Back to Journals » Risk Management and Healthcare Policy » Volume 15
Assessment of Potentially Inappropriate Medications Using the EU (7)-PIM List, in a Sample of Portuguese Older Adults’ Residents in Nursing Homes
Authors Plácido AI, Aguiar A, Piñeiro-Lamas M, Varallo F, Figueiras A, Herdeiro MT, Roque F
Received 3 December 2021
Accepted for publication 5 July 2022
Published 13 July 2022 Volume 2022:15 Pages 1343—1352
DOI https://doi.org/10.2147/RMHP.S346300
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Kent Rondeau
Ana Isabel Plácido,1 Ana Aguiar,1 María Piñeiro-Lamas,2,3 Fabiana Varallo,4 Adolfo Figueiras,2,3,5 Maria Teresa Herdeiro,6 Fátima Roque1,7
1Research Unit for Inland Development, Polytechnic of Guarda (UDI-IPG), Guarda, Portugal; 2Consortium for Biomedical Research in Epidemiology and Public Health (CIBER en Epidemiología y Salud Pública-CIBERESP), Madrid, Spain; 3Institute of Health Research of Santiago de Compostela (IDIS), Santiago de Compostela, Spain; 4Department of Pharmaceutical Sciences, School of Pharmaceutical Sciences of Ribeirão Preto, University of São Paulo, Ribeirão Preto, São Paulo, Brazil; 5Department of Preventive Medicine and Public Health, Faculty of Medicine, University of Santiago de Compostela, Santiago de Compostela, Spain; 6Institute of Biomedicine (iBiMED-UA), Department of Medical Sciences, University of Aveiro, Aveiro, 3810-193, Portugal; 7Health Sciences Research Centre, University of Beira Interior (CICS-UBI) Covilhã, Covilhã, Portugal
Correspondence: Fátima Roque, Research Unit for Inland Development, Polytechnic of Guarda (UDI-IPG), Avenida Dr. Francisco Sá Carneiro, no. 50, Guarda, 6300-559, Portugal, Tel +35 1-271205220, Email [email protected]
Abstract: Prescription of potentially inappropriate medication (PIM) in older adults is associated with poor clinical outcomes. The EU (7)-PIM list was created for the European market to improve pharmacotherapy in older adults.
Purpose: This work aims to characterize the medication profile and assess the presence of PIM, using the EU (7)-PIM list in older adults’ residents at nursing homes.
Methods: Retrospective data were collected from the anonymized nursing home records. After PIM identification, a descriptive analysis was performed, and a generalized linear model for dependent negative binomial-type variables was constructed to assess the risk of PIM.
Results: Of the 210 participants (mean age 85.10), 82.40% were polymedicated. PIM was observed in 86.4% participants (mean per patient = 2.30± 0.10). The most common PIM were proton pump inhibitors (n = 121, 57.62%), followed by anxiolytics (n = 96, 45.71%). 64.30% of all patients take 2– 4 PIM and 5.80% take five or more PIM. The occurrence of PIM is influenced by the number of prescribed medicines (RR 1.14; 95% CI 1.1.-1.17) and the presence of digestive system diseases (RR 1.05; 95% CI 1.0– 1.09).
Conclusion: The high prevalence of PIM observations highlights the necessity of the implementation of guidelines to prevent PIM.
