically. Inclusion criteria for patients were age between 18 to 65 years, not treated with oral and topical steroids, immunosuppressants and vitamin D supplements, not undergoing current phototherapy and presence of chronic inflammatory diseases like systemic lupus erythematosus, multiple sclerosis, inflammatory disease and malignancy. For control subjects, all inclusion criteria were the same as for cases except for the absence of psoriasis. The study was approved by the ethical committee of BPKIHS, Dharan. Full written informed consent was obtained from all patients and control subjects.
Clinical and Laboratory Parameters
Details of age, sex, sun exposure per day, history of physical exercise, history of smoking, history of alcohol intake and associated disease were recorded for both patients and control subjects. Details of the duration of psoriasis, age at onset and positive family history were recorded. The severity of psoriasis was assessed according to the PASI.15 Fitz Patrick’s skin type was assessed. The weight, height and waist circumference were measured. Body mass index (BMI) was calculated.16 The blood pressure of the patient was taken after 5 minutes of rest and the average of two readings was recorded. Five milliliters of blood were drawn and fasting blood sugar (FBS), triglycerides (TAG) and high density lipoproteins (HDL) were analyzed. Serum 25(OH) D was assessed by chemiluminescent immunoassay in the biochemistry lab of our hospital and classified according to severity.17
Statistical Analysis
Data were entered in Microsoft Excel 2010 and were further analyzed in SPSS version 10.5. For epidemiological results, graphical and tabular presentations were done. Continuous variables were expressed as the mean (±SD) or as median whereas categorical variables were expressed as number (%). The normality of continuous variables was analyzed by using a Kolmogorov–Smirnov test. To compare the mean values of quantitative variables between the psoriasis patients and healthy controls, Student’s t-test was used. The qualitative variables were analyzed by Chi-squared test or Fisher’s exact test when one cell had an expected count of less than 5. A multivariable logistic regression analysis was done to measure the association between psoriasis and vitamin D insufficiency (<30ng/mL). Odds ratios (ORs) were determined by the Wald Chi-squared test and predictors with P <0.20 were assessed for multivariate analysis. P value of <0.05 was considered statistically significant.
Results
We studied 120 patients (63 males and 57 females) with chronic plaque psoriasis. The mean age was 42.93 ± 14.282 years and the median age 43.50 years. The duration of disease ranged from 1 month to 37 years with a mean duration of 5.69 years. The mean age at onset of disease was 37.05 years. A family history of psoriasis was mentioned by 16 (13.3%). The mean PASI was 1.92 (± 0.705). The majority of cases (60, 50%) had moderate disease while 35 (29.2%) and 25 (20.8%) had mild and severe disease respectively. Among 120 patients with psoriasis, scalp involvement was seen in 59 (49.2%), nail involvement was seen in 42 (35%), and palmoplantar involvement was seen in 18 (15%) cases. Flexural and genital involvement was seen in 5 (4.2%) and 2 (1.7%) of total cases only. Among those with nail involvement, pits were present in 26 (39.4%), subungual hyperkeratosis in 20 (30.3%), and onycholysis in 13 (19.7%). Other nail signs included oil droplet sign in 2 (3.03%), transverse ridging in 3 (4.54%), and leukonychia in 2 (3.03%) cases.
The control group subjects enrolled comprised 60 outpatient (32 males and 28 females) healthy volunteers who were the attendants of patients other than psoriasis. The two groups did not significantly differ in age, sex, Fitzpatrick skin phototype, sun exposure per day, history of smoking and alcohol intake, DBP, FBS, TAG, HDL, calcium (Table 1). But they significantly varied in BMI (OR 2.142, 95% CI 1.025–4.477, P= 0.043), SBP (OR 5.745, 95% CI, P= 0.022) and metabolic syndrome (OR 4.634, 95% CI 1.020–21.058, P=0.047).
 |
Table 1 Summary of Demographic Characteristics and Biochemical Parameters of Cases and Controls |
The mean serum 25(OH) D concentration in cases was 19.57 ± 6.85 ng/mL and the mean level of serum 25(OH) D level in control group was 23.63 ± 6.40 ng/mL and was statistically significant (p= 0.001) (Table 2) (Figure 1).Vitamin D deficiency (<20 ng/dl) was observed in 57.5% of patients with psoriasis versus 30% of control subjects (P<0.001, OR 3.720, 95% CI 1.890–7.322). Regarding vitamin D insufficiency (<30 ng/dl), it was observed among 87.5% of patients with psoriasis and 83.3% of control subjects. Multivariate studies with binary logistic regression showed a strong association between the presence of psoriasis and vitamin D deficiency (<20 ng/dl) even after adjusting for age, sex, BMI, sun exposure, physical exercise and Fitzpatrick skin phototype (OR 2.929, 95% CI 1.376–6.230, P<0.005) (Table 3).
