Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 17
Assessment of the Negative Factors for the Clinical Outcome in Patients with SARS-CoV-2 Infection and Type 2 Diabetes Mellitus
Authors Albai O, Braha A, Timar B, Sima A, Deaconu L, Timar R
Received 15 November 2023
Accepted for publication 13 January 2024
Published 22 January 2024 Volume 2024:17 Pages 271—282
DOI https://doi.org/10.2147/DMSO.S447835
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Juei-Tang Cheng
Oana Albai,1– 3 Adina Braha,1,2 Bogdan Timar,1– 3 Alexandra Sima,1– 3 Loredana Deaconu,1 Romulus Timar1– 3
1Department of Second Internal Medicine- Diabetes, Nutrition, Metabolic Diseases, and Systemic Rheumatology, ”victor Babes” University of Medicine and Pharmacy, Timisoara, 300041, Romania; 2Department of Diabetes, Nutrition and Metabolic Diseases Clinic, ”Pius Brînzeu” Emergency Clinical County University Hospital, Timisoara, 300723, Romania; 3Centre for Molecular Research in Nephrology and Vascular Disease/MOL-NEPHRO-VASC, ”Victor Babes” University of Medicine and Pharmacy, Timisoara, 300041, Romania
Correspondence: Adina Braha, Department of Second Internal Medicine- Diabetes, Nutrition, Metabolic Diseases, and Systemic Rheumatology, Victor Babes” University of Medicine and Pharmacy, 2 Eftimie Murgu Square, Timisoara, 300041, Romania, Email [email protected]
Purpose: Patients with diabetes mellitus (DM) are more susceptible to viral and bacterial infections, facing a more severe prognosis and higher mortality rates. The study’s main aim was to evaluate the survival and mortality rates of patients with type 2 diabetes (T2DM) and SARS-CoV-2 virus infection alongside the main factors influencing the prognosis.
Patients and Methods: The present study included 186 patients with T2DM and SARS-CoV-2 virus infection admitted to the COVID-19 Department of the “Pius Brînzeu” Emergency Clinical County University Hospital between November 2020 and March 2021. Patients had investigations performed upon arrival in the emergency room and during hospitalization. We analyzed the risk of negative prognosis based on clinical data (oxygen saturation (SatO2), respiratory rate (RR), lung damage), glycemic control (HbA1c, glycemia at hospital admission), and the duration of T2DM.
Results: The mortality rate in the studied group was 36.6%. All deceased patients had previously been diagnosed with hypertension; 95.58% had a body mass index (BMI) greater than 25 kg/m2, and 79.41% presented with cardiovascular disease (CVD). Compared to those who recovered, statistically significant differences were observed in BMI, glycemic levels at admission, glycosylated hemoglobin levels (HbA1c), SatO2, RR, and lung damage. Valid statistically significant predictors for death in T2DM patients with COVID-19 were hyperglycemia at admission > 198mg/dl, HbA1c> 8.6%, and SatO2≤ 87%.
Conclusion: SatO2, glycemia at hospital admission, and HbA1c had the highest sensitivity and specificity to predict the prognosis of T2DM patients with SARS-CoV-2 infection. Glycemic control is essential in the prognosis of patients with DM and COVID-19 infection. The prognosis was worse if other comorbidities were associated, especially hypertension and CVD.
