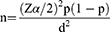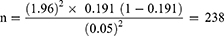Back to Journals » Infection and Drug Resistance » Volume 15
Assessment of Bacterial Profiles and Antimicrobial Susceptibility Pattern of Isolates Among Patients Diagnosed with Surgical Site Infections at Mizan-Tepi University Teaching Hospital, Southwest Ethiopia: A Prospective Observational Cohort Study
Authors Abayneh M, Asnake M , Muleta D, Simieneh A
Received 10 January 2022
Accepted for publication 12 March 2022
Published 13 April 2022 Volume 2022:15 Pages 1807—1819
DOI https://doi.org/10.2147/IDR.S357704
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Mengistu Abayneh,1 Molla Asnake,2 Dassalegn Muleta,1 Asnake Simieneh1
1College of Medical and Health Science, Department of Medical Laboratory Sciences, Mizan-Tepi University, Mizan-Aman, Southwest Ethiopia; 2College of Medical and Health Science, Department of Medicine, Mizan-Tepi University, Mizan-Aman, Southwest Ethiopia
Correspondence: Mengistu Abayneh, Email [email protected]
Background: Although emergency health-care services, particularly clinical and surgical care, are an important part of the provision of high quality health care in Ethiopia, infections related with surgical care are still the most well-known medical services-related diseases. This study aimed to assess the bacterial profiles and antimicrobial susceptibility pattern of isolates among patients diagnosed with surgical site infections at Mizan-Tepi university teaching hospital, southwest Ethiopia.
Methodology: A prospective observational cohort study was conducted from June to September 2021. Patient data were collected using a structured questionnaire. Follow-up of patients who had undergone a surgical procedure was conducted for at least 30 days. Wound swabs were collected from patients suspected to have surgical site infections (SSIs) and cultured onto appropriate culture media. The antimicrobial susceptibility testing was done using the disk diffusion technique. Data were analyzed using SPSS software version 25.0. Frequencies and cross-tabulation were used to summarize descriptive statistics.
Results: In this study, the postoperative SSIs rate was 12.6%. All patients with SSIs were culture positive, and a total of 41 bacterial isolates were detected. Of these, 73.2% were Gram-negative, 26.8% were Gram-positive and 24.2% were a mixture of two bacterial growths. Escherichia coli accounted for 29.3%, followed by Staphylococcus aureus (19.5%), Proteus species (14.6%) and Pseudomonas aeruginosa (12.2%). With the exceptions of amikacin and meropenem, which exhibited very high sensitivity, ranging from 33.3– 100.0% isolates was resistant against all other tested antibiotics. The resistance rate to three or more classes of antibiotics was 100.0%.
Conclusion: In this study, the most isolated bacteria causing SSIs were Gram-negative and multidrug-resistant strains. This event highlights that surveillance of the bacterial profile and antibiotic susceptibility pattern coupled with the implementation of the strict protocol for antibiotic use and operative room regulations is important to minimize the burden of SSIs.