Keywords: elderly, institutionalized older adults, medication profile, prescription
Introduction
World populations are getting old, and the Portuguese population is not an exception, according to the United Nations (UN) in 2019, Portuguese older people (aged 65 or over) represented 22.40% of all population, and it is expected that in 2030 this number will rise by 27.00%. 1 Medication is undoubtedly the health technology that contributes most to the increased longevity of populations.2 Due to (1) increased susceptibility to develop comorbid conditions, (2) altered pharmacokinetics and pharmacodynamics, and (3) polypharmacy (according to the most used definitions: the concomitant use of five or more medicines) older adults are more vulnerable to suffering adverse drug reactions (ADRs) related to the use of some medicines.3,4 Because of this, the concept of potentially inappropriate medication (PIM) in the geriatric population has emerged.5–7 PIM is defined as “medications that have more potential risk than the potential benefit or prescribing that does not agree with accepted medical standards”,7 and are associated with increased hospital admissions and ultimately higher healthcare costs.8–10
Healthcare systems have made efforts to reduce the prescription of these high-risk drugs and consequently decrease ADRs, hospital admission, mortality, and healthcare costs.11–13
According to the literature, the overall prevalence of PIM used in nursing homes is 43.2%, with a higher prevalence in Europe (49.00%) than in the rest of the world.12
Despite the scarcity of studies to evaluate the presence of PIM in Portuguese nursing homes, a recent study suggests that 60.30% to 75.40% of the Portuguese nursing home residents had at least one PIM.14 Another study suggests that the percentage of older adults who took at least one PIM in Portuguese nursing homes ranges from 56.60-to 85.50.15
In the last decade, several criteria have been developed to identify PIM among older adults.16 In 2015, a group of experts from seven European countries created the EU (7)-PIM list, which comprises 282 chemical substances from 34 therapeutic groups.17 This list has recently been adapted to the reality of medicines marketed in Portugal.18
Considering that, the inner center region of Portugal is one of the most aged regions of the country and the scarcity of studies that evaluate the prevalence of PIM in Portuguese nursing home residents, we developed this work that aims to characterize the medication profile and assess the presence of PIM, using the EU (7)-PIM list in older adults’ residents at nursing homes. This list has the advantage to be appropriate for pharmacoepidemiology applications using administrative databases or surveys without any clinical information because it is only necessary to know the mediation profile of the patients including the duration of treatment and dosage of some medicine.
Materials and Methods
Ethical Approval
This study obtained ethical approval from the Ethics Committee of Polytechnic Institute of Guarda (IPG) on 30 January of 2020 (Register n° 2/2020). Consent for data collection was obtained from those responsible for the nursing homes. The retrospective data were collected by the nursing home staff in an anonymized form and the way they were delivered to us did not allow any identification or traceability. Due to the retrospective nature of the study, no contact with the patients, and the anonymity of the data the ethics review board of IPG did to require consent from the patients for the use of these data in this study, maintaining the impossibility of traceability. During data treatment, the General Data Protection Regulation was followed.
Setting, Study Population, and Sample Selection
A retrospective cross-sectional study was conducted on a convenience sample of four nursing homes located in the inner center region of Portugal. It was included retrospective data from 210 residents from four nursing homes with age ≥65 years old, Data were collected, in February 2020, by the nursing home staff.
In Portugal, nursing homes can be private profit institutions or not-for-profit private institutions of social solidarity (IPSS). IPSS can work in collaboration with the Portuguese social security that supports partially the costs with the institutionalized older adult. In 2015, social security support includes less than 13% of Portuguese older adults. Within 32.40% (57,570 older adults) of them were residents of IPSS.19
Regarding Portuguese Healthcare costs, older adults with an average salary of 1.5 times below the value of the social support index or with certain medical conditions are exempt from co-payment for any publicly provided services.19–21 However, the bulk of co-payments are made for medicines to which different levels (ranging from 15% to 90%) of co-insurance are applied according to their therapeutic value.22
Data Collection
Retrospective data were collected by the responsible nurse and provided in an anonymized form. The data included pathologies, medication prescribed, and duration of treatment. The chronic medication (ie Medication that is taken daily for a period exceeding 6 months is) was classified according to Anatomical Therapeutic Classification (ATC) code, using the WHO Collaborating Centre for Drug Statistics Methodology’s website.23 The pathologies were classified according to the International Statistical Classification of Diseases and Related Health Problems, 10th Revision (ICD-10).24 All the medication was analyzed by applying the EU(7)-PIM list 17 adapted for the Portuguese reality.18
Statistical Analysis
Data analysis was executed using the Statistical Package for Social Sciences (SPSS 25, IBM Corp., New York, NY, USA) and the free statistical software R (v4.0.3). All p values ≤0.05 were considered statistically significant. Age, number of used drugs, PIM, and the number of pathologies were classified as continuous variables. Sex, age category, PIM according to EU(7)-PIM list, pathologies according to ICD-10, and drugs used according to ATC classification were considered categorical variables. Numerical and ordinal data were analyzed using descriptive statistics and presented in frequency, percentage, using mean, and standard deviation. To assess the risk of PIM associated with the independent variables, adjusted relative risk (RR) and their 95% confidence intervals (95% CIs) were estimated using generalized linear models for dependent negative binomial-type variables (count variable: number of PIM for each patient).