Keywords: SARS-CoV-2 infection, type 2 diabetes mellitus, negative prognostic factors, mortality rate
Introduction
On March 11, 2020, the World Health Organization (WHO) declared the Coronavirus Infectious Disease 2019 (COVID-19) a global pandemic due to the rapid spread of this virus. Three years after the first case of COVID-19 was identified in the city of Wuhan in China, several questions related to the SARS-CoV-2 virus need to be answered.1,2 Worldwide, nearly 700 million people have been affected, and approximately 7 million have died, causing a severe crisis in many areas.3
Some individuals have been more severely affected by the new SARS-CoV-2 coronavirus, namely the elderly, those with chronic diseases, respiratory diseases, cardiovascular diseases (CVD), DM, and even those with obesity presenting a higher risk of developing severe forms, even death.4–6 T2DM represent an independent predictor for morbidity and mortality in patients with SARS-CoV-2 infection. Patients with obesity frequently present respiratory dysfunction, with changes in respiratory mechanisms, altered gas exchange, and decreased muscle strength. They risk developing more frequent severe pneumonia associated with hypoventilation and pulmonary hypertension than those without obesity. Obesity is also associated with DM, CVD, renal impairment, comorbidities that increase susceptibility to cardiovascular events, infection, and an altered immune response.7–10
Like all other coronaviruses, SARS-CoV-2 has four structural proteins: E (coat protein), M (membrane protein), N (nucleocapsid protein), S (spike protein), and eight accessory proteins. The spike surface glycoprotein plays an essential role by promoting the attachment of the virus to its receptor on host cells. Thus, it can determine host tropism and transmissibility.11
SARS-CoV-2 uses ACE-2 as a cellular entry receptor. The ACE-2 receptor is a type I transmembrane glycoprotein (mono-carboxypeptidase) composed of 805 amino acids. The first step of the viral entry process is represented by binding the N-terminal portion of the S1 viral protein to a site of the ACE-2 receptor.12,13 After entering the host cell, the spike protein of SARS-CoV-2 is cleaved by cellular proteases, causing fusion of the viral and cellular membranes. Priming the protein spike by the transmembrane protease, serine 2 (TMPRSS2), is essential for SARS-CoV-2 infection and spreads throughout the body.14–16
ACE-2 receptors are present in the heart (coronary artery endothelium, myocytes, fibroblasts, epicardial adipocytes), blood vessels (endothelial and smooth vascular cells), intestinal epithelial cells, lungs (tracheal and bronchial epithelial cells, type 2 pneumocytes, macrophages), kidneys (luminal surface of tubular cells), testis and brain. The large surface area of alveolar epithelial cells could explain the increased vulnerability and severe consequences of virus invasion in the lungs.17–23 By binding the ACE-2 receptors in the pancreas, the SARS-CoV-2 virus could change carbohydrate metabolism. Another possible explanation is the body’s exaggerated response, with high antibody production that excessively acts on essential organs to maintain a normal blood glucose level.10,24–26
Our study aimed to evaluate the mortality and recovery rate of the negative prognostic factors in patients with T2DM and COVID-19. We analyzed the comorbidities and the cardiometabolic parameters in those who died and recovered.
Materials and Methods
We identified 1207 adult patients hospitalized in the COVID-19 Department of the “Pius Brînzeu” Emergency Clinical County University Hospital in Timisoara between November 2020 and March 2021 for a retrospective, non-randomized, non-interventional study. Patients were included in the final analysis based on the inclusion criteria: age > 18 years, previous T2DM diagnosis, confirmed SARS-COV2 infection through real-time reverse transcription–polymerase chain reaction (RT–PCR) method, pulmonary clinical signs (acute or clinically manifest respiratory failure, lung damage on the computer tomography (CT)). Patients without pulmonary clinical signs or previously known T1DM were excluded from the analysis (Figure 1). Assuming a diabetes prevalence of 10.5%, we calculated that a minimum of 145 sample size is needed to reach a confidence level of 95%. The informed consent was waived due to the retrospective study design. The study followed the ethical principles of the current Helsinki Declaration (2013 version) for medical research on human subjects and was approved by the Ethical Committee of “Pius Brînzeu” Emergency Clinical County University Hospital (416/15.11.2023), respecting the confidentiality of patients’ personal data, according to General Data Protection Regulation (GDPR) Compliance.
 |
Figure 1 Diagram of the study design. |
The patients were evaluated in the hospital’s emergency department before admission. The evaluation consisted of laboratory analyses, RT–PCR SARS-COV2, and pulmonary CT interpreted by artificial intelligence to establish the lung damage of COVID-19. All the clinical data of interest in this study were extracted from the clinical observation sheets of hospitalized patients: age, sex, BMI, diabetes duration, RR, oxygen saturation levels at admission (SatO2), lung damage, admission glycemia (randomly measured), HbA1c, C-reactive protein (CRP), fibrinogen, blood count, D-Dimers, associated comorbidities, and the weather patients were vaccinated for COVID-19 (data about type of vaccine or doses were not available), or required ventilatory support in intensive care units, were collected. The associated conditions sought were hypertension, CVD, heart failure, bronchial asthma, chronic obstructive pulmonary disease (COPD), cancers, and chronic kidney disease.
