Back to Journals » Journal of Pain Research » Volume 16
Assessment of Ultrasound-Guided Continuous Low Serratus Anterior Plane Block for Pain Management After Hepatectomy: A Randomized Controlled Trial
Authors Jiang F, Wu A, Liang Y, Huang H, Tian W, Chen B, Liu D
Received 31 January 2023
Accepted for publication 6 July 2023
Published 14 July 2023 Volume 2023:16 Pages 2383—2392
DOI https://doi.org/10.2147/JPR.S406498
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jinlei Li
Fei Jiang,* Ailing Wu, Yan Liang, Hui Huang, Wei Tian, Bogang Chen, Di Liu*
Department of Anesthesiology, The First People’s Hospital of Neijiang, Neijiang, Sichuan, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Di Liu, Department of Anesthesiology, The First People’s Hospital of Neijiang, No. 1866 Han’an Dadao West Section, Shizhong District, Neijiang, Sichuan, People’s Republic of China, Tel +86 3541633502, Email [email protected]
Purpose: Adequate postoperative analgesia is a key to earlier recovery from open surgery. This work investigated the pain control and quality of patient recovery after hepatectomy to evaluate the modified continuous serratus anterior plane block (called low SAPB) for postoperative analgesia.
Patients and Methods: This single-center, blinded, randomized, controlled study included 136 patients who underwent hepatectomy under general anesthesia. For postoperative analgesia, the patients in the SAPB group were given a continuous low SAPB at the 7th intercostal space in the right mid-axillary line, and the patients in the control group were given continuous intravenous opioid analgesia. The numeric pain rating scale (NPRS) was used for pain assessment. The postoperative assessment focused on the remedial drug consumption, the occurrence of adverse postoperative analgesic reactions, and the quality of patient recovery evaluated with the QoR-15 questionnaire.
Results: Compared to the controls, the SAPB patients had significantly lower NPRS scores at 12 h and 24 h at rest and 6 h, 12 h, and 24 h in motion, and a longer time to first use of remedial analgesics at 24 h, and higher overall QoR-15 scores at 24 h [124 (121, 126) vs 121 (120, 124)] and 48 h [129 (126, 147) vs 126 (125, 128)], after surgery. There was no significant difference in the incidence of analgesia-related adverse reactions between the two groups.
Conclusion: The continuous low SAPB could achieve superior pain control, especially for motor pain, to intravenous opioid analgesia during the first 24 h post-surgery. Even with no significant difference in the incidence of postoperative adverse reactions, patients with continuous low SAPB appeared to have a higher quality of recovery in the first two days post-surgery than patients with continuous intravenous analgesia.
Keywords: liver resection, postoperative analgesia, serratus anterior plane block
Introduction
Liver cancer is one of the most frequent fatal malignancies that threaten human health.1 Liver resection remains the optimal choice for the treatment of liver cancer in current clinical practice.2 Due to the invasive procedure, drainage tube placement, liver regeneration following damage or resection, and inflammatory response, patients commonly experience moderate-to-severe acute pain (defined as a visual analogue scale score greater than 3) lasting several days after hepatectomy.3,4 The incidence of moderate-to-severe acute pain in patients on day 1 after hepatectomy is as high as 72%.4,5 The stress response to painful surgical trauma is associated with the increased release of inflammatory factors and hormones including cortisol and adrenocorticotropic hormones, which can induce cardiovascular and metabolic adverse effects. These pains reduce patients’ quality of life, raise postoperative stress, prolong surgical wound healing time, aggravate patients’ anxiety and depression, and even evolve into chronic postsurgical pain, which hinders the rapid recovery and rehabilitation process.5 As reported, 55–80% of patients undergoing hepatectomy have inadequate postoperative analgesia.6 Multimodal analgesia after open surgery is essential.4 Intravenous analgesia, epidural analgesia, and peripheral nerve blocks are common postoperative analgesia modalities in clinical practice.7–9 Systemic intravenous analgesia could contribute to side effects including respiratory depression, nausea, vomiting, and pruritus.10,11 Whereas sympathetic blockade (hypotension, bradycardia) or patient coagulation dysfunction limits its use, epidural analgesia may provide better pain management.5
Ultrasound-guided serratus anterior plane block (SAPB) provides accurate ultrasound localization for block catheter placement and has been gradually applied for pain management after upper abdominal surgery as a new fascial plane block technique in recent years.12–14 It was first described by Blanco in 2013 and has a high block success rate in contrast to thoracic epidural analgesia and paravertebral block. Ultrasound-guided SAPB not only reduces surgical stress response, inflammatory factor release, and opioid use15 but also avoids hemodynamic fluctuations and serious respiratory and intraspinal sequelae.12,16–18 In clinical practice, 30 mL of 0.25% ropivacaine is administered via an injection into the deep fascial plane under the serratus anterior muscle at the 7th intercostal space in the mid-axillary line for SAPB, which provides good analgesia to the anterolateral chest wall and upper abdominal wall. Analgesic evaluation of such a modified low SAPB after hepatectomy in terms of pain management and quality of patient recovery has not been reported to date. Therefore, this prospective, randomized, controlled study aimed to evaluate the clinical analgesic efficacy and safety of ultrasound-guided continuous low SAPB after hepatectomy to provide a clinical reference.