To select the model covariables, we developed Directed Acyclic Graph (DAG). The starting DAG was based on our framework that visualizes the relationship between variables (confounders, intermediate variables, exposure, and outcome). To construct the definitive DAG, we evaluated the conditional independencies and the adjustment sets using the dagify function (ggdag library of software R). The variables included in the final DAG were considered for the multivariate analysis. Then, the variables were eliminated one by one, evaluating (1) the coefficients of the principal variables of exposure changed by no more than 10% and (2) the Schwartz’s Bayesian Information Criterion (BIC) improvement. The glm.nb function was used for estimation of the models (implemented in the MASS library of the software R).
Results
Characteristics of Older Adult Patients
The mean (± SD) age of the 210 participants was 85.10 (±6.98, Min 65, Max 102) years and 75.20% were women (Table 1). Most of the participants have three or fewer chronic conditions. Diseases of the circulatory system, mental and behavioral disorders, and endocrine, nutritional, and metabolic diseases were the most prevalent diseases, affecting, respectively, 62.40%, 50.00%, and 33.80% of patients (Table 1). On average, the number of prescribed medicines per patient was 7.10 ± 2.99 (Table 1). 60.50% of the participants took between 5 and 9 medicines, and 21.90% take 10 or more medicines.
 |
Table 1 Characteristics of Older Adult Patients |
The most frequent pharmacological subgroups (third ATC level) of prescribed medicines were anxiolytics (n = 151), drugs for peptic ulcer, and gastro-oesophageal reflux disease (n = 122), antithrombotic agents (n = 119), and high-ceiling diuretics (n = 95). Within these, benzodiazepine derivatives (n = 132), proton pump inhibitors (n = 121), platelet aggregation inhibitors excl. Heparin (n = 79) and Sulfonamides, plain (n = 94) respectively, were the fourth ATC level subgroups most frequently consumed by patients (Figure 1).
 |
Figure 1 Most frequent pharmacological subgroups (third level), according to the ATC classification system of prescribed. |
Potentially Inappropriate Medication Identified
The application of the EU (7)-PIM list allowed the identification of 469 PIM, corresponding to 32.20% of medication analyzed. The PIM occurred in 190 patients, representing 86.40% of all participants (mean per patient = 2.30± 0.10). Nine polymedicated patients do not take any PIM. In patients without and with PIM, the average number of prescribed medicines was 3.20 and 7.15, respectively. The more common PIM were detected in the proton pump inhibitors subgroup (n = 121), followed by anxiolytics (n = 96) (Table 2). We also observed that 20.50% of the sample take one PIM, 64.30% take 2–4 PIM, and 5.8% take five or more PIM.
 |
Table 2 Most Frequent PIM According to the EU (7)-PIM List |
Potentially Inappropriate Medication-Related Factors
Figure 2A shows the proposed DAG for a set of available covariates. When evaluating the conditional independence with the data, it was observed that age is not independent of PIM, or even conditioned to health problems, and sex is independent of both health problems and PIM. So, the DAG was refined (see Figure 2B). This DAG satisfies all the conditional independence relations that it carries implicitly. In Figure 2B, you can see the appropriate adjustment set for the DAG.
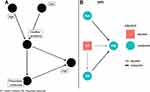 |
Figure 2 Directed acyclic graphs. Proposed relationship between the different variables (A) and the relationship between the different variables after the appropriate adjustment (B). |
In the adjusted regression model, it was observed that the number of prescribed medicines (RR 1.15; 95% CI 1.10–1.19), and the presence of digestive system diseases (RR 1.05; 95% CI 1.01–1.09) influenced the occurrence of PIM (Table 3). It was also observed that sex and other diseases did not influence PIM occurrence.
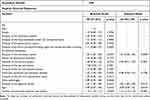 |
Table 3 Factors Influencing the Presence of PIM |
Discussion
This study reports the prevalence of PIM in institutionalized older adults. We observed that 86.40% of nursing home residents take at least one PIM, according to the EU (7)-PIM list. We also observed that the occurrence of PIM is influenced by the number of prescribed medicines, age, and by the presence of diseases in the digestive system.
