Back to Journals » HIV/AIDS - Research and Palliative Care » Volume 9
Assessment of the effect of antiretroviral therapy on renal and liver functions among HIV-infected patients: a retrospective study
Authors Wondifraw Baynes H , Tegene B , Gebremichael M, Birhane G, Kedir W, Biadgo B
Received 29 August 2016
Accepted for publication 18 November 2016
Published 22 December 2016 Volume 2017:9 Pages 1—7
DOI https://doi.org/10.2147/HIV.S120979
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Bassel Sawaya
Habtamu Wondifraw Baynes,1 Birhanemeskel Tegene,2 Mikiyas Gebremichael,3 Gebrehawaria Birhane,3 Wabe Kedir,3 Belete Biadgo1
1Department of Clinical Chemistry, 2Department of Medical Microbiology, 3Department of Medical Laboratory Science, School of Biomedical and Laboratory Sciences, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia
Background: The emergence of highly active antiretroviral therapy (HAART) has dramatically improved quality of life in prolonging survival of human immunodeficiency virus (HIV)-infected patients on treatment in developed as well as developing countries. However, the main shortcoming of HAART in long-term use is its potential to cause liver and kidney derangements that may be life threatening. The drugs are actively accumulated in the proximal renal tubule resulting in functional disturbance with mitochondrial injury being one of the most important targets recognized. Therefore, the aim of this study was to assess the adverse effects of HAART on kidney and liver functions among HIV-infected patients presenting to the University of Gondar Hospital, Ethiopia.
Materials and methods: An institution-based retrospective study was conducted from 2010 to 2015 on a subset of HIV-infected patients. Data were collected from the registration book of the University of Gondar Hospital antiretroviral clinic laboratory after checking the completeness of age, gender, creatinine, blood urea nitrogen, and alanine aminotransferase level. Data were entered and analyzed using SPSS version 20. Descriptive statistics, chi-square test, one-way analysis of variance, and logistic regression were done to determine associations. A P-value <0.05 was considered statistically significant.
Results: A total of 275 study subjects were included in the study. Of these, 62.2% were females, and the overall prevalence of chronic kidney disease (CKD) before and after treatment was 3.6% and 11.7%, respectively. A majority of the CKD patients were in stage 3 for patients after treatment. The overall prevalence of hepatotoxicity was 6.5% and 16.7% before and after treatment, respectively. A majority of the patients developed Grade 2 hepatotoxicity 66.7% and 65.2% before and after treatment, respectively. Binary and multiple logistic regression analysis indicated that the female gender was a risk factor for CKD.
Conclusion: The prevalence of nephrotoxicity and hepatotoxicity were high among patients who took HAAR. Stage 3 nephrotoxicity and Grade 2 hepatotoxicity had the highest incidences of the total toxicities, and the female gender was a risk factor for nephrotoxicity. Further prospective studies are recommended to determine the effect of HAART and contributing factors.
Keywords: CKD, hepatotoxicity, nephrotoxicity, HAART, Gondar, Ethiopa
Introduction
Human immunodeficiency virus (HIV) is known to be the cause of a major public health problem worldwide from the start of the 21st century. If left untreated, within a decade the vast majority of HIV-infected individuals would develop fatal opportunistic infections as a result of HIV-induced deficiencies in the immune system.1,2 An estimated 36.9 million people are living with HIV worldwide, with ~15.8 million people having access to highly active antiretroviral therapy (HAART) based on a 2015 report.3 HAART was developed for managing retroviral infections such as HIV in order to prolong life. The primary goal of HAART is maximal and durable suppression of viral load, preservation and restoration of immunologic function, improvement of quality of life, and reduction of HIV-related morbidity and mortality.4
HAART over different durations of time has an effect on the nephron as well as on liver hepatocytes by inducing toxicity.5 Renal dysfunction by HAART has been associated primarily with tenofovir disoproxil fumarate, which is actively accumulated in the proximal renal tubule.6 Liver disease is known to be caused by a number of antiretrovirals. Following exposure to the drug, the toxic moiety induces some type of stress or functional disturbance, with mitochondrial injury being one of the most important targets recognized.7,8 For instance, nevirapine (NVP) and efavirenz (EFV) can cause hepatotoxicity via a hypersensitivity syndrome reaction that can result in acute liver necrosis and death.9
The overall rate of severe hepatotoxicity with nucleoside reverse transcriptase inhibitors (NRTI) therapy has been reported by Reisler et al10 as 12%, which highlights the complexity and difficulty in evaluating and managing hepatotoxicity associated with antiretroviral therapy (ART). Acute renal failure is a common complication in ambulatory HIV-infected patients treated with HAART and has recently been associated with AIDS, hepatitis C virus coinfection, and liver disease.11,12 An overall incidence of HAART related with hepatotoxicity in observational studies was found to vary from study to study and increase the risk of hepatitis C virus coinfection.13,14 Therefore, the aim of this study was to assess the adverse effects of HAART on kidney and liver functions among HIV-infected patients presenting to the University of Gondar Hospital, Ethiopia.