Statistical Analysis
Statistical analysis was performed using SPSS statistics software (Statistical Package for the Social Science version 26, for Windows; SPSS Inc., Chicago, IL, USA). The continuous variables were presented as mean and standard deviation, median with minimum, and maximum values based on their distribution. Categorical variables were presented as absolute values and percentages. For the comparison of two sets of continuous, non-normal distributed variables, the Mann–Whitney test was applied. The chi-squared or Fisher’s exact test was used to compare categorical variables. To analyze the distribution of different grades of weight status among survivors or deceased groups, Chi-squared for trend was used. Statistical logistic regression analysis was used to identify death predictors. We performed receiver operating characteristic (ROC) analysis to evaluate the performance of predictive factors for evaluating the accuracy of logistic regression models that classify subjects as recovered or deceased. The area under the receiver operating characteristic (AUROC) was presented to show how much the model could distinguish between those two groups. We calculated the risk ratio of death in patients exposed to the SARS-CoV-2 vaccine prior to infection and the odds ratio for death in vaccinated and unvaccinated groups. Missing data were introduced as empty cells and ignored by the statistical analysis program. Variables with more than 5% missing data in the studied group were not considered for analysis. A p-value < 0.05 was considered statistically significant.
Results
The final analysis included 186 patients, 54.3% men (101/186) and 45.7% women (85/186), with a mean age of 68±10 years and a median diabetes duration of 8 years. 35.4% of the subjects required intensive care during hospitalization. Regarding other comorbidities, 98.3% were hypertensive, and 96.7% had a BMI over 25 kg/m2. Also, six patients (3.2%) were diagnosed with cancers (Table 1).
 |
Table 1 The Main Characteristics of the Patients |
The mortality rate in the study group was 36.6% (68/186), p< 0.0001, with a similar rate among both men and women (Table 2). Analyzing the people who died, compared to those who recovered, we observed statistically significant differences in age, BMI, glycemia at admission, HbA1c, SatO2, RR, and lung damage (Table 2). Deceased patients were older, with a higher BMI, higher glycemia at admission in the hospital, poorer glycemic control according to HbA1c, higher CRP levels, tachypneic, with more severe lung damage and acute respiratory failure, and required ventilatory support in intensive care units in a higher percentage (96.7%, p< 0.0001, Table 2) compared to recovered patients. Also, 43% of analyzed patients were vaccinated against SARS-CoV-2 infection. The mortality rate among vaccinated patients was 30.9% (21/68). In contrast, among the unvaccinated ones, the mortality rate was significantly higher at 69.1% (p< 0.0001). Overall, the survival rate was significantly higher for patients who received the COVID-19 vaccine; the relative risk of death was 0.59, p= 0.01, and the odds ratio was 0.44, p= 0.01.
 |
Table 2 Comparison of Anthropometric, Clinical, and Biological Parameters in Recovered Patients, Compared to Deceased |
Most patients have been previously diagnosed with hypertension at a similar rate regardless of gender (p< 0.3). However, 79.4% of the deceased subjects had CVD, compared to a lower percentage of 50.8% in the survivor group (p< 0.001). The distribution of weight status was similar in both groups for normal weight, overweight, or obesity grade (p= 0.8, chi-squared for trend). Of the patients who survived, only one patient was diagnosed with cancer; compared to the deceased group, there was a higher cancer rate (p= 0.02), and 16.1% had known lung disease, similar to deceased subjects (p= 0.1). The frequency of comorbidities in the studied group is presented in Table 3.
 |
Table 3 The Frequency of the Patient’s Comorbidities in Patients Who Recovered Compared to Those Deceased |
To evaluate the association of death with possible predictive factors included in the analysis, measured on a continuous scale, we built univariate and multivariate logistic regression models with potential risk factors such as SatO2, lung damage, glycemia at admission, HbA1c, RR, BMI and age, respectively outcome, death. The results indicated that for every increase of 1% in SatO2, the probability of death decreases by 54% (Nagelkerke R2= 0.88). All the other potential risk factors were directly associated with the relative risk of death, as presented in Table 4.