The high prevalence of PIM observed in this study is corroborated by other studies that use the EU (7)-PIM list in nursing home patients.25–27 In nursing home patients from Serbia, it was observed that only 14.75% of the patients did not use any PIM.27 Moreover, two studies in France concluded that more than 70% of the patients use at least one PIM.25,26
According to the literature, the regular use of PIM affects almost one-half of nursing home residents, double the estimated in home-dwelling older adults.28,29 In European older adults, the prevalence of PIM ranges from 20% in home-dwelling older adults30 to approximately 80% in hospitalized patients, depending on the criteria used.31 The high prevalence of PIM in Portuguese nursing home residents was observed by others and, depending on the tools used, the percentage of older adults with at least one PIM ranged from 38.90% to 59.80%.14,15
Furthermore, in primary healthcare centers, 68.60% of the Portuguese older adults use at least one PIM.31
Across the literature, the correlation between settings and the likelihood of using a PIM lacks consensus: some authors reported that nursing home residents had an improved risk of using at least one PIM compared with home-dwelling patients,12,29,32–35 while others reported an opposite tendency36 or no association between the residence and the prevalence of PIM.35
According to the literature, the high number of prescribed medicines was described as a player in the occurrence of PIM and is associated with the occurrence of adverse reactions, increased hospitalizations, and increased healthcare costs.25,37–39 A systematic review concluded that up to 74% of nursing home residents use at least 10 medicines.40 In our sample, we observed that 82.40% of the older adults were polymedicated, and 21.90% used at least 10 medicines. These observations were corroborated by some reports32,41,42 but are higher than observed in other European countries, such as the Czech Republic, England, Finland, France, Germany, Italy, and the Netherlands where polypharmacy was observed in 49.70% of residents in the nursing home.43 Others observed that 51% of the European older adults consumed at least six medicines per day.30 It was described that in nursing home residents, there is an association between polypharmacy and the prevalence of PIM.29,44,45 In our sample, we observed that, in patients without PIM, the average number of prescribed medicines was 3.20, whereas patients with at least one PIM consumed an average of 7.15 medicines per day.
According to multivariate analyses, age is associated with the prevalence of PIM, this observation is not surprising, a recent review pointed out that in Europe, 49.00% of the older adults use at least one PIM.30
The profile of disease observed by us is similar to other studies;15,46,47 we observed that up that 50.00% of the patients were diagnosed with diseases of the circulatory system and/or mental and behavioral disorders. Multivariate analyses revealed that only digestive system diseases that are present in less than 25.50% of the patients are associated with the use of PIM. Others observed that mental, neurological, and genitourinary system diseases were the diseases associated with a high incidence of PIM.47
The most common PIM used were related to the long-term use (>8 weeks) of proton pump inhibitors, the consumption of anxiolytics (alprazolam and lorazepam), and the dosage >325 mg/d of iron preparations.
High consumption of proton pump inhibitors is frequently observed.15,44,48,49 According to a critical review 25% to 75% of the prescriptions of roton inhibitors prescription have no indication of use.50 These drugs are used to reduce gastric acid production and are associated with an increased risk of Clostridioides difficile infections and hip fractures.17,53 The prolonged use of these medicines has also been associated with an increased risk of dementia by others.52
An inappropriate use of anxiolytics (lorazepam, alprazolam) was observed in almost 45% of the sample, a higher value in comparison with other European studies.14,15,53 Prolonged consumption of these substances can be associated with an increase in the risk of falls complicated with hip fractures and can cause depression in older patients.17
The high prevalence of polypharmacy and PIM in nursing home residents confirms that healthcare systems need to be prepared to respond to the consequences of the increased number of age-related comorbidities.54–56 The identification of high-risk medicines in older adults using explicit criteria is a possible strategy to reduce the consequences of inappropriate prescribing.57 Although the appropriate medication for older adult patients should be based on evidence-based clinical guidelines, the implementation of screening tools in clinical practice, as well as frequent reviews of medication58 could ameliorate the prescribing process in older patients, promote the quality of pharmacotherapy in older adults, and ultimately favor better health outcomes.
To avoid confounding effects among the independent variables, PIM influencers were determined using a generalized linear model for dependent negative binomial-type variables.