Materials and methods
Study design, period, and area
An institution-based retrospective study was conducted from records of 2010–2015 data during March 2016 at the University of Gondar Hospital ART clinic. The hospital is located in North Gondar around 747 km away from the capital city, Addis Ababa, which acts as the referral center for four district hospitals.
Data collection technique and study participants
The study participants comprised of a subset of 275 HIV-infected patients who were on HAART for at least 3 years and registered for primary care at the University of Gondar Hospital anti retro viral therapy (ART) clinic. These patients had been previously screened for renal and liver dysfunction when they were initiated for HAART. Age, gender, and other clinical characteristics and laboratory data were gathered in patient records. Participants who had regular follow-up and those having creatinine, blood urea nitrogen (BUN), and alanine aminotransferase (ALT) results during initiation for HAART were included in the study. However, those who missed any of the above data were excluded from the study. For supplying quality test results and for the care of HIV patients, the ART clinic personnel performed the clinical chemistry tests using Mindray BS-200 chemistry analyzer (Shenzen Mindray Bio-Medical electronics Co. Ltd, Nanshan, People’s Republic of China). The quality of the results were assured by running daily quality control checks and regular calibration of the instruments used.
Interpretation for the hepatic enzyme was based on the AIDS Clinical Trial Group grading system with ALT elevations >40.0 U/L, which is the upper limit of normal (ULN) range in individuals with normal ALT values at baseline, for adults.15
Hepatotoxicity grades were categorized as Grade 1 when a ALT value lies between 1.25 and <2.5 × ULN, Grade 2 when a ALT value lies between 2.5 and <5.0 × ULN, Grade 3 when a ALT value lies between 5.0 and <10 × ULN, and Grade 4 when a ALT value lies ≥10 × ULN.16
The glomerular filtration rate (GFR) was estimated using the Modification of Diet in Renal Disease Study equation:17 186 × (serum creatinine [mg/dL])−1.154 × age−0.203 × 0.742 (if female) × 1.210 (if African American). Chronic kidney disease (CKD) was defined by either kidney damage or GFR <60 mL/min/1.73 m2 for ≥3 months, regardless of the cause. CKD stages were categorized based on the classification system established by the National Kidney Foundation – Kidney Disease Outcomes Quality Initiative classification. For this study, CKD was defined as Kidney Disease Outcomes Quality Initiative CKD stages 3, 4, and 5 (estimated glomerular filtration rate [eGFR] <60 mL/min/1.73 m2): with eGFR 30–59, 15–29, and <15 mL/min/1.73 m2, respectively. Stage 3 was further classified into 3A (45–59.9) and 3B (30–44.9).18,19
Data analysis and interpretation procedure
Data were collected from the registration book of the University of Gondar Hospital ART clinic laboratory after checking the completeness of patient’s age, gender, creatinine, BUN, and ALT level. The data were then coded and analyzed using the Statistical Package for the Social Sciences (SPSS) version 20 software program (IBM Corporation, Armonk, NY, USA). Descriptive statistics, chi-square test, one-way analysis of variance, and logistic regression were done to determine associations. Those variables having a P-value <0.2 in bivariate regression were further analyzed by multiple logistic regression for strength of association between variables using 95% confidence interval (CI) and a P-value <0.05 was considered statistically significant.