 |
Table 4 Logistic Univariate Regression Analysis of Possible Risk Factors for Death in Patients with SARS-COV2 Infection and T2DM |
To see to what extent the above factors influence the risk of death, we performed a multiple logistic regression analysis, in which the dependent variable was patients who died. The independent variables were the factors mentioned in Table 4. We included the variables if p<0.05 and gradually eliminated if p> 0.1. In the regression equation, SatO2 and glycemia at admission were retained in the model as valid predictors, with Nagelkerke R2= 0.95 (Table 5).
 |
Table 5 Multiple Logistic Regression Analysis of Possible Risk Factors for Death in Patients with SARS-COV2 Infection and T2DM |
We constructed ROC models to evaluate the possibility of predicting death based on possible factors studied, for which the outcome was death. SatO2, glycemia at hospital admission, and HbA1c had the highest sensitivity and specificity (Figures 2–4). A glycemia >198 mg/dl at admission represents a statistically significant predictive factor of death, with a sensitivity of 82.4 and specificity of 92.4, according to the ROC curve (AUROC 0.880, p< 0.001, Figure 2).
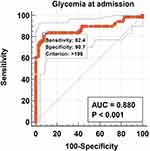 |
Figure 2 Graphical representation of the ROC curve of the glycemia at hospital admission for the prediction of death. |
 |
Figure 3 Graphical representation of the ROC curve of the HbA1c for the prediction of death. |
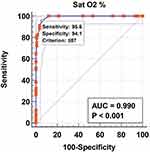 |
Figure 4 Graphical representation of the ROC curve of the SatO2 for the prediction of death. |
According to the ROC curve, an HbA1c > 8.6% represents a statistically significant predictive factor of death, with a sensitivity of 75.0 and specificity of 92.4 (AUROC 0.846, p< 0.001, Figure 3).
SatO2≤ 87% at admission in the hospital represents a statistically significant predictive factor of death, with a sensitivity of 95.6 and specificity of 94.1, according to the ROC curve (AUROC 0.990, p< 0.001, Figure 4).
Antidiabetic medications were tested as possible predictive factors of the outcome, but the analysis could not be performed due to the small number of patients treated with different combinations between the drug classes.
Discussion
In the present research, we analyzed the demographic, clinical, biological characteristics, associated comorbidities, and antidiabetic medications of T2DM patients and pulmonary insufficiency due to the infection with SARS-CoV2 in a Romanian cohort. We identified the predictors of in-hospital death of these patients. We identified an HbA1c> 8.6%, with a random glycemia at hospital admission above 198 mg/dL and SatO2≤ 87% at admission in the hospital, representing statistically significant predictive factors of in-hospital death.
It is known that vulnerable people (elderly, those with immune depression, comorbidities like cancers, DM, hypertension, CVD, and kidney disease) have a higher risk of infections and developing severe forms of the disease.27,28
Hyperglycemia occurs in approximately 25–30% of patients without DM, in critical conditions, especially under stress, through many mechanisms: the release of counter-regulatory hyperglycemic hormones, changes in insulin receptors caused by the inflammation process, and reduction of insulin secretion in pancreatic β-cells.29 It has been shown that some Coronaviridae, such as SARS-CoV-1 or MERS-CoV, destroy Langerhans cells with the activation of the enzyme dipeptidyl peptidase 4 (DPP-4), thus causing the inhibition of insulin secretion.30–32 Hyperglycemia was also detected in more than 50% of patients without previous metabolic disorders and infected with the SARS-CoV-2 virus.33–35 Regardless of the pathophysiological mechanism involved, stress-induced hyperglycemia by an exaggerated inflammatory response is associated with endothelial dysfunction and increased oxidative stress due to the production of free radicals. These changes induce a prothrombotic state that causes cellular and tissue damage, especially in critical patients with multiple comorbidities.36–38 In our study, patients with CVD and hypertension had a severe outcome and higher mortality rate. Patients who died had greater than 50% lung damage and SatO2 less than 80%.