Keywords: surgical site infections, bacterial profile, antimicrobial resistance, Ethiopia
Introduction
Surgical site infections (SSIs) are the most widely recognized medical services-related infections around the world. A World Health Organization (WHO) report showed that SSIs are the most detailed and frequent types of health care-associated infections (HAIs) in low- and middle-income countries and affect up to one third of patients who have undergone a surgical procedure. The SSIs are the most recognized nosocomial infections, accounting for 15% of all nosocomial infections. The risk is that most SSIs are caused by bacteria which are resistant to commonly used antimicrobial agents, and can be multidrug-resistant pathogens.1–3
In developing countries, the risk of getting SSIs associated with surgical care is high due to various types of risk factors, including low adherence to safety precautions at the level of the patient, procedure, hospital setting, or surgical team.2,4 Hence, the risk of getting nosocomial infections in a developing country is 2–20 times higher than in developed nations.5 For instance, the pooled prevalence of HAI is 15.5%, where surgical site infections are the leading infection with a pooled incidence of 5.6%, which is significantly higher than the proportions in developed countries (Europe and the USA).6 In Africa alone, the cumulative incidence of surgical site infection ranged from 2.5–30.9%.7 There have been a number of deaths and disabilities related to post-operative complications, such as an additional need for treatment of SSIs, prolonged hospital stays, and even death.8,9
The most common bacteria isolated from SSIs are S. aureus including methillin and vancomycin resistant S. aureus, Enterobacteriaceae, coagulase-negative Staphylococci (CoNS), Enterococci and Pseudomonas aeruginosa. These microorganisms can enter a surgical wound either by direct contact with airborne dispersal10,11 or by contamination.12 The native flora of the skin and mucous membranes of the patients are the most common source of causative agents of SSIs.12 SSIs may also be caused by organisms present in the hospital environment, including medical equipment.12–14
Substantial research has been conducted to prevent SSIs, and, as a result, recommendations have been published as guidelines for SSIs. Conducting continuous and high-quality surveillance on SSIs is crucial to determine the extent of the problem and to assess the impact of any existing prevention/improvement intervention.2,15 In Ethiopia, though some studies have been conducted on the prevalence of SSIs, which ranged from 11.0–25.5%,16,17 still there has been scarce updated evidence data on the bacterial causes of SSIs and their antimicrobial susceptibility patterns to give emphasis to the situation and to design local and national strategies to prevent and control its burden. Moreover, it is useful to collect data on the risk factors in order to analyze SSI outcomes by subgroup, identify high-risk patients, and control for differences in the patient-level risk. Thus, this study was conducted to assess the bacterial profile and antimicrobial susceptibility pattern of isolates among patients diagnosed with SSIs at Mizan-Tepi university teaching hospital, southwest Ethiopia.
Materials and Methods
Study Area and Period
The study was conducted at Mizan-Tepi university teaching hospital, in the southwest of Ethiopia, from June–September, 2021. Mizan-Tepi university teaching hospital is found in Mizan-Aman town which is located 561 km southwest of Addis Ababa. Currently, it is the second teaching hospital with more than 139 beds in the southwestern part of the country, providing services for approximately 8000 inpatients, 57,184 outpatient attendants, 14,508 emergency cases, and 4080 births in a year coming to the hospital from the catchment population of 5 million people.
Study Design and Study Subjects
A prospective observational cohort study design was conducted. All patients who underwent a surgical procedure at Mizan-Tepi university teaching hospital during the study period were included in this study. Then we followed the key criteria of the CDC-NHSN and WHO for the definition of a SSI which is the fixed time period of 30 days of follow-up (where day 1 = the procedure date) for superficial SSI,2,18 which would normally include both inpatient and outpatient periods. Those patients who developed postoperative wound infections later than 30 days and patients who had infected burn wounds as well as those with an initial diagnosis suggestive of infection were excluded.
Sampling Size Determination
The required sample size was calculated using the single population proportion formula by assuming a confidence interval (CI) of 95%, margin error (d) of 5%, and the rate of SSIs (p) of 19.1%,19 which is reported in a previous study in Ethiopia.
Therefore, the value of n will be:
By considering a 10% non-response rate, the final sample size for this study was 262.
Data Collection Tool and Data Collection
Socio-Demographic and Risk Factors Data Collection
Patient data were collected using a structured and pretested questionnaire. The variables and some of the method descriptions were customized from previous studies and protocols.19,20 Socio-demographic, procedural, and clinical data were collected by interviewing patients or guardians using structured questionnaires and from patients’ medical recording charts and patients’ operation notes using a data abstraction tool. Short training and orientations were given to two nurses and one laboratory technologist who were recruited as data collectors. Surgeons and attending physicians have undertaken the clinical evaluation of SSIs. All patients who had undergone surgical procedures were followed and the charts were reviewed before, during, and after the operation until the patients were discharged from the hospital and after discharge till 30 days since the operations were done. After discharge, the follow-up of the operation wound was made with mobile telephone calls, and if a SSI was suspected, the patient was asked to return to the hospital for confirmation of the diagnosis. The CDC-NHSN and WHO criteria for the definition of a SSI2,18 were applied to recruit those patients suspected of developing SSIs.