Ethical considerations
Data were collected after ethical clearance was obtained from the School of Biomedical and Laboratory Sciences, College of Medicine and Health Science, University of Gondar. Consent was not required from patients because it was retrospective study and patients were not available during the study period. The School of Biomedical and Laboratory Sciences, University of Gondar waived this requirement for patient consent. After discussing the purpose and aim of the study, permission was obtained from the Head of the University of Gondar Hospital laboratory before data collection who confirmed that all results obtained would be kept confidential.
Results
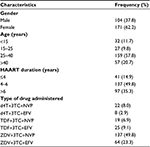
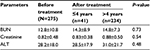
A total of 275 study participants, 104 (37.8%) males and 171 (62.2%) females were included in the study. A majority (218 [79.2%]) of the study participants were in the age group ≤40 years old and most of them (234 [85.1%]) took HAART for an average of >4 years (Table 1). The mean values of creatinine (0.83±0.38 vs. 0.88±0.50; for ≤4 years and >4 years of therapy, respectively), BUN (14.3±8.9 vs. 14.8±7.3), and ALT (28.5±17.9 vs. 31.0±21.7) for patients after treatment were higher than the values before treatment (0.82±0.48, 12.8±10.8, and 28.2±18.0 for creatinine, BUN, and ALT, respectively) but were not statistically significant (Table 2).
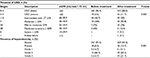
For patients on zidovudine-based regimens the mean values for creatinine (0.87±0.38), BUN (14.86±7.53), and ALT (32.2±23) were higher than the values for patients on stavudine-based (0.85±0.25, 13.85±6.5, and 23.0±12.3 for creatinine, BUN, and ALT, respectively) and tenofovir-based (0.83±0.36, 11.74±4.17, and 31.1±4.2 for creatinine, BUN, and ALT, respectively) regimen users from the nucleoside analog. Furthermore, ALT was higher for NVP-based (29.8±16.6) regimen users than for EFV-based (28.17±21.0) regimen users from the nonnucleoside analogs. Only creatinine was statistically significant (P=0.002; Table 3).
The overall prevalence of CKD before and after treatment was 3.6% and 11.7%, respectively. A majority of the CKD cases were observed in stage 3; 30 (93.8%) after treatment and 10 (100%) before treatment. Moreover, 3.1% of the patients had renal failure after treatment. The overall prevalence of hepatotoxicity was 18 (6.5%) and 46 (16.7%) among patients before and after treatment, respectively. A majority of the patients developed Grade 2 hepatotoxicity: 12 (66.7%) and 30 (65.2%) before and after treatment, respectively (Table 4).
Binary logistic regression analysis showed female gender, increase in age, and taking stavudine-based regimen had a P-value <0.2; of these, only female gender was significantly associated with CKD (Tables 5 and 6).
Discussion
In this study, we recruited patients who were actively taking HAART. The mean values of serum BUN, creatinine, and ALT were increased among patients on HAART over different durations of time has an effect on the nephron as well as on liver hepatocytes by inducing toxicity that may not related to the virus it self only.5 Regarding the effect of different HAART regimens, in this study, the ZDV-based regimen has the greatest impact on alteration of creatinine, BUN, and ALT values compared with other nucleoside analogs, while the tenofovir-based regimen is the safest. Patients who were on EFV-based regimens showed a significant effect on liver and kidney parameters than those who were on NVP-based regimen from the nonnucleoside analog with a P-value of (0.029 vs 0.002) for BUN and creatinine, respectively. This is supported by the findings from two studies in Ghana and one in Cameroon.20–22 Another similar study also reported that renal dysfunction as a side effect of HAART, that has been associated primarily with the parent tenofovir, which is accumulated in proximal renal tubule.23
CKD is the major problem in HAART patients and can lead to loss of kidney function, leading to complications and kidney failure, and development of cardiovascular disease.19 The prevalence of CKD in our study was 11.7% among HAART-treated individuals, using the Modification of Diet in Renal Disease method of estimation, which is in line with the prevalence of 9.9% in a study conducted in Ghana20 but lower than that in studies conducted in Nigeria and Burundi with CKD prevalence of 47.6% and 45.7% among HIV patients, respectively.24,25