Numerous studies have demonstrated that stress hyperglycemia detected at hospital admission was associated with a worse prognosis.39 The negative prognosis can be explained by the fact that ACE-2 is the main entry receptor for SARS-CoV-2 infection, and ACE-2 glycosylation is induced by hyperglycemia.40,41 This was also observed by Faldini et al in a retrospective analysis of 413 patients with COVID-19, in which hyperglycemia on admission was directly correlated with clinical severity and poor prognosis, especially in patients without previous DM.42 Similar data were also demonstrated by Copelli et al.43
In a multivariate logistic regression analysis, Liu et al demonstrated that fasting glycemia is an independent risk factor for severe diseases.44 Smith et al found that glycemia on hospital admission was significantly higher in patients who required oro-tracheal intubation than those who did not require ventilatory support.45 In our group, patients who died had a statistically significant higher glycemia at hospital admission than those who recovered: 309 mg/dL compared to 138 mg/dL.
Metabolic imbalance and impaired immune system increase the susceptibility of DM patients to SARS-CoV-2 infection. A more profound knowledge of the pathophysiology of COVID-19, with the identification of metabolic mechanisms, is essential to provide specific ways to prevent and improve the consequences of SARS-CoV-2 infection.46,47
A significant increase in glycemic values was observed in patients with previously good glycemic control and COVID-19 infection, needing treatment with insulin therapy in higher doses. This suggests the possibility of pancreatic invasion by SARS-CoV-2. Possible mechanisms that cause damage to the pancreas are the direct cytopathic effect of SARS-CoV-2 replication, systemic response to respiratory failure, and the harmful immune response induced by SARS-CoV-2 infection.48 Hyperglycemia activates neutrophils, contributing to the cytokine storm and sepsis in COVID-19, confirmed by increased inflammatory markers (erythrocyte sedimentation rate, CRP, ferritin, fibrinogen, D-dimers, lactate dehydrogenase).49 A similar study shows strong positive correlations between high white blood cell counts at admission and poor outcomes. Therefore, to predict in-hospital mortality in these patients, a panel of investigations (total blood count, CRP, ferritin, systemic immune-inflammation index) is recommended.50
In our study, in logistic univariate regression models, we found that BMI and age are possible risk factors for in-hospital death of the study patients. Our subjects had a median BMI of 30 kg/m2, about 35% needed intensive therapy. Similarly, in another study, COVID-19 patients with diabetes and grade I obesity were admitted to the ICU.51 The relation between diabetes, obesity, and COVID-19 could be explained by the upregulation of ACE2 expression52 and altered lipid synthesis and clearance, which could accelerate the inflammatory response in these patients.53
Age was a contributing factor to the worse outcome in the present research. Subjects who died during the hospital admission were significantly older than the survivors, with a mean difference of 2.5 years (p< 0.01). However, no differences across genders were observed. In an epidemiological research of 72,314 cases, 44.1% of them were elderly above 60 years old, with an increased mortality rate directly proportionally with age.54 Researchers suggest that age, frailty, and diabetes represent the triad that aggravates the prognosis of a COVID-19 infection because of the aging immune system that makes the patient more susceptible to a severe form of infection.54 In our study, deceased patients presented significantly higher values of CRP 135 mg/dL at the hospital admission compared to the recovered patients at 96.5 mg/dL (p= 0.003).
Although vaccination for preventing severe outcomes in COVID-19 was highly recommended, the vaccination status did not influence the outcome in our analysis. However, no conclusion could be drawn since we could not collect the exact data about the type of vaccine, doses, and immune response to the vaccine prior to infection with SARS-COV2.
The present study has several limitations to a lesser or greater extent. The single-center study design was retrospective observational on a relatively small number of patients. Based on the literature and policy, patients were treated according to the standard of care for their comorbidities and to the hospital protocol for COVID-19. The duration of specific symptoms before presenting at the hospital could have also been a confounding factor in the outcome. Also, data about previously administered vaccines were not available. Moreover, some patients could have been coinfected with a bacteria that influenced the prognosis.
Conclusion
SatO2, glycemia at hospital admission, and HbA1c had the highest sensitivity and specificity in predicting the prognosis of T2DM patients with SARS-CoV-2 infection. Glycemic control is essential in the prognosis of DM patients with COVID-19 infection. An optimal glycemic control may cause a lower release of inflammatory cytokines and a lower ability to bind ACE-2 to the virus, leading to improved prognosis in those affected by the SARS-CoV-2 virus. Also, SatO2 and lung damage are extremely useful factors for assessing COVID-19 prognosis. The prognosis of patients with T2DM and COVID-19 infection is worse if other comorbidities are associated, especially hypertension and CVD.