Materials and Methods
This prospective, randomized, controlled, single-blind trial was approved by the Institutional Ethics Committee (Approval number: 2021-01) of the First People’s Hospital of Neijiang (Sichuan Province, China) and was conducted following the principles outlined in the Declaration of Helsinki. This trial was preregistered on clinicaltrials.gov (Trial registration number: NCT05531864). Written informed consent was obtained from all participants for the enrolment and interventions in the study.
Participants and Grouping
The patients diagnosed with hepatocellular carcinoma who were scheduled for hepatectomy (open surgery) between June 18, 2021 and July 24, 2022 in our hospital were included in the present study. All diagnoses met the World Health Organization’s diagnostic criteria for liver cancer. The included patients were aged 25 to 75 years with an ASA (American Society of Anesthesiologists) level of I, II, or III, and a body mass index (BMI) of 18.5 to 29 kg/m2. Patients with cardiac, cerebral, hepatic, or renal failure, comorbid psychiatric or neuromuscular disorders, or coagulation disorders were excluded. Other exclusion criteria included a history of allergy to anesthetic drugs or local anesthetics, a history of long-term opioid or drug use, an inability to cooperate with the researchers in this study for various reasons, and participation in other synchronous clinical or drug trials.
Participants were randomized into two groups: the SAPB group (general anesthesia with continuous low SAPB) and the control group (general anesthesia with continuous intravenous opioid analgesia). Each patient was randomly assigned to the SAPB group or control group according to a random number table. Random numbers were generated using IBM SPSS 26.0 software. Group allocations were kept in serially numbered opaque envelopes and sealed. After baseline assessment and recruitment, one patient’s envelope was sent to an anesthetist. The anesthetist opened the envelope to determine the group allocation and performed the interventions pre-established for the indicated group. The anesthetist who performed the blocks did not participate in the intraoperative pain management of the patients.
General Anesthesia and Postoperative Analgesia
General anesthesia and surgical technique were the same for all patients included. General anesthesia was actively induced by administering 0.02 mg/kg midazolam, 0.4 μg/kg sufentanil, 0.3 mg/kg cis-atracurium, and 0.3 mg/kg etomidate, and maintained by propofol, remifentanil, cis-atracurium, and sevoflurane. In the SAPB group, continuous low SAPB and patient-controlled nerve analgesia (0.25% ropivacaine hydrochloride, 5 mL/h) were used. In the control group, patient-controlled continuous intravenous analgesia (100 mL dilution containing 2 μg/kg sufentanil, 0.25 mg/kg dezocine, and 10 mg toltestrone) was given. The analgesia pump parameters were set as background dose 2 mL/h, patient-controlled analgesia dose 0.5 mL, and locking time 15 min. At the end of the surgery, the patients were monitored in the recovery room and received pain assessment using the 11-point NPRS (the numeric rating scale for clinical pain measurement), which ranges from “0” (indicating “no pain”) to “10” (indicating “pain as bad as you can imagine”). When the postoperative NPRS score was greater than 3, remedial analgesia (100 mg tramadol) was administered by a ward physician who was blind to the grouping information.