However, this study had higher prevalence than the study conducted in Tanzania (1.2%).26 In this study, stage 3 (eGFR, 30–59.9) was the highest (100% vs. 93.7%) among the CKD patients before and after treatment, but the results of previous studies did not support our finding; they found a high prevalence of CKD in stage 2 (eGFR, 60–89.9).20,26 The reason for this difference could be that in our study eGFR values between 60–89.9 were not considered as presence of CKD because we could not measure urine albumin as another alternative mean values for determining CKD when eGFR values were >60. In our study, a 3.1% prevalence of severe kidney disease and a 3.1% prevalence of renal failure were observed. Another study of hospitalized patients with complications of HIV reported increased risk for acute renal failure and is associated with liver disease, CKD, and increased mortality related to volume depletion, hemodynamic stress, and the acute administration of nephrotoxic medications or radio contrast.12 Taking gender into account, a higher proportion of females (62.2%) were recruited; this higher proportion might indicate their ease of susceptibility to HIV infection due to biological and other factors. In the logistic regression analysis, female gender was found to be a risk factor for CKD with an AOR of 2.5 (1.0–6.0), which agrees with the study conducted in San Diego, California, which reported a reduction in GFR for female gender,27 but does not agree with the study conducted in Tanzania26 with an odds ratio of 1.5 (0.7–3.3). This study shows an increment for patients aged >40 years old in the likelihood of developing CKD (8.8% vs. 17.5%) before and after treatment, which is high when compared with that for those <40 years of age, which agrees with the study conducted in San Diego, California, reported as per 10-year increase there was a significant development of CKD.27
With regard to HAART regimen, a majority of the participants (73%) in this study were ZDV-based regimen users, but a high proportion of patients (13.3%) develop CKD among stavudine based regimen users, which is supported by a clinical trial and animal study conducted elsewhere28,29 that induce to some extent for mitochondrial toxicity.
In our study, patients who took HAART for >4 years have shown a strong association with CKD, which is supported by a previous study, that indicates longer duration of treatment as significantly associated with CKD.22 Furthermore, another cross-sectional study has demonstrated that subjects who took HAART for longer periods showed strong association with renal dysfunction.26 Different studies in sub-Saharan Africa reported that long-term infection with HIV is associated with a wide spectrum of renal diseases with variable prevalence of these diseases in HIV-infected patients: 6% in South Africa, 38% in Nigeria, 26% in Côte d’Ivoire, 28% in Tanzania, 25% in Kenya, 20%–48.5% in Uganda, and 33.5% in Zambia and Nigeria. Results from these histopathological studies also suggested that broader spectrum of tissue damage in HIV-associated kidney disease, exists in the African population than was previously thought,30 whereas in our study it did not show significant association (95% CI=0.52–3.57); the reason might be that a majority of the participants took HAART for a lower duration in the category that we used to explain the results.
Hepatotoxicity may result from HIV infection itself and other factors including hepatitis B and C virus infections, and systemic opportunistic infections, if patients are coinfected.31 Moreover, the impact is severe in patients undergoing long-term treatment with different combinations of HAART and is associated with substantial toxicity, adherence difficulties, and, finally, drug resistance with variable prevalence from different studies.32 In this study, we found a 16.7% and 6.5% prevalence of hepatotoxicity after and before treatment, respectively. This study has a slightly lower prevalence than the prevalence from the cohort study conducted in Atlanta, Georgia, which had a total prevalence rate of 23%.33 Another similar study reported that the overall rate of severe hepatotoxicity with NRTI therapy of 12% reported by Reisler et al10 is lower than in our study which might be due to the ability of NVP and EFV to cause hepatotoxicity via a hypersensitivity syndrome reaction that can result in acute liver necrosis and death. The variation in prevalence in the stated country might be due to the presence of coinfection by hepatitis virus which has the capability of increasing hepatotoxicity. Grade 2 hepatotoxicity accounts for the majority of the hepatotoxicity grades in this study (63%), which is similar to the results of the study conducted in Atlanta, Georgia, that had a high prevalence rate for Grade 2 hepatotoxicity among the four grades of toxicities,33 while it differs from the findings of two studies conducted in Cameroon.22,34
Limitations
The limitations of this study were the cross-sectional nature of the study, the underlying etiology of liver and renal disease was unknown in study participants, and the results of urine specimens were not available to evaluate for albuminuria, all of which underestimate the prevalence.