Acknowledgments
The authors gratefully acknowledge the professionals from the Diabetes, Nutrition and Metabolic Diseases Clinic, ”Pius Brînzeu” Emergency Clinical County University Hospital Timisoara, Romania, for their support in data acquisition.
Disclosure
The authors report no conflicts of interest in this work.
References
1. World Health Organization. WHO Director-General’s opening remarks at the media briefing on COVID-19 –; 2020. Available from: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020.
2. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi:10.1056/NEJMoa2001017
3. Worldometer. COVID-19: coronavirus pandemic; 2021. Available from: https://www.worldometers.info/coronavirus.
4. Jordan RE, Adab P, Cheng KK. Covid‐19: risk factors for severe disease and death. BMJ. 2020;368:m1198. doi:10.1136/bmj.m1198
5. Abbas AM, Sayed R, Omar F, Ahmed L. Bidirectional relationship between COVID‐19 and diabetes. AJBSR. 2020;9(6):424–426. doi:10.34297/AJBSR.2020.09.001442
6. Kalligeros M, Shehadeh F, Mylona EK, et al. Association of obesity with disease severity among patients with COVID‐19. Obesity. 2020;28(7):1200–1204. doi:10.1002/oby.22859
7. Abbas AM, Fathy SK, Fawzy AT, Salem AS, Shawky MS. The mutual effects of COVID-19 and obesity. Obes Med. 2020;19:100250. doi:10.1016/j.obmed.2020.100250
8. Youssef ME, Yahya G, Popoviciu MS, Cavalu S, Abd-Eldayem MA, Saber S. Unlocking the Full Potential of SGLT2 Inhibitors: expanding Applications beyond Glycemic Control. Int J Mol Sci. 2023;24(7):6039. doi:10.3390/ijms24076039
9. Iacobellis G, Penaherrera CA, Bermudez LE, Mizrachi EB. Admission hyperglycemia and radiological findings of SARS-COv2 in patients with and without diabetes. Diab Res Clin Pract. 2020;164:108185. doi:10.1016/j.diabres.2020.108185
10. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID‐19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):
11. Walls AC, Park YJ, Tortorici MA, Wall A, McGuire AT, Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181(2):281–92 e286. doi:10.1016/j.cell.2020.02.058
12. Tipnis SR, Hooper NM, Hyde R, Karran E, Christie G, Turner AJ. A human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase. J Biol Chem. 2000;275(43):33238–33243. doi:10.1074/jbc.M002615200
13. Donoghue M, Hsieh F, Baronas E, et al. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ Res. 2000;87:E1–E9.
14. Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181(2):271–280.e8. doi:10.1016/j.cell.2020.02.052
15. Hussain M, Jabeen N, Raza F, et al. Structural variations in human ACE2 may influence its binding with SARS-CoV-2 spike protein. J Med Virol. 2020;92(9):1580–1586. doi:10.1002/jmv.25832
16. Glowacka I, Bertram S, Muller MA, et al. Evidence that TMPRSS2 activates the severe acute respiratory syndrome coronavirus spike protein for membrane fusion and reduces viral control by the humoral immune response. J Virol. 2011;85(9):4122–4134. doi:10.1128/JVI.02232-10
17. Li M-Y, Li L, Zhang Y, Wang X-S. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect Dis Poverty. 2020;9(1):45. doi:10.1186/s40249-020-00662-x
18. Popoviciu MS, Kaka N, Sethi Y, Patel N, Chopra H, Cavalu S. Type 1 diabetes mellitus and autoimmune diseases: a critical review of the association and the application of personalized medicine. J Pers Med. 2023;13(3):422. doi:10.3390/jpm13030422
19. Batlle D, Wysocki J, Satchell K. Soluble angiotensin-converting enzyme 2: a potential approach for coronavirus infection therapy? Clin Sci. 2020;134(5):543–545. doi:10.1042/CS20200163
20. Crețu OM, Dan RG, Blidişel IAC, Sima LV, Munteanu M, Păun I. Hemobilia through aneurysm of the right hepatic artery, 22 months after laparoscopic cholecystectomy: case presentation. Rom J Morphol Embryol. 2017;58(1):197–199.