SAPB patients were treated in the supine position with the right upper limb abducted at 90°, and the nerve block catheter was positioned at the 7th intercostal space in the right mid-axillary line, which could block the lateral cutaneous branches of the 4th to 11th intercostal nerves (T4–T11) and provide good analgesia to the anterolateral chest wall and upper abdominal wall. In detail, after routine skin preparation, the linear ultrasound transducer probe (S9, SonoScape, Shenzhen, China) was placed over the thoracic cage in a sagittal plane to identify the superficial latissimus dorsi and the deep serratus anterior muscle (as shown in Figure 1). Subsequently, using an in-plane approach, a nerve block puncture needle (Stimuplex, B. Braun Medical, Melsungen, Germany) was inserted caudally from the skin above the 6th or 7th rib to the 8th intercostal space in the right mid-axillary line, namely, through the latissimus dorsi and the serratus anterior muscle to reach the deep fascial plane between the serratus anterior muscle and the external intercostal muscles and ribs in the 7th intercostal space. About 2 mL of normal saline solution was used for the injection test. After a black appearance of fluid spreading caudally along the rib surface was visible on ultrasound and no blood or gas was found after pulling back the plunger, 30 mL of AstraZeneca 0.25% ropivacaine was injected into the deep fascial plane. Besides, a puncture needle was inserted caudally along the 6th, 7th, or 8th rib surface through the external and internal intercostal muscles in the 6th, 7th, or 8th intercostal space, respectively, to reach the plane between the internal and the innermost intercostal muscles. About 2 mL of 0.25% ropivacaine was injected into the plane each in the 6th, 7th, and 8th intercostal spaces after an injection test as above described. Afterward, a nerve block puncture needle was inserted caudally as above described, and the nerve block catheter was kept at the deep fascial plane (a depth of approximately 5 cm; T7) in the 7th intercostal space for continuous postoperative analgesia. At the end of one surgery, the patient-controlled analgesia pump (0.25% ropivacaine, 5 mL/h) was connected to the nerve block catheter. The patient-activated dose was 8 mL per time, and the lockout interval was set at one hour.
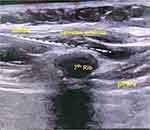 |
Figure 1 Sonographic anatomy of the low serratus anterior plane block. 7th rib, the seventh rib. |
Primary and Secondary Endpoints
The primary outcomes were the numeric pain rating scale (NPRS) scores at rest and in motion at various postoperative time points (0 h, 2 h, 6 h, 12 h, 24 h, and 48 h). The secondary outcomes included the quality of patient recovery after surgery which was evaluated with the global Quality of Recovery-15 (QoR-15) questionnaire,19 time to the first use of remedial analgesics (tramadol) after various postoperative analgesia interventions, adverse postoperative analgesic reactions, or nerve block-related complications (nausea, vomiting, etc.) within the postoperative 24 h.
Statistical Analysis
All data were collected by an Excel program and then analyzed using GraphPad Prism 9.0. The Shapiro–Wilk test was used to determine if the data followed a normal distribution. The normally distributed data (including age, height, BMI, duration of anesthesia, and duration of surgery) were expressed as mean ± standard deviation (SD) and analyzed using an independent samples t-test. Fisher’s exact test was used for non-normally distributed quantitative data (eg, gender). The skewed data were expressed as the median and interquartile range (IQR). The Mann–Whitney U-test was applied to detect the between-group differences in NPRS scores at 0 h, 2 h, 6 h, 12 h, 24 h, and 48 h after surgery. The relationship between qualitative variables and independent samples (eg, ASA classification) was analyzed using a chi-square test. P values <0.05 were considered statistically significant.