Laboratory Data Collections
Swab samples were collected aseptically using sterile cotton swabs from SSIs before the wound was cleaned with an antiseptic solution. The surrounding areas of the surgical wound were cleaned with 70% ethyl alcohol and excess debris from the wound base was removed by irrigating with normal saline to avoid contaminating the swab with skin flora. Then, two swab samples were collected from at least 1 cm2 area of viable wound tissue using a sterile cotton swab from the infected site, one for Gram stain and another for culture. The deepest parts of the wounds were sampled, to avoid superficial micro-flora. All the collected samples were kept in test tubes containing 1.0 mL of normal saline and taken to the microbiology laboratory of Mizan-Tepi university for analysis within 3 hours after collection. An aseptic technique was applied to avoid cross-contamination at all stages.
Culturing and Identification Procedures
All the swab samples were cultured onto MacConkey and mannitol salt agar (Oxoid, UK) by rolling the swab over the agar and streaking from the primary inocula. The inoculated plates were kpt for 24–48 h at 35–37°C for the organisms to be grown. Isolation and identification were done as per standard techniques.21 First, screening was made by Gram staining and with their colony characteristics. Members of the family Enterobacteriaceae were differentiated from other Gram-negative bacilli by performing two rapid catalase and oxidase tests. Biochemical tests such as citrate utilization test, Triple Sugar Iron (TSI) agar test, Sulphite-Indole–Motility (SIM) test, and urease tests were used to differentiate enteric bacteria. Staphylococcus aureus were identified from CoNS with their colony characteristics on the mannitol salt agar plate and by performing coagulase tests. All the isolates were kept at 2–8°C in nutrient broth until antimicrobial sensitivity tests were done.
Antibiotic Sensitivity Testing
Kirby–Bauer disc diffusion technique was employed to determine antimicrobial susceptibility patterns of all isolates. The selection of the tested antibiotics depended on the isolated bacteria and the local antibiotics regimen. Standard suspension of a pure colony was done in sterile normal saline. Sterile cotton-tipped applicator sticks were used to spread the suspensions onto Muller-Hinton agar (Oxoid, UK). Antibiotics disks, such as ampicillin (10 µg), amoxicillin-clavulanic acid (30 µg), ceftriaxone (30 μg), cefoxitin (30 μg), gentamicin (10 μg), amikacin (30 μg), ciprofloxacin (5 μg), doxycycline (30 μg), trimethoprim-sulfamethoxazole (1.25/23.75 μg), clindamycin (2 μg), chloramphenicol (30 µg), erythromycin (15 µg) and meropenem (30 µg) were placed using sterile forceps. The zone of inhibition was measured and interpreted by comparing the zone with Kirby–Bauer chart as per CLSI 2018.22 Multidrug-resistance (MDR) is considered as resistance to three or more classes of antibiotics.
Quality Assurance
The manufacturer’s instructions on all the materials, culture media, and reagents were strictly followed. The ability of culture media to support the growth of common organisms causing SSIs was determined by inoculating the media with a typical stock culture. Escherichia coli ATCC*25922 and Staphylococcus aureus ATCC25923 were used as quality control strains.
Data Analysis
The data were analyzed for descriptive statistics using SPSS version 25.0. Descriptive statistics, such as count and percentage were calculated to describe bacterial profile, antimicrobial susceptibility patterns, demographic of the subjects, and clinical and procedural related variables. Frequencies and cross-tabulation were employed to describe the magnitude of SSIs in relation to each patient’s demographic, clinical and procedural characteristics. Statistically significant differences were determined at P-values <0.05. Finally, the data were presented in tables and graphs as appropriate.
Results
Socio-Demographic Characteristics and Magnitude of SSIs
In this study, a total of 262 patients underwent surgical procedures, all of whom were followed up during the study. Of these, 33/262 (12.6%) developed SSIs within 30 days of the procedure. Most, 145/262 (55.3%), of the patients were in the age group 19–35 years and just over half of the participants, 156/262 (59.6%), were females. A higher proportion of SSIs was observed among the age group of >50 years (8/20; 40.0%), a difference which was statistically significant (p = 0.001). More than half, 135/262 (51.5%), of the participants were from rural areas and over half 141/262 (53.8%) had completed their primary school education (Table 1).
 |
Table 1 Socio-Demographic and Clinical Characteristics of Study Participants |
The rate of SSIs among consumers who drank alcohol and consumers who did not was 11/64 (17.2%) and 22/198 (11.1%), respectively, and the difference was not statistically significant (p >0.05). Only 3/17 (17.6%) patients with smoking habits developed SSIs, which did not show a statistically significant difference with non-smokers, 30/245 (12.2%) (p >0.05) (Table 1).