This study has some limitations, associated with the lack of both representativeness and randomization of the sample, which prevents the generalization of the results in the Portuguese nursing homes and other healthcare models. However, the information collected in this study reinforces the need to frequently review the medication in older adults and suggests that the integration of tools, which identify possible PIM, in health informatics systems as part of Clinical Decision Support to alert general practitioners (GP) could improve medication prescription in older adults.
Conclusion
A great prevalence of PIM prescriptions was observed in institutionalized older patients, and the most frequent were proton pump inhibitors followed by benzodiazepines. It is important to note that the use of PIM classification criteria is an important alert during the prescription and the medication review process; however, the decision of prescribing or not a medicine should be taken by the prescribers, considering the specificities of each patient.
Abbreviations
ADRs, adverse drug reactions; ATC, corresponding Anatomical Therapeutic Classification; DAG, directed acyclic graph; GP, general practitioners; ICD-10, International Statistical Classification of Diseases and Related Health Problems, 10th Revision; IPSS, not-for-profit private institutions of social solidarity; PIM, potentially inappropriate medication; UN, United Nations.
Data Sharing Statement
Datasets generated and analyzed within this study are available upon reasonable request.
Acknowledgments
We would like to thank all nursing home professionals.
Funding
This work was financially supported by the APIMedOlder project [PTDC/MED-FAR/31598/2017], funded by the operational programme of competitiveness and internationalization (POCI), in its FEDER/FNR component POCI-01-0145-FEDER-031598, and the Foundation for Science and Technology (Fundação Para a Ciência e Tecnologia – FCT), in its state budget component (OE).
Disclosure
The authors report no conflicts of interest in this work. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
1. World Population Ageing 2019 Highlights. World Population Ageing 2019 Highlights; 2019. doi:10.18356/9DF3CAED-EN
2. da Costa FA, Silvestre L, Periquito C, et al. Drug-related problems identified in a sample of Portuguese institutionalised elderly patients and pharmacists’ interventions to improve safety and effectiveness of medicines. Drugs. 2016;3:1. doi:10.1007/s40801-016-0061-x
3. Plácido AI, Marante N, Morgado M, Herdeiro MT, Roque F. Intervenção farmacêutica na detecção de problemas relacionados com medicamentos em idosos institucionalizados: revisão sistemática [Pharmacists' interventions in medication-related problems in institutionalized elderly people: A systematic review]. Egitania Sci. 2020;2:25. Portuguese. doi:10.46691/es.v2i25.269
4. Masnoon N, Shakib S, Kalisch-Ellett L, Caughey GE. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017;17:1. doi:10.1186/s12877-017-0621-2
5. Abdulah R, Insani WN, Destiani DP, Rohmaniasari N, Mohenathas ND, Barliana MI. Polypharmacy leads to increased prevalence of potentially inappropriate medication in the Indonesian geriatric population visiting primary care facilities. Ther Clin Risk Manag. 2018;14:1591–1597. doi:10.2147/TCRM.S170475
6. Anathhanam S, Powis RA, Cracknell AL, Robson J. Impact of prescribed medications on patient safety in older people. Ther Adv Drug Saf. 2012;3:4. doi:10.1177/2042098612443848
7. Hanlon JT, Schmader KE, Ruby CM, Weinberger M. Suboptimal prescribing in older inpatients and outpatients. J Am Geriatr Soc. 2001;49:2. doi:10.1046/j.1532-5415.2001.49042.x