Results
After random allocation and several rounds of exclusion, a total of 136 participants were included in our analysis. Of the 139 patients who were eligible and underwent open surgery for hepatocellular carcinoma, there were two patients lost to follow-up, and one was excluded due to the occurrence of postoperative thrombosis (Figure 2). Basic information about the enrolled patients is detailed in Table 1.
 |
Table 1 Patient Demographic and Clinical Parameters |
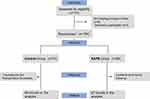 |
Figure 2 CONSORT (Consolidated Standards of Reporting Trials) flow diagram. The SAPB group underwent general anesthesia and continuous low serrate anterior plane block, while the control group underwent general anesthesia and continuous intravenous opioid analgesia. Abbreviations: SAPB, serratus anterior plane block; control, the control group. Notes: Adapted from Schulz KF, Altman DG, Moher D, CONSORT 2010 Statement: Updated Guidelines for Reporting Parallel Group Randomised Trials. PLoS Med. 2010;7(3): e1000251. Copyright: © 2010 Schulz et al. Creative Commons Attribution License.20 |
As shown in Table 2 and Figure 3, after various postoperative analgesia interventions, the patients with continuous low SAPB (SAPB group) had a significantly greater reduction in NPRS scores at rest and in motion compared to the control patients within 24 h post-surgery (p<0.05). In addition, we also used a survival curve to depict the time to the first use of remedial analgesics after various postoperative analgesia interventions (Figure 4). We found a significant difference between the two groups in the time to the first use of remedial analgesics (p=0.017). Besides, as shown in Table 3, there was no significant difference between the two groups in terms of complications such as incisional infection and hypotension (requiring intervention), implying that continuous low SAPB did provide better pain control on the first postoperative day than other analgesia modalities. The SAPB patients had fewer postoperative adverse reactions (nausea, vomiting, and pruritus) than the control patients.
 |
Table 2 Assessment of Numeric Pain Rating Scale Scores of the Two Groups at Different Postoperative Time Points |
 |
Table 3 Comparison of Incidence of Adverse Effects Between the Two Groups |
As shown in Figure 5, the SAPB patients had significantly higher QoR-15 scores at 24 h [124 (121, 126) vs 121 (120, 124)] and 48 h [129 (126, 147) vs 126 (125, 128)] post-surgery than the control patients; both groups of patients also had significantly higher QoR-15 scores in the first two postoperative days compared to their baseline levels (p<0.05). However, such significant differences were not maintained on the third postoperative day.
Discussion
This prospective, randomized, controlled study evaluated the analgesic effect of ultrasound-guided continuous low SAPB and the quality of patient recovery after hepatectomy. We found that ultrasound-guided continuous low SAPB significantly reduced pain at 12 h and 24 h post-surgery at rest and in motion and reduced the administration of remedial analgesics at 24 h post-surgery compared to intravenous analgesia alone. The patients with continuous low SAPB had higher global QoR-15 scores at 48 h post-surgery, indicating a clinically meaningful improvement in the quality of recovery. However, there was no statistically significant difference in analgesia-related adverse reactions between SAPB patients and control patients.
In the present study, the ultrasound-guided continuous low SAPB used as an analgesic protocol for postoperative pain management after hepatectomy is a modified SAPB modality. Unlike Blanco’s classical SAPB with the puncture points located at the 4th and 5th intercostal spaces in the mid-axillary line, the puncture points in our study were located above the 6th or 7th rib in the mid-axillary line, and the analgesic (ropivacaine) was injected into and the nerve block catheter was placed into the deep fascial plane between the deep surface of the serratus anterior muscle and external intercostal muscles and ribs at the 7th intercostal space in the mid-axillary line. Our SAPB modality used a lower concentration (0.25%) and a higher dose (30 mL) of ropivacaine. Our continuous low SAPB could maximize the spread of local anesthetic to the lateral cutaneous branches, mainly T4–T11, of the intercostal nerves. The hyperalgesia of dermatomal block levels T4–T11 can be examined in the lateral region of the abdomen at 30 min. Previous studies17,21–24 have shown that SAPB is more frequently used in thoracic anesthesia, and relatively few studies have reported its application in hepatectomy. Elsharkaw et al25 reported a case series of the application of rhomboid intercostal and sub-serratus plane blocks for postoperative pain management in upper abdominal surgery, which could better block the lateral cutaneous branches of the thoracic intercostal nerves and be used for thoracic and upper abdominal analgesia in various clinical situations. This block modality is highly similar to the SAPB. In the present study, we put forward our modified continuous low SAPB for postoperative analgesia. We chose the puncture point and the block catheter placement site more distant from the hepatic region to avoid their contributing surgical incisional contamination. Besides, the local anesthetic was injected into the plane between the internal and innermost intercostal muscles to achieve a selective block of the anterior cutaneous branch of the 6th, 7th, and 8th intercostal nerves. This would avoid unnecessary medical disputes caused by incision infections caused by the rectus abdominis sheath block. Our study showed that the block levels of ultrasound-guided unilateral SAPB at the 7th or 8th intercostal space were concentrated at T4–T11, which provided good postoperative analgesia of incisions for liver resection.