Procedural and Clinical Characteristics and Magnitude of SSIs
As shown in Table 2, cesarean section (C/S) accounted for 150/262 (57.3%), followed by gastrointestinal tract, 53/262 (20.2%), and genitourinary tract, 21/262 (8.0%). The rate of SSIs in cesarean section (C/S) was 14/150 (9.3%), followed by 9/53 (17.0%) in the gastrointestinal tract and 5/21 (23.8%) in the genitourinary tract. About the types of surgery, just over half of the participants, 145/262 (55.3%), had emergency surgery, and the rate of SSIs among patients with emergency surgery was 19/145 (13.1%) and among elective surgery, it was 14/117 (12.0%).
 |
Table 2 Procedure and Clinical Related Characteristics of Study Participants |
Regarding the types of wounds, a large number of patients, 234/262 (89.3%), had clean or clean-contaminated wounds. The rate of SSIs was 22/234 (9.4%) in patients with clean or clean- contaminated wounds, compared with 11/28 (39.3%) in patients with contaminated wounds, a statistically significant difference (p = 0.001) (Table 2).
The rate of SSIs in patients with ASA score <III was 28/251 (11.2%), while the rate of SSIs in patients with ASA score ≥III was 5/11 (45.4%), the difference was statistically significant (p = 0.001). Regarding types of provided antibiotics prophylaxis, the rate of SSIs among patients taking ceftriaxone alone or with metronidazole as antibiotic prophylaxis was 14/164 (8.5%), and for those taking ampicillin the rate was 19/98 (19.4%), this difference was statistically significant (p = 0.010) (Table 2).
Prevalence of Co-Morbid Conditions
In this study, co-morbidities were defined as the presence of one or more other conditions co-occurring with the disease of primary concern during surgery. Accordingly, the overall presence of co-morbid conditions was 31/262 (11.8%) and the rate of SSIs among patients with co-morbidity and those without co-morbidity was 11/31 (35.5%) and 22/231 (9.5%), respectively, which showed a statistically significant difference (p = 0.010). The most common co-morbidity was anemia in 8/262 (3.1%), followed by cardiac disorder in 6/262 (2.3%), diabetes in 5/262 (1.9%), respiratory disease in 4/262 (1.5%), malignancy in 4/262 (1.5%) and HIV/AIDS in 4/262 (1.5%). The rate of SSIs in anemic patients was 4/8 (50.0%) and 29/254 (11.4%) in non-anemic patients, the difference was statistically significant (p = 0.001). In addition, the rate of SSIs in diabetic patients was 3/5 (60.0%) and 30/257 (11.7%) in non-diabetic patients, the difference was statistically significant (p = 0.001) (Tables 1 and 3).
 |
Table 3 Co-Morbidity Conditions Among Study Participants |
Bacterial Isolates
For patients who developed SSIs (n = 33), wound swabs were collected and cultured on appropriate media. All SSIs patients had culture positive at least for one bacterial growth giving a total of 41 bacterial isolates. Out of a total of 41 bacterial isolates, 73.2% (30/41) of these were Gram-negative, 26.8% (11/41) were Gram-positive and 24.2% (8/33) were a mixture of two bacterial growths. Among the types of bacteria identified, Escherichia coli accounted for 29.3% (12/41), followed by Staphylococcus aureus at 19.5% (8/41), Proteus species at 14.6% (6/41), Pseudomonas aeruginosa at 12.2% (5/41), and Klebsiella species at 9.8% (4/41) (Figure 1).