8. Weeda ER, AlDoughaim M, Criddle S. Association between potentially inappropriate medications and hospital encounters among older adults: a meta-analysis. Drugs Aging. 2020;37:7. doi:10.1007/s40266-020-00770-1
9. Hedna K, Hakkarainen KM, Gyllensten H, Jönsson AK, Petzold M, Hägg S. Potentially inappropriate prescribing and adverse drug reactions in the elderly: a population-based study. Eur J Clin Pharmacol. 2015;71:12. doi:10.1007/s00228-015-1950-8
10. Chang CB, Lai HY, Hwang SJ, et al. Prescription of potentially inappropriate medication to older patients presenting to the emergency department: a nationally representative population study. Sci Rep. 2018;8(1). doi:10.1038/s41598-018-30184-4
11. Do Nascimento MMG, Mambrini JV, Lima-Costa MF, Firmo JO, Peixoto SW, de Loyola Filho AI. Potentially inappropriate medications: predictor for mortality in a cohort of community-dwelling older adults. Eur J Clin Pharmacol. 2017;73(5):615–621. doi:10.1007/s00228-017-2202-x
12. Morin L, Laroche ML, Texier G, Johnell K. Prevalence of potentially inappropriate medication use in older adults living in nursing homes: a systematic review. J Am Med Dir Assoc. 2016;17:9. doi:10.1016/j.jamda.2016.06.011
13. Albert SM, Colombi A, Hanlon J. Potentially inappropriate medications and risk of hospitalization in retirees: analysis of a US retiree health claims database. Drugs Aging. 2010;27:5. doi:10.2165/11315990-000000000-00000
14. da Costa FA, Periquito C, Carneiro MC, Oliveira P, Fernandes AI, Cavaco-Silva P. Potentially inappropriate medications in a sample of Portuguese nursing home residents: does the choice of screening tools matter? Int J Clin Pharm. 2016;38(5):1103–1111. doi:10.1007/s11096-016-0337-y
15. Monteiro C, Canário C, Ribeiro MÂ, Duarte AP, Alves G. Medication evaluation in Portuguese elderly patients according to beers, stopp/start criteria and EU(7)-PIM list – an exploratory study. Patient Prefer Adherence. 2020;14:795–802. doi:10.2147/PPA.S247013
16. Dimitrow MS, Airaksinen MSA, Kivelä SL, Lyles A, Leikola SNS. Comparison of prescribing criteria to evaluate the appropriateness of drug treatment in individuals aged 65 and older: a systematic review. J Am Geriatr Soc. 2011;59:8. doi:10.1111/j.1532-5415.2011.03497.x
17. Renom-Guiteras A, Meyer G, Thürmann PA. The EU(7)-PIM list: a list of potentially inappropriate medications for older people consented by experts from seven European countries. Eur J Clin Pharmacol. 2015;71:7. doi:10.1007/s00228-015-1860-9
18. Rodrigues DA, Herdeiro MT, Thürmann PA, Figueiras A, Coutinho P, Roque F. Operacionalização para Portugal da Lista EU(7)-PIM para Identificação de Medicamentos Potencialmente Inapropriados nos Idosos [Operationalisation for Portugal of the EU(7)-PIM List for Identification of Potentially Inappropriate Medicines in Older Adults]. Acta Med Port. 2021;34:3. Portuguese. doi:10.20344/amp.13618
19. Relatório de Portugal Comissão Económica das Nações Unidas para a Europa (UNECE) terceiro ciclo de revisão e avaliação da Estratégia de Implementação Regional (RIS) do plano Internacional de ação de Madrid Sobre o Envelhecimento (MIPAA) [Portuguese Department of Economic and Social Affairs. Portuguese report third cycle of review and evaluation of regional Implementation of the Madrid International Plan of Action on Ageing]; 2017. Portuguese.
20. State of health in the EU the country health profile series. Available from: http://www.who.int/bulletin/disclaimer/en/.
21. Nunes AM, Ferreira DC. Reforms in the Portuguese health care sector: challenges and proposals. Int J Health Plan Manag. 2019;34:1. doi:10.1002/hpm.2695
22. Atualizado D. Revisão de categorias de isenção e atualização de valores das taxas moderadoras [ACSS– central administration of the health system. Review of exemption categories and update of moderation fees values]; 2016. Portuguese