With our modified SAPB, the SAPB patients had significantly lower NPRS scores at rest and in motion than the control patients at 12 h and 24 h after hepatectomy for hepatocellular carcinoma. Besides, the SAPB patients had no significantly lower resting NPRS scores at 6 h post-surgery than the control patients, which may be attributed to the small sample size. In addition, the median NPRS scores at rest and in motion after surgery were less than 4 at most times in both groups, indicating clinically acceptable analgesia could be achieved in both groups using the multimodal analgesia protocol in this study. Of note, SAPB with ropivacaine alone could only control the incisional pain and did not improve the outcome of postoperative visceral pain, so flurbiprofen ester was administered intravenously 30 min before the end of surgery to achieve better postoperative analgesia.
With survival curve analysis, we investigated the time to the first use of remedial analgesia after various postoperative analgesia interventions in the two groups and found a statistically significant difference between the two groups. The SAPB patients required significantly less opioid analgesia after the same procedure since our study used a higher dose of local anesthetic (30 mL ropivacaine). Notably, the trigger points for postoperative analgesia intervention by the anesthetists differed between studies. The patients in our study had an NPRS score greater than 3 as the intervention threshold, and the other studies used different intervention thresholds and anesthetics. This may partially explain the significant difference in opioid use between the studies.
In any case, the administration of analgesics should not be the main criterion for assessing patient comfort in the perioperative period. Therefore, unlike previous studies that simply assessed postoperative pain severity and the use of postoperative remedial analgesics, this study also assessed patients’ QoR-15 scores on the three postoperative days, which is an internationally recognized and validated patient-centered instrument for assessing the quality of patient postoperative recovery. This study found that the SAPB patients had significantly higher QoR-15 scores on the first and second postoperative days than the control patients, and there was no difference in the scores on the third postoperative day between the two groups, mainly due to the good analgesic effect of SAPB on the first postoperative day and the reduction in the use of postoperative remedial analgesics and less resulting nausea and vomiting and other adverse effects, thus improving the quality of recovery and patient satisfaction.
Ropivacaine is a long-acting amide local anesthetic and has a longer duration of action, a more rapid onset of action, a higher maximal dose, and lower toxicity to the central nervous system and cardiovascular system compared with other local anesthetics. With the available reports and our clinical practice, this study selected 30 mL of 0.25% ropivacaine for ultrasound-guided low SAPB and 5 mL/h of 0.25% ropivacaine for continuous SAPB pump after surgery. It was found that ropivacaine could alter the growth, apoptosis, and proliferation of cancer cells through a variety of mechanisms.26–28 Ropivacaine reduced the mRNA levels of key cell cycle regulators and the expression of MK167, a marker of cell proliferation, in hepatocellular carcinoma cells.26 It could promote apoptosis and inhibit the development of hepatocellular carcinoma by targeting the IGF-1 R/PI3K/AKT/mTOR signaling pathway.27 It could also disrupt mitochondrial function and activate caspase-3 activity to promote apoptosis in hepatocellular carcinoma cells.28 Taken together, ropivacaine can inhibit the biological properties of tumors by blocking sodium channels and is suitable for analgesia after surgery for liver cancer.
Our study is a single-center study, and further confirmation of our findings in other centers is required, although the team of surgeons and anesthetists ensured the standardization and consistency of all procedures. Besides, due to ethical constraints, 0.9% saline was not used in this study as a negative control in control patients. As SAPB for postoperative analgesia after hepatic lobectomy is less studied at present, the low SAPB we applied was slightly modified from the traditionally described modality and was compared with patient-controlled analgesia in the present study to observe whether such SAPB was effective in postoperative analgesia. It may be better to compare it with the classical thoracic paravertebral nerve block for pain management after hepatectomy. Given the small sample size, larger relevant studies are warranted to assess the analgesic effect of SAPB more accurately in hepatocellular carcinoma patients undergoing hepatectomy and the quality of patient recovery.