 |
Figure 1 Proportion and types of bacteria isolated from patients diagnosed with SSIs. |
Antimicrobial Resistance Profiles
In this study, antimicrobial resistance analysis was performed on microorganisms isolated from patients with surgical site infections. Hence, all (100.0%) Gram-negative isolates showed resistance toward ampicillin, and 91.7% of isolates were resistant toward amoxicillin-clavulanic acid. In addition, ranging from 50.0% to 83.3%, Gram-negative isolates were resistant to ceftriaxone, cefoxitin, gentamicin, ciprofloxacin, doxycycline, cotrimoxazole, and chloramphenicol. Gram-positive isolates of S. aureus showed higher resistance towards ampicillin (87.5%) followed by amoxicillin-clavulanic acid, doxycycline, cotrimoxazole, clindamycin, and erythromycin, all with resistance rate of 75.0%. S. aureus also showed resistance to gentamicin and chloramphenicol, each with a resistance rate of 62.5%. Using a cefoxitin disk as a surrogate marker, 3/8 (37.5%) of S. aureus were methicillin-resistant S. aureus (MRSA). Two out of three (66.7%) isolates of CoNS showed resistance to the above antibiotics, except cefoxitin, in which one out of three (33.3%) CoNS showed resistance. Multidrug-resistance (MDR) towards three or more classes of antibiotics was 100.0%. None of the isolates were resistant to amikacin and only (7.3%) of Gram-negative isolates were resistant to meropenem (Table 4).
 |
Table 4 Prevalence of Antimicrobial Resistance Pattern of Bacteria Isolated from Patients Diagnosed with SSIs (n = 41) |
Discussion
This is the first SSIs surveillance study, which has attempted to estimate the burden of SSIs by determining not only their magnitude but also the etiological bacterial agents causing SSIs and their antimicrobial susceptibility patterns at Mizan-Tepi university teaching hospital in southwest districts of Ethiopia. The rate of SSIs in this study was 12.6% and among cesarean sections, it was 9.3% (14/150). The current finding is slightly higher than those found in studies from different parts of the developing world, including in Ethiopia.23–27 In low- and middle-income countries (LMICs), the combined incidence of SSIs is 11.8%.6,28 However, this finding is lower than the study finding in other parts of Ethiopia, in which the average rates of SSIs was 21.5%.16,29–32 Compared with developed countries, this finding is higher than those of several European countries and the USA, in which the cumulative incidence of SSIs ranges from 0.8–9.5% in Europe33 and is 0.9% in the USA.34
HAIs may be caused by infectious agents from endogenous or exogenous sources, such as normal flora of the skin, nose, mouth, gastrointestinal tract, or vagina and those external to the patient, such as patient care personnel, visitors, patient care equipment, medical devices, or the health-care environment.2,4,18 For example, studies have reported detection of microorganisms from inanimate contact surfaces in various medical devices and health-care settings.35–37 Hence, the rate of SSIs observed in this study as well as in other studies conducted in the country may be related to lack of or inappropriate use of medical equipment and materials necessary to maintain strict guidelines for asepsis, poor hygienic conditions of patients, and hospital set-ups, poorly trained staff, and poor implementation and follow-up strategies in providing high-quality health care.
This study also tried to assess the rate of SSIs in relation to patients’ socio-demographic, procedural, and clinical characteristics. Many factors in the patient’s journey through surgery have been identified as contributing to the risk of SSIs. Accordingly, a significantly higher rate of SSIs was observed in the age group of >50 years, presence of co-morbid conditions such as anemia and diabetic mellitus, post-operative hospital stay greater than 14 days, ASA class ≥III, contaminated wound procedure, and providing ampicillin as antibiotic prophylaxis (p <0.05).
The present findings seem to be consistent with other research findings which highlighted some SSI risk factors, such as age, ASA class, wound classification, skill and experience of the surgeon, duration of surgery, blood transfusion, and emergency surgery.16,19,26,29,31 Therefore, the prevention of these infections is complex and requires a comprehensive set of preventive measures before, during, and after surgery.