 |
Table 2 Mean Vitamin D Level in Cases and Controls |
 |
Table 3 Multivariate Analysis of Cases and Controls |
 |
Figure 1 Comparision of mean serum 25–0H vitamin D concentration between psoriasis and control groups. |
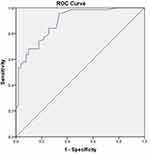
Among the cases with severe PASI, 25 (96%) had vitamin D deficiency and 1 normal vitamin D level. With moderate disease, 41 (71.9%), 12 (21.1%), and 4 (7%) had deficient, insufficient, and normal levels of vitamin D respectively. Ten (27%), 24 (64.9%), and 3 (8.1%) had a normal, insufficient, and deficient vitamin D in mild disease. A significant negative correlation was found between serum 25(OH) D level and PASI (r= −0.628, P= 0.01) (Figure 2). A receiver operating characteristic curve was performed and an optimal cutoff PASI value of 4.05 (AUC=0.889, P<0.001) was obtained. Above this value, patients had a high risk of vitamin D deficiency with the sensitivity of 84.1% and specificity of 82.4% (Figure 3). No significant differences were found in occupation, duration of sun exposure, history of smoking and alcohol intake, age at onset, duration of disease, associated comorbidities, BMI, presence of metabolic syndrome, SBP, DBP, FBS, TAG and serum calcium even after conducting a binary logistic regression model for vitamin D deficiency.
 |
Figure 2 Negative correlation between 25 OH vitamin D serum level and PASI (r = 0.628). |
Discussion
This study showed that serum 25(OH) D levels were significantly lower in psoriatic patients than in control subjects after adjusting for confounding factors. The serum 25(OH) D levels were negatively associated with PASI in multiple linear regression analysis. Patients with psoriasis with PASI greater than or equal to 4.05 were found to have a higher risk of vitamin D deficiency with high sensitivity and specificity. The strength of this study is that multiple confounding factors were taken into consideration along with biochemical parameters which can affect the serum level of vitamin D.
The presence of vitamin D receptors in keratinocytes was first established from in vitro study of cultured keratinocytes in 19882 and afterward many in vitro and clinical studies have well-documented the role of vitamin D in the proliferation and differentiation of keratinocytes. Vitamin D inhibits keratinocytes’ proliferation and induces their differentiation. Besides this, vitamin D has anti-inflammatory effects by downregulating the production of various pro-inflammatory cytokines.3 Low levels of vitamin D levels in psoriatic patients could be relevant in a few ways. It has been observed that low levels of vitamin D may have implications for the pathogenesis of the disease as well as its association with metabolic syndrome and associated comorbidities. In another way, it is still a matter of debate if it is one of the consequences of the chronic inflammatory state of the disease itself.
The major finding of this study is the significantly reduced serum vitamin D level among psoriasis patients in comparison with control groups even after adjusting for confounding factors as well as various cardiovascular risk factors. Similar findings were also put forward in studies by Orgaz Molina et al1 and Chandrasekhar et al.18 A similar finding was also observed in Prabha et al5 and Hassan et al’s6 studies conducted in India which has a similar geographical latitude as Nepal. But the limitation of these studies is that they did not consider the confounding factors and other cardiovascular risk factors that influence the serum 25(OH) D level greatly.
In our study, we observed a significant negative correlation between serum 25(OH) D level and PASI defining the severity of psoriasis. Similar observations have been made in a study conducted by Chandrasekhar et al18 where PASI was found to be correlated with inflammatory and oxidative parameters. Thus, this finding of our study points out that low vitamin D levels can be due to chronic systemic inflammation that is found to be associated with psoriasis. This study showed that there were significant differences between obesity and the presence of metabolic syndrome among psoriasis and control subjects, as has been put forward as a clear fact by recent reviews.19 But both obesity and metabolic syndrome had not been observed as independent predictors of hypovitaminosis D in multivariate linear regression analysis. Though a negative correlation between 25(OH) D and BMI has been revealed, the relationship between vitamin D deficiency and obesity as well as the underlying mechanisms like insulin resistance, low-grade systemic inflammation and oxidative stress are complex and need further investigation.
In conclusion, serum 25(OH) D levels are significantly lower in psoriatic patients than in healthy control subjects. These levels also vary according to the severity of the disease as determined by PASI in our study and those patients with PASI ≥ 4.05 are likely to have vitamin D deficiency with the sensitivity of 0.841 and specificity of 0.824. The strength of this study is that various confounding factors might affect serum levels of Vitamin D as well as various metabolic parameters. But the factors like use of sunblock and parathyroid hormone status that could affect vitamin D have not been assessed, which is one of the major limitations of this study. However, this study has strongly supported more interventional studies with oral supplementation of Vitamin D in psoriasis patients and emphasizes the need for further studies to explore the relationship of vitamin D deficiency with obesity and metabolic syndrome.