Conclusion
In this work, our continuous low SAPB within the first 24 h post-surgery provided better postoperative analgesia compared to continuous intravenous analgesia. Continuous low SAPB appears to be superior to continuous intravenous analgesia regarding postoperative analgesia and quality of patient recovery from hepatectomy in the first two days post-surgery, while no significant difference exists between both in the incidence of postoperative adverse effects. More randomized controlled trials with larger samples need to be conducted to confirm these observations.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics Approval and Informed Consent
This prospective, randomized, controlled, single-blind trial was approved by the Institutional Ethics Committee (approval number: 2021-01) of the First People’s Hospital of Neijiang (Sichuan, China) and was conducted in accordance with the principles outlined in the Declaration of Helsinki. This trial was preregistered on clinicaltrials.gov (Trial registration number: NCT05531864).
Funding
This work was supported by the Research Program of the Sichuan Provincial Health Commission, Sichuan, China (No. 20PJ288), and the Sichuan Medical Association Youth Research Innovation Fund Project (No. Q22022).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Anwanwan D, Singh SK, Singh S, et al. Challenges in liver cancer and possible treatment approaches. Biochim Biophys Acta Rev Cancer. 2020;1873(1):188314. doi:10.1016/j.bbcan.2019.188314
3. Joshi GP, Kehlet H. Postoperative pain management in the era of ERAS: an overview. Best Pract Res Clin Anaesthesiol. 2019;33(3):259–267. doi:10.1016/j.bpa.2019.07.016
4. Agarwal V, Divatia JV. Enhanced recovery after surgery in liver resection: current concepts and controversies. Korean J Anesthesiol. 2019;72(2):119–129. doi:10.4097/kja.d.19.00010
5. Dieu A, Huynen P, Lavand’homme P, et al. Pain management after open liver resection: procedure-specific postoperative pain management (PROSPECT) recommendations. Reg Anesth Pain Med. 2021;46(5):433–445. doi:10.1136/rapm-2020-101933
6. Chou R, Gordon DB, De Leon-Casasola OA, et al. Management of postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain. 2016;17(2):131–157. doi:10.1016/j.jpain.2015.12.008
7. Mungroop TH, Geerts BF, Veelo DP, et al. Fluid and pain management in liver surgery (MILESTONE): a worldwide study among surgeons and anesthesiologists. Surgery. 2019;165(2):337–344. doi:10.1016/j.surg.2018.08.013
8. Dudek P, Zawadka M, Andruszkiewicz P, et al. Postoperative analgesia after open liver surgery: systematic review of clinical evidence. J Clin Med. 2021;10(16):3662. doi:10.3390/jcm10163662
9. Zhou L, Huang J, Chen C. Most effective pain-control procedure for open liver surgery: a network meta-analysis. ANZ J Surg. 2018;88(12):1236–1242. doi:10.1111/ans.14456
10. Salicath JH, Yeoh EC, Bennett MH. Epidural analgesia versus patient-controlled intravenous analgesia for pain following intra-abdominal surgery in adults. Cochrane Database Syst Rev. 2018;8(8):Cd010434. doi:10.1002/14651858.CD010434.pub2
11. Bongiovanni T, Lancaster E, Ledesma Y, et al. Systematic review and meta-analysis of the association between non-steroidal anti-inflammatory drugs and operative bleeding in the perioperative period. J Am Coll Surg. 2021;232(5):765–790.e1. doi:10.1016/j.jamcollsurg.2021.01.005
12. Tiwari AK, Mar AA, Fairley MA. Serratus anterior plane block for upper abdominal incisions. Anaesth Intensive Care. 2019;47(2):197–199. doi:10.1177/0310057x19842461