The other objective of the present study was to evaluate bacterial profiles causing SSIs and their antimicrobial resistance patterns. Accordingly, 73.2% Gram-negative and 26.8% Gram-positive SSIs-causing bacteria were detected. Of the Gram-negatives, Escherichia coli accounted for 29.3%, followed by Proteus spp. (14.6%), Pseudomonas aeruginosa (12.2%), Klebsiella spp. (9.8%) and Citrobacter spp. (7.3%). From Gram-positive bacteria, Staphylococcus aureus is the predominant (19.5%) isolate, followed by CoNS (7.3%). The predominance of the above isolates is inconsistent with study findings in other parts of Ethiopia,29,30,38,39 as well as in other developing countries.26,40 The advantages of the above isolates have also been reported in several European countries.41 Although there are no clear explanations about the main cause of SSIs by these bacteria in the current study, it is possible that the presence of these microorganisms as normal flora of the patient’s body and in a hospital environment, including different types of medical equipment may lead to post-procedural contamination of surgical wounds. For instance, many studies have reported that these microorganisms were detected from different medical devices and inanimate contact surfaces in the health-care environment.35–37
In this study, high rates of resistance to commonly used antibiotics were observed in bacterial isolates causing SSIs. All (100.0%) Gram-negative isolates were resistant to ampicillin, while 91.7% of isolates were resistant to amoxicillin-clavulanic acid. In addition, 50.0–83.3% of isolates were resistant to ceftriaxone, cefoxitin, gentamicin, ciprofloxacin, doxycycline, cotrimoxazole, and chloramphenicol. Multidrug-resistance (MDR) towards more commonly used three or more classes of antibiotics was 100.0%. High resistance rates toward the above antibiotics were also reported in other studies.26,29,30,38,39 A study in Tanzania also showed that the majority (97%) of the Gram-negative bacteria were resistant to more than four classes of antibiotics.40 This can pose a huge challenge for the prevention of SSIs, especially in our case where ampicillin and ceftriaxone are the most commonly used antibiotics in all patients undergoing surgery.
Among Gram-positive bacteria, 87.5% of Staphylococcus aureus showed resistance to ampicillin, and 75.0% were resistant to amoxicillin-clavulanic acid, doxycycline, cotrimoxazole, clindamycin, and erythromycin. In addition, 37.7–62.5% of S. aureus was resistant to cefoxitin, gentamicin, and chloramphenicol. The presence of highly resistant bacteria causing SSIs has also been reported in other studies.26,29,30,38–40 This significantly higher resistance may be related to the frequent and inappropriate use of these antibiotics for therapeutic and prophylactic purposes in human infections. The studies have demonstrated almost linear relationships between antibiotic consumption and the prevalence of antimicrobial resistance (AMR).42,43 For instance, in Ethiopia, many studies confirmed that ceftriaxone is the most prescribed antibiotic prophylaxis.44,45 The use of broad-spectrum antibiotics increases the selective pressure of bacteria and stimulates the emergence of multi-drug resistant pathogens.
Limitations of This Study
In this study, blood agar medium was not used to detect SSI-causing pathogens, which could undermine the prevalence and types of isolated bacteria. The overall number of patients with SSIs was small, which limits the ability to estimate the true prevalence of AMR. Due to resource issues, molecular techniques to provide more information to indicate specific antibiotic resistance genes have not been performed. Furthermore, it should be noted that these results may not be generalizable to other settings.
Conclusion
The prevalence of SSIs observed in the study remains high, despite this being one of the priority areas for the provision of high-quality surgical care in our country, Ethiopia. All patients with SSIs were culture positive for at least one bacterial species. Multidrug-resistance was seen in almost all of the isolates leaving clinicians with few choices of drugs for the treatment of patients with SSIs. Moreover, an increased prevalence of SSIs was observed in older patients, the presence of co-morbid conditions such as anemia and diabetes mellitus, postoperative hospital stay, types provided for antibiotic prophylaxis, and types of wound and ASA class. Therefore, periodic surveillance of the bacterial profile and antibiotic susceptibility testing coupled with the implementation of a strict protocol for antibiotic administration and operative room regulations is important to minimize the burden of SSIs with resistant bacteria pathogens.
Data Sharing Statement
All the data supporting our findings were incorporated within the manuscript.