Abbreviations
25(OH) D, 25hydroxy vitamin D3; PASI, psoriasis area and severity index; BMI, body mass index; DM, diabetes mellitus; FBS, fasting blood sugar; HDL, high density lipoprotein; TAG, triacylglycerides; P, level of significance.
Ethics Approval
It has been received from Institutional Review Committee of BPKIHS, Dharan.
Acknowledgments
Sincere word of gratitude to Mr. Dharanidhar Baral and Dr. Rajesh MD for their support in statistical analysis in results.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Orgaz-Molina J, Buendía-Eisman A, Arrabal-Polo MA, et al. Deficiency of serum concentration of 25-Hydroxyvitamin D in psoriatic patients: a case-Control study. J Am Acad Dermatol. 2012;67(5):931–938. doi:10.1016/j.jaad.2012.01.040
2. Bikle DD, Gee E, Pillai S. Regulation of keratinocyte growth, differentiation, and vitamin D metabolism by analogs of 1,25-dihydroxyvitamin D. J Invest Dermatol. 1993;101(5):713–718. doi:10.1111/1523-1747.ep12371681
3. Craik C. NIH Public Access. Rev Endocr Metab Disord. 2012;23(1):1–7.
4. Pitukweerakul S, Thavaraputta S, Prachuapthunyachart S, et al. Hypovitaminosis D is Associated with Psoriasis: a Systematic Review and Metaanalysis. Kans J Med. 2019;12(4):103–108. doi:10.17161/kjm.v12i4.13255
5. Radha Raja Prabha K, Mohana Lakshmi PC, Chittambalam P, et al. Case control evaluation of serum vitamin D levels in psoriasis. IP Indian J Clin Exp Dermatol. 2019;5(1):1–5. doi:10.18231/2581-4729.2019.0001
6. Hassan R, Kallan F, Subramanian S, et al. Vitamin d deficiency in psoriatic patients -A case control study. IP Indian J Clin Exp Dermatol. 2019;5(1):37–40. doi:10.18231/2581-4729.2019.0009
7. Malavika S, Nirmali S. A study of serum vitamin D in psoriatic patients in a tertiary care hospital of Assam. Indian J Res. 2019;8(9):10–11.
8. Farnandes ADE, Listiawan MY, Evy Ervianti TS. The Analysis of Serum Vitamin D (25 [OH] D) Level in Psoriasis Patients Comparing with Control Subjects. Periodical DermatologyVenereol. 2020;32(2):111–118.
9. Mohammed Q, Mathkhor AJ, SKhudhairy A. Vitamin D deficiency in patients with psoriasis and psoriatic arthritis. Asian J Pharmaceutical Clin Res. 2020;13(11):168–170. doi:10.22159/ajpcr.2020.v13i11.39653
10. Nayak PB, Girisha BS, Noronha TM, et al. Low Vitamin D in psoriasis: reality or myth? Indian J Dermatol. 2018;63(3):255–260. doi:10.4103/ijd.IJD_35_18
11. Diniz S, Pinto JM, Marta M, et al. Serum Levels of 25-Oh Vitamin D in Psoriatic Patients and Control Subjects. JoJ Dermatology Cosmetics. 2019;1(4):65–68.
12. Allayali A, Niaz G, Al HK, et al. Association between Vitamin D Deficiency and Psoriasis: a Case-Control Study. J Clin Exp Dermatol Res. 2018;9(2):442. doi:10.4172/2155-9554.1000442
13. El-farargy SM, Ghanayem NM, Elrashidy AM. Correlation between vitamin D serum level and severity of psoriasis. Menoufia Med J. 2020;3:1016–1020.
14. Yosuf Abd Mallick AJ. Serum Concentration of 25-Hydroxy Vitamin D in Patients with Chronic Plaque Psoriasis:.A case control study. J Dow Univ Health Sci. 2020;14(2):47–53.
15. Feldman SR, Krueger GG. Psoriasis assessment tools in clinical trials. Ann Rheum Dis. 2005;64:65–68. doi:10.1136/ard.2004.031237
16. Nishida C, Barba C, Cavalli-Sforza T, et al. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363(9403):157–163.
17. Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911–1930. doi:10.1210/jc.2011-0385
18. Chandrashekar L, Krishna Kumari GR, Rajappa M, et al. 25-hydroxy Vitamin D and ischaemia-modified albumin levels in psoriasis and their association with disease severity. Br J Biomed Sci. 2015;72(2):56–60. doi:10.1080/09674845.2015.11666797
19. Singh S, Paulina Young AWA. An update on psoriasis and metabolic syndrome.A meta-analysis of observational studies. PLoS One. 2017;12:e0181039. doi:10.1371/journal.pone.0181039
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.