13. FernÁndez MT, LÓpez S, Aguirre JA, et al. Serratus intercostal interfascial plane block in supraumbilical surgery: a prospective randomized comparison. Minerva Anestesiol. 2021;87(2):165–173. doi:10.23736/s0375-9393.20.14882-x
14. Fernández Martín MT, López Álvarez S, Mozo Herrera G, et al. Ultrasound-guided cutaneous intercostal branches nerves block: a good analgesic alternative for gallbladder open surgery. Rev Esp Anestesiol Reanim. 2015;62(10):580–584. doi:10.1016/j.redar.2015.02.011
15. Lillemoe HA, Marcus RK, Day RW, et al. Enhanced recovery in liver surgery decreases postoperative outpatient use of opioids. Surgery. 2019;166(1):22–27. doi:10.1016/j.surg.2019.02.008
16. Semyonov M, Fedorina E, Grinshpun J, et al. Ultrasound-guided serratus anterior plane block for analgesia after thoracic surgery. J Pain Res. 2019;12:953–960. doi:10.2147/jpr.S191263
17. Finnerty DT, Mcmahon A, Mcnamara JR, et al. Comparing erector spinae plane block with serratus anterior plane block for minimally invasive thoracic surgery: a randomised clinical trial. Br J Anaesth. 2020;125(5):802–810. doi:10.1016/j.bja.2020.06.020
18. Blanco R, Parras T, Mcdonnell JG, et al. Serratus plane block: a novel ultrasound-guided thoracic wall nerve block. Anaesthesia. 2013;68(11):1107–1113. doi:10.1111/anae.12344
19. Myles PS, Shulman MA, Reilly J, et al. Measurement of quality of recovery after surgery using the 15-item quality of recovery scale: a systematic review and meta-analysis. Br J Anaesth. 2022;128(6):1029–1039. doi:10.1016/j.bja.2022.03.009
20. Schulz KF, Altman DG, Moher D, CONSORT Statement: Updated Guidelines for Reporting Parallel Group Randomised Trials. PLoS Med. 2010;7(3):e1000251.
21. Jack JM, Mclellan E, Versyck B, et al. The role of serratus anterior plane and pectoral nerves blocks in cardiac surgery, thoracic surgery and trauma: a qualitative systematic review. Anaesthesia. 2020;75(10):1372–1385. doi:10.1111/anae.15000
22. Kim D-H, Oh YJ, Lee JG, et al. Efficacy of ultrasound-guided serratus plane block on postoperative quality of recovery and analgesia after video-assisted thoracic surgery: a randomized, triple-blind, placebo-controlled study. Anesth Analg. 2018;126(4):1353–1361. doi:10.1213/ane.0000000000002779
23. Fernández Martín MT, López Álvarez S, Fajardo Pérez M, et al. Serratus-intercostal interfascial plane block: alternative analgesia for open nephrectomy? Minerva Anestesiol. 2018;84(7):872–873. doi:10.23736/s0375-9393.18.12808-2
24. Xiao Y-K, She S-Z, Xu L-X, et al. Serratus anterior plane block combined with general analgesia and patient-controlled serratus anterior plane block in patients with breast cancer: a randomized control trial. Adv Ther. 2021;38(6):3444–3454. doi:10.1007/s12325-021-01782-y
25. Elsharkawy H, Maniker R, Bolash R, et al. Rhomboid intercostal and subserratus plane block: a cadaveric and clinical evaluation. Reg Anesth Pain Med. 2018;43(7):745–751. doi:10.1097/aap.0000000000000824
26. Le Gac G, Angenard G, Clément B, et al. Local anesthetics inhibit the growth of human hepatocellular carcinoma cells. Anesth Analg. 2017;125(5):1600–1609. doi:10.1213/ane.0000000000002429
27. Zhang R, Lian Y, Xie K, et al. Ropivacaine suppresses tumor biological characteristics of human hepatocellular carcinoma via inhibiting IGF-1R/PI3K/AKT/mTOR signaling axis. Bioengineered. 2021;12(2):9162–9173. doi:10.1080/21655979.2021.1995103
28. Wang W, Zhu M, Xu Z, et al. Ropivacaine promotes apoptosis of hepatocellular carcinoma cells through damaging mitochondria and activating caspase-3 activity. Biol Res. 2019;52(1):36. doi:10.1186/s40659-019-0242-7
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