23. Anon. WHO ATC/DDD Index 2020. WHO Collaborating Centre for Drugs Statistics Methodology; 2020.
24. World Health Organization. International classification of diseases (ICD-10). Fam Pract Manag. 2010;18:6.
25. Caucat M, Zacarin A, Rousseau V, Montastruc JL, Bagheri H. The cost of potentially inappropriate medications in nursing homes in West Occitanie. Pharmacy. 2020;8:1. doi:10.3390/pharmacy8010039
26. Bourrel C, Zacarin A, Rousseau V, Montastruc JL, Bagheri H. Are potentially inappropriate and anticholinergic medications being prescribed for institutionalized elderly subjects? Fundam Clin Pharmacol. 2020;34(6):743–748. doi:10.1111/fcp.12560
27. Stojanović M, Vuković M, Jovanović M, Dimitrijević S, Radenković M. Potentially InappropriateMedications in Belgrade, Serbia Nursing home residents: a comparison of two approaches. Eval Health Prof. 2021;44:2. doi:10.1177/0163278719900653
28. Opondo D, Eslami S, Visscher S, et al. Inappropriateness of medication prescriptions to elderly patients in the primary care setting: a systematic review. PLoS One. 2012;7:8. doi:10.1371/journal.pone.0043617
29. Morin L, Fastbom J, Aroche ML, Johnell K. Potentially inappropriate drug use in older people: a nationwide comparison of different explicit criteria for population-based estimates. Br J Clin Pharmacol. 2015;80:2. doi:10.1111/bcp.12615
30. Fialová D, Laffon B, Marinković V, et al. Medication use in older patients and age-blind approach: narrative literature review (insufficient evidence on the efficacy and safety of drugs in older age, frequent use of PIMs and polypharmacy, and underuse of highly beneficial nonpharmacological strategies). Eur J Clin Pharmacol. 2019;75:4. doi:10.1007/s00228-018-2603-5
31. Gallagher P, Lang PO, Cherubini A, et al. Prevalence of potentially inappropriate prescribing in an acutely ill population of older patients admitted to six European hospitals. Eur J Clin Pharmacol. 2011;67:11. doi:10.1007/s00228-011-1061-0
32. Simões PA, Santiago LM, Maurício K, Simões JA. Prevalence of potentially inappropriate medication in the older adult population within primary care in Portugal: a nationwide cross-sectional study. Patient Prefer Adherence. 2019;13:1569–1576. doi:10.2147/PPA.S219346
33. Piecoro LT, Browning SR, Prince TS, Ranz TT, Scutchfield ED. A database analysis of potentially inappropriate drug use in an elderly Medicaid population. Pharmacotherapy. 2000;20:2. doi:10.1592/phco.20.3.221.34779
34. Shah SM, Carey IM, Harris T, DeWilde S, Cook DG. Quality of prescribing in care homes and the community in England and Wales. Br J Gen Pract. 2012;62:598. doi:10.3399/bjgp12X641447
35. Bony H, Lloyd RA, Hotham ED, et al. Differences in the prescribing of potentially inappropriate medicines in older Australians: comparison of community dwelling and residential aged care residents. Sci Rep. 2020;10:1. doi:10.1038/s41598-020-66991-x
36. Lane CJ, Bronskill SE, Sykora K, et al. Potentially inappropriate prescribing in Ontario community-dwelling older adults and nursing home residents. J Am Geriatr Soc. 2004;52:6. doi:10.1111/j.1532-5415.2004.52250.x
37. Henschel F, Redaelli M, Siegel M, Stock S. Correlation of incident potentially inappropriate medication prescriptions and hospitalization: an analysis based on the PRISCUS list. Drugs. 2015;2:3. doi:10.1007/s40801-015-0035-4
38. Laroche ML, Charmes JP, Merle L. Potentially inappropriate medications in the elderly: a French consensus panel list. Eur J Clin Pharmacol. 2007;63:8. doi:10.1007/s00228-007-0324-2
39. Moriarty F, Cahir C, Bennett K, Fahey T. Economic impact of potentially inappropriate prescribing and related adverse events in older people: a cost-utility analysis using Markov models. BMJ Open. 2019;9:1. doi:10.1136/bmjopen-2018-021832
40. Jokanovic N, Tan ECK, Dooley MJ, Kirkpatrick CM, Bell JS. Prevalence and factors associated with polypharmacy in long-term care facilities: a systematic review. J Am Med Dir Assoc. 2015;16:6. doi:10.1016/j.jamda.2015.03.003
41. Advinha AM, de Oliveira-Martins S, Mateus V, Pajote SG, Lopes MJ. Medication regimen complexity in institutionalized elderly people in an aging society. Int J Clin Pharm. 2014;36:4. doi:10.1007/s11096-014-9963-4
42. Pinto I, Nascimento L, Pereira F, Azevedo J, Pereira S. Polypharmacy in institutionalized elderly in nursing homes of Northern Portugal. 2nd World Congress of Health Research; 2014. Vol 46.