Conclusion
The mean values of creatinine, BUN, and ALT were higher among HAART-treated patients, and there was a significant difference in the mean values of BUN and creatinine with respect to the different regimens used. The prevalences of nephrotoxicity and hepatotoxicity were high among HAART patients in this study. Stage 3 nephrotoxicity and Grade 2 hepatotoxicity were the commonly observed toxicities. Female gender was a risk factor for nephrotoxicity.
Acknowledgment
We express our deepest gratitude to all the study participants and the staff at the University of Gondar Hospital ART laboratory for their cooperation during data collection.
Author contributions
All authors were responsible for writing the proposal, collection of data, designing the study, and analysis and interpretation of the data. All authors read and approved the final manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
Okolie MN, Eghafona NO, Omoregie R. Anti-human immunodeficiency virus agents. J Med Lab Sci. 2003;12(1):1–14. | ||
Brooks GF, Carroll KC, Butel JS, Morse SA, Mietzner TA. Jawetz, Melnick, & Adelberg’s Medical Microbiology. 25th ed. New York: McGraw-Hill; 2010:406–407. | ||
Global AIDS Response Progress Reporting (GARPR) estimates. Geneva, Switzerland: UNAIDS; 2016. | ||
Hawkins T. Understanding and managing adverse effects of antiretroviral therapy. Antiviral Res. 2010;85(1):201–209. | ||
Peters PJ, Moore DM, Mermin J, et al. Antiretroviral therapy improves renal function among HIV-infected Ugandans. Kidney Int. 2008;74(7):925–929. | ||
Cihlar T, Ho ES, Lin DC, Mulato AS. Human renal organic anion transporter 1 (hOAT1) and its role in the nephrotoxicity of antiviral nucleotide analogs. Nucleosides Nucleotides Nucleic Acids. 2001;20(4–7):641–648. | ||
Ong MM, Latchoumycandane C, Boelsterli UA. Troglitazone induced hepatic necrosis in an animal model of silent genetic mitochondrial abnormalities. Toxicol Sci. 2007;97(1):205–213. | ||
Hanawa N, Shinohara M, Saberi B, Gaarde WA, Han D, Kaplowitzn N. Role of JNK translocation to mitochondria leading to inhibition of mitochondria bioenergetics in acetaminophen-induced liver injury. J Biol Chem. 2008;283(20):13565–13577. | ||
Reust CE. Common adverse effects of antiretroviral therapy for HIV disease. Am Fam Physician. 2011;83(12):1443–1451. | ||
Reisler R, Liou S, Servoss JC, Robbins G, Theodore D, Murphy R. Incidence of hepatotoxicity and mortality in 21 adult antiretroviral treatment trials. ACTG Liver Diseases Focus Group. 2001; 1st International AIDS Society Conference on HIV Pathogenesis and Treatment; Buenos Aires, Argentina. | ||
Franceschini N, Napravnik S, Eron JJ Jr, Szczech LA, Finn WF. Incidence and etiology of acute renal failure among ambulatory HIV-infected patients. Kidney Int. 2005;67(4):1526–1531. | ||
Wyatta CM, Arons RR, Klotmana PE, Klotmanb ME. Acute renal failure in hospitalized patients with HIV: risk factors and impact on hospital mortality. AIDS. 2006;20(4):561–565. | ||
Kalyesubula R, Kagimu M, Opio KC, Kiguba R, Semitala CF, Schlech WF, Katabira ET. Hepatotoxicity from first line antiretroviral therapy: an experience from a resource limited setting. Afr Health Sci. 2011;11(1):16–23. | ||
Wit FW, Weverling GJ, Weel J, Jurriaans S, Lange JM. Incidence of and risk factors for severe hepatotoxicity associated with antiretroviral combination therapy. J Infect Dis. 2002;186(1):23–31. | ||
Servoss JC, Kitch DW, Andersen JW, Reisler RB, Chung RT, Robbins GK. Predictors of antiretroviral-related hepatotoxicity in the adult AIDS Clinical Trial Group (1989–1999). J Acquir Immune Defic Syndr. 2006;43(3):320–323. | ||