21. Patel VB, Zhong JC, Grant MB, Oudit GY. Role of the ACE2/Angiotensin 1-7 axis of the renin-angiotensin system in heart failure. Circ Res. 2016;118(8):1313–1326. doi:10.1161/CIRCRESAHA.116.307708
22. Turner AJ, Hiscox JA, Hooper NM. ACE2: from vasopeptidase to SARS virus receptor. Trends Pharmacol Sci. 2004;25(6):291–294. doi:10.1016/j.tips.2004.04.001
23. Zhang H, Penninger JM, Li Y, Zhong N, Slutsky AS. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46(4):586–590. doi:10.1007/s00134-020-05985-9
24. Sardu C, D’Onofrio N, Balestrieri ML, et al. Hyperglycaemia on admission to hospital and COVID-19. Diabetologia. 2020;63(11):2486–2487. doi:10.1007/s00125-020-05216-2
25. ADA. How COVID-19 impacts people with diabetes. Available from: https://www.diabetes.org/coronavirus-covid-19/how-coronavirus-impacts-people-with-diabetes.
26. Moore JB, June CH. Cytokine release syndrome in severe COVID-19. Science. 2020;368(6490):473–474. doi:10.1126/science.abb8925
27. Albai O, Frandes M, Timar B, Paun DL, Roman D, Timar R. Long-term risk of malignant neoplastic disorders in type 2 diabetes mellitus patients with metabolic syndrome. Diabetes Metab Syndr Obes. 2020;13:1317–1326. doi:10.2147/DMSO.S243263
28. Albai O, Timar B, Paun DL, Sima A, Roman D, Timar R. Metformin treatment: a potential cause of megaloblastic anemia in patients with type 2 diabetes mellitus. Diabetes Metab Syndr Obes. 2020;13:3873–3878. doi:10.2147/DMSO.S270393
29. Ali Abdelhamid Y, Kar P, Finnis ME, et al. Stress hyperglycaemia in critically ill patients and the subsequent risk of diabetes: a systematic review and meta-analysis. Crit Care. 2016;20(1):301. doi:10.1186/s13054-016-1471-6
30. Ilias I, Zabuliene L. Hyperglycemia and the novel covid-19 infection: possible pathophysiologic mechanisms. Med Hypotheses. 2020;139:109699. doi:10.1016/j.mehy.2020.109699
31. Yang JK, Lin SS, Ji XJ, Guo LM. Binding of sars coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol. 2021;47(3):193–199. doi:10.1007/s00592-009-0109-4
32. Kleine-Weber H, Schroeder S, Kruger N. Polymorphisms in dipeptidyl peptidase 4 reduce host cell entry of Middle East respiratory syndrome coronavirus. Emerg Microbes Infect. 2020;9(1):155–168. doi:10.1080/22221751.2020.1713705
33. Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934–943. doi:10.1001/jamainternmed.2020.0994
34. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi:10.1016/S0140-6736(20)30211-7
35. Sardu C, D’Onofrio N, Balestrieri ML, Barbieri M, Rizzo MR. Messina, Maggi P, Coppola, Paolisso G, Marfella R: outcomes in patients with hyperglycemia affected by covid-19: can we do more on glycemic control? Diabetes Care. 2020;43(7):1408–1415. doi:10.2337/dc20-0723
36. Umpierrez GE, Isaacs SD, Bazargan N, You X, Thaler LM, Kitabchi AE. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab. 2002;87(3):978–982. doi:10.1210/jcem.87.3.8341
37. Whitcomb BW, Pradhan EK, Pittas AG, Roghmann MC, Perencevich EN. Impact of admission hyperglycemia on hospital mortality in various intensive care unit populations. Crit Care Med. 2005;33(12):2772–2777. doi:10.1097/01.CCM.0000189741.44071.25
38. Albai O, Frandes M, Sima A, Timar B, Vlad A, Timar R. Practical applicability of the ISARIC-4C score on severity and mortality due to SARS-CoV-2 infection in patients with type 2 diabetes. Medicina. 2022;58(7):848. doi:10.3390/medicina58070848