43. Onder G, Liperoti R, Fialova D, et al. Polypharmacy in nursing home in Europe: results from the SHELTER study. J Gerontol. 2012;67 A(6). doi:10.1093/gerona/glr233
44. Jankyova S, Rubintova D, Foltanova T. The analysis of the use of potentially inappropriate medications in elderly in the Slovak Republic. Int J Clin Pharm. 2020;42:1. doi:10.1007/s11096-019-00944-x
45. Paque K, Elseviers M, Vander Stichele R, et al. Associations of potentially inappropriate medication use with four year survival of an inception cohort of nursing home residents. Arch Gerontol Geriatr. 2019;80:82–87. doi:10.1016/j.archger.2018.10.011
46. Pretorius RW, Gataric G, Swedlund SK, Miller JR. Reducing the risk of adverse drug events in older adults. Am Fam Physician. 2013;87:5.
47. Wang TC, Ku PJ, Lu HL, Hsu KC, Trezise D, Wang HY. Association between potentially inappropriate medication use and chronic diseases in the elderly. Int J Environ Res Public Health. 2019;16:12. doi:10.3390/ijerph16122189
48. Alturki A, Alaama T, Alomran Y, Al-Jedai A, Almudaiheem H, Watfa G. Potentially inappropriate medications in older patients based on Beers criteria: a cross-sectional study of a family medicine practice in Saudi Arabia. BJGP Open. 2020;4:1. doi:10.3399/bjgpopen20X101009
49. Sönnerstam E, Sjölander M, Gustafsson M. An evaluation of the prevalence of potentially inappropriate medications in older people with cognitive impairment living in Northern Sweden using the EU(7)-PIM list. Eur J Clin Pharmacol. 2017;73:6. doi:10.1007/s00228-017-2218-2
50. Jaynes M, Kumar AB. The risks of long-term use of proton pump inhibitors: a critical review. Ther Adv Drug Saf. 2018;10. doi:10.1177/2042098618809927
51. Yang YX, Lewis JD, Epstein S, Metz DC. Long-term proton pump inhibitor therapy and risk of Hip fracture. J Am Med Assoc. 2006;296:24. doi:10.1001/jama.296.24.2947
52. Gomm W, von Holt K, Thomé F, et al. Association of proton pump inhibitors with risk of dementia: a pharmacoepidemiological claims data analysis. JAMA Neurol. 2016;73:4. doi:10.1001/jamaneurol.2015.4791
53. Ravera S, Hummel SA, Stolk P, Heerdink RE, de Jong-Van Den Berg LTW, de Gier JJ. The use of driving impairing medicines: a European survey. Eur J Clin Pharmacol. 2009;65(11):1139–1147. doi:10.1007/s00228-009-0695-7
54. Prados-Torres A, Del Cura-González I, Prados-Torres D, et al. Effectiveness of an intervention for improving drug prescription in primary care patients with multimorbidity and polypharmacy: study protocol of a cluster randomized clinical trial (Multi-PAP project). Implement Sci. 2017;12:1. doi:10.1186/s13012-017-0584-x
55. Wastesson JW, Morin L, Laroche ML, Johnell K. How chronic is polypharmacy in old age? A Longitudinal Nationwide Cohort Study. J Am Geriatr Soc. 2019;67:3. doi:10.1111/jgs.15717
56. Feng X, Tan X, Riley B, Zheng T, Bias T, Sambamoorthi U. Polypharmacy and multimorbidity among Medicaid enrollees: a multistate analysis. Popul Health Manag. 2018;21:2. doi:10.1089/pop.2017.0065
57. Karandikar YS, Chaudhari SR, Dalal NP, Sharma M, Pandit VA. Inappropriate prescribing in the elderly: a comparison of two validated screening tools. J Clin Gerontol Geriatr. 2013;4:4. doi:10.1016/j.jcgg.2013.04.004
58. Stuhec M, Bratović N, Mrhar A. Impact of clinical pharmacist’s interventions on pharmacotherapy management in elderly patients on polypharmacy with mental health problems including quality of life: a prospective non-randomized study. Sci Rep. 2019;9:1. doi:10.1038/s41598-019-53057-w
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