Division of AIDS (DAIDS), National Institute of Allergy and Infectious Diseases National Institutes of Health US Department of Health and Human Services. Table for Grading the Severity of Adult and Pediatric Adverse Events. November 2014. Available from: http://rcc.tech-res.com/DAIDS%20RCC%20Forms/. | ||
Levey AS, Greene T, Kusek JW, Beck GL. A simplified equation to predict glomerular filtration rate from serum creatinine. J Am Soc Nephrol. 2000;11:A0828. | ||
Hogan M. KDIGO conference proposes changes to CKD classification, but not to the definition. Nephrol. 2009;2(12):9–10. | ||
National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2002; 39(2 Suppl 1):S1–S266. | ||
Obirikorang C, Osakunor DNM, Ntaadu B, Adarkwa OK. Renal function in Ghanaian HIV-infected patients on highly active antiretroviral therapy: a case control study. PLoS One. 2014;9(6):e99469. | ||
Osakunor DNM, Obirikorang C, Fianu V, Asare I, Dakorah M. Hepatic enzyme alterations in HIV patients on antiretroviral therapy: a case-control study in a hospital setting in Ghana. PLoS One. 2015;10(8):e0134449. | ||
Fokunang CN, Banin AN, Kouanfack C, Ngogang JY. Evaluation of hepatotoxicity and nephrotoxicity in HIV patients on highly active anti-retroviral therapy. J AIDS HIV Res. 2010;2(3):048–057. | ||
Cihlar T, Ho ES, Lin DC, Mulato AS. Human renal organic anion transporter 1 (hOAT1) and its role in the nephrotoxicity of antiviral nucleotide analogs. Nucleosides Nucleotides Nucleic Acids. 2001;20(4–7):641–648. | ||
Ayokunle DS, Olusegun OT, Ademola A, Adindu C, Olaitan RM, Oladimeji AA. Prevalence of chronic kidney disease in newly diagnosed patients with human immunodeficiency virus in Ilorin, Nigeria. J Bras Nefrol. 2015;37(2):177–184. | ||
Cailho J, Nkurunziza B, Izzedine H, et al. Prevalence of chronic kidney disease among people living with HIV/AIDS in Burundi: a cross-sectional study. BMC Nephrol. 2011;12:40. | ||
Mpondo BC, Kalluvya SE, Peck RN, et al. Impact of antiretroviral therapy on renal function among HIV-infected Tanzanian adults: a retrospective cohort study. PLoS One. 2014;9(2):e89573. | ||
Crum-Cianflone N, Ganesan A, Teneza-Mora N, Riddle M, Medina S, Barahona I, Brodine S. Prevalence and factors associated with renal dysfunction among HIV-infected patients. AIDS Patient Care STDs. 2010;24(6):353–360. | ||
Ahmed M. Abacavir induced reversible fanconi syndrome with nephrogenic diabetes insipidus in patients with acquired immunodeficiency syndrome. J Postgrad Med. 2006;52(4):296–297. | ||
Krishnan M, Nair R, Haas M, Atta M. Acute renal failure in HIV positive 50 year old man. Am J Kidney Dis. 2000;36(5):1072–1078. | ||
Fabian J, Naicker S. HIV and kidney disease in sub-Saharan Africa. Nat Rev Nephrol. 2009;5(10):591–598. | ||
Ugiagbe RA, Eze EU. Effect of anemia on hepatotoxicity of HAART in HIV patients in Benin City. Niger Med J. 2011;52(3):167–172. | ||
Johnson VA, Brun-Vezinet F, Clotet B, Conway B, D’Aquila RT, Demeter LM. Update of the drug resistance mutations in HIV-1. Top HIV Med. 2004;12:119–124. | ||
Ofotokun I, Smithson SE, Lu C, Easley KA, Lennox JL. Liver enzymes elevation and immune reconstitution among treatment-naïve HIV-infected patients instituting antiretroviral therapy. Am J Med Sci. 2007;334(5):334–341. | ||
Lucien K, Clement A, Fon N, Weledji P, Ndikvu C. The effect of antiretroviral treatment on liver function enzymes among HIV infected out patients. Afr J Clin Exp Microbiol. 2010;11(3):174–178. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.