39. Dungan KM, Braithwaite SS, Preiser JC. Stress hyperglycaemia. Lancet. 2009;373(9677):1798–1807. doi:10.1016/S0140-6736(09)60553-5
40. Yang JK, Feng Y, Yuan MY. Plasma glucose levels and diabetes are independent predictors for mortality and morbidity in patients with SARS. Diabet Med. 2006;23(6):623–628. doi:10.1111/j.1464-5491.2006.01861.x
41. Singh AK, Singh R. Hyperglycemia without diabetes and new-onset diabetes are both associated with poorer outcomes in COVID-19. Diabet Res Clin Pract. 2020;167:108382. doi:10.1016/j.diabres.2020.108382
42. Fadini GP, Morieri ML, Boscari F, et al. Newly-diagnosed diabetes and admission hyperglycemia predict COVID-19 severity by aggravating respiratory deterioration. Diabet Res Clin Pract. 2020;168:108374. doi:10.1016/j.diabres.2020.108374
43. Coppelli A, Giannarelli R, Aragona M, et al.; Pisa COVID-19 Study Group. Hyperglycemia at hospital admission is associated with severity of the prognosis in patients hospitalized for COVID-19: the Pisa COVID-19 Study. Diabetes Care. 2020;43(10):2345–2348. doi:10.2337/dc20-1380
44. Liu Q, Chen H, Li J. Fasting blood glucose predicts the occurrence of critical illness in COVID-19 patients: a multicenter retrospective cohort study. J Infect. 2020;81(3):e20–e23. doi:10.1016/j.jinf.2020.07.006
45. Smith SM, Boppana A, Traupman JA, et al. Impaired glucose metabolism in patients with diabetes, prediabetes, and obesity is associated with severe COVID-19. J Med Virol. 2021;93(1):409–415. doi:10.1002/jmv.26227
46. Mahrooz A, Muscogiuri G, Buzzetti R, Maddaloni E. The complex combination of COVID-19 and diabetes: pleiotropic changes in glucose metabolism. Endocrine. 2021;2:1–9.
47. Poly TN, Islam MM, Li YJ, Lin MC, Hsu MH, Wang YC. Metformin use is associated with decreased mortality in COVID-19 patients with diabetes: evidence from retrospective studies and biological mechanism. J Clin Med. 2021;10(16):3507. doi:10.3390/jcm10163507
48. Ugwueze CV, Ezeokpo BC, Nnolim BI, et al. COVID-19 and diabetes mellitus: the link and clinical implications. Dubai Diabetes Endocrinol J. 2020;26(2):69–77. doi:10.1159/000511354
49. Santos A, Magro DO, Evangelista-Poderoso R, Saad MJA. Diabetes, obesity, and insulin resistance in COVID-19: molecular interrelationship and therapeutic implications. Diabetol Metab Syndr. 2021;13(1):23. doi:10.1186/s13098-021-00639-2
50. Cocoş R, Mahler B, Turcu-Stiolica A, et al. Risk of death in comorbidity subgroups of hospitalized COVID-19 patients inferred by routine laboratory markers of systemic inflammation on admission: a retrospective study. Viruses. 2022;14(6):1201. doi:10.3390/v14061201
51. Lighter J, Phillips M, Hochman S, et al. Obesity in patients younger than 60 years is a risk factor for Covid‐19 hospital admission. Clinl Infect Dis. 2020;71(15):896–897. doi:10.1093/cid/ciaa415
52. Kruglikov IL, Scherer PE. The role of adipocytes and adipocyte‐like cells in the severity of COVID‐19 infections. Obesity. 2020;28(7):
53. Heialy SA, Hachim M, Senok A, et al. Regulation of angiotensin converting enzyme 2 (ACE2) in obesity: implications for COVID‐19. bioRxiv. 2020;3:046938.
54. CDC Weekly C. The novel coronavirus pneumonia emergency response epidemiology team The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) —China, 2020. China CDC Weekly. 2020;2(8):113–122. doi:10.46234/ccdcw2020.032
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