Ethical Approval and Consent to Participate
Ethical approval was obtained first from Mizan-Tepi university Ethical Review Board. Official permission was sought from the Mizan-Tepi university teaching hospital administration. Before data collection, written informed consent was obtained from respondents and/or legal guardians. This study complied with the Declaration of Helsinki, and all methods were performed in accordance with the relevant guidelines and regulations.
Acknowledgments
We would like to thanks Mizan-Tepi university for giving permission of the laboratory set-up with necessary supplies. We also thank all the data collectors and study participants for participating in this study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
No funding was allocated for this study.
Disclosure
The authors declare that they have no competing interest in this work.
References
1. World Health Organization. Global Guidelines for the Prevention of Surgical Site Infection.
2. World Health Organization. Protocol for Surgical Site Infection Surveillance with a Focus on Settings with Limited Resources. Geneva: World Health Organization; 2018. Licence:CC BY-NC-SA 3.0 IGO.
3. Owens CD, Stoessel K. Surgical site infections: epidemiology, microbiology and prevention. J Hosp Infect Suppl. 2008;2:3–10. doi:10.1016/S0195-6701(08)60017-1
4. Asaad AM, Badr SA. Surgical site infections in developing countries: current burden and future challenges. Clin Microbiol. 2016;5:e136. doi:10.4172/2327-5073.1000e136
5. World Health Organization. 2015. Infection prevention and control recovery plans and implementation: Guinea, Liberia, and Sierra Leone inter-country meeting: final report, 20–22 July 2015, Monrovia, Liberia. World Health Organization. Available from: https://apps.who.int/iris/handle/10665/204370.
6. Allegranzi B, Bagheri Nejad S, Combescure C, et al. Burden of endemic health-care-associated infection in developing countries: systematic review and meta-analysis. Lancet. 2011;377(9761):228–241. doi:10.1016/S0140-6736(10)61458-4
7. Bagheri Nejad S, Allegranzi B, Syed SB, Ellis B, Pittet D. Health-care-associated infection in Africa: a systematic review. Bull World Health Organ. 2011;89(10):757–765. doi:10.2471/BLT.11.088179
8. Coello R, Charlett A, Wilson J, et al. Adverse impact of surgical site infections in English hospitals. J Hosp Infect. 2005;60(2):93–103. doi:10.1016/j.jhin.2004.10.019
9. Bashaw MA, Keister KJ. Peri-operative strategies for surgical site infection prevention. AORN J. 2018;109(1):68–78. doi:10.1002/aorn.12451
10. Fischer S, Thieves M, Hirsch T, et al. Reduction of airborne bacterial burden in the OR by installation of Unidirectional Displacement Airflow (UDF) systems. Med Sci Monit. 2015;21:2367–2374. doi:10.12659/MSM.894251
11. Nichols RL. Techniques known to prevent postoperative wound infection. Infect Control. 1982;3:34–37. doi:10.1017/S0195941700057088
12. Solomkin J, Gastmeier P, Bischof P, et al. WHO global guidelines for the prevention of surgical site infection Geneva. Switzerland. Lancet Infect Dis. 2017;17(3):262–264. doi:10.1016/S1473-3099(17)30081-6
13. Sievert DM, Ricks P, Edwards JR, et al. Antimicrobial resistant pathogens associated with health care associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009–2010. Infect Control Hosp Epidemiol. 2013;34(1):1–14. doi:10.1086/668770
14. Weiner LM, Webb AK, Limbago B, et al. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2011–2014. Infect Control Hosp Epidemiol. 2016;37(11):1288–1301. doi:10.1017/ice.2016.174
15. Seale AC, Hutchison C, Fernandes S, et al. Supporting surveillance capacity for antimicrobial resistance: laboratory capacity strengthening for drug resistant infections in low and middle income countries. Wellcome Open Res. 2017;2:91. doi:10.12688/wellcomeopenres.12523.1
16. Afenigus AD, Shbabawu AT, Melese TG, et al. Surgical site infection and associated factors among adult patients admitted in west and east Gojjam zone hospitals, Amhara region, Ethiopia. Nurse Care Open Acces J. 2019;6(3):
17. Samuel W, Mehretu B, Samson G. Magnitude and factors associated with post-cesarean surgical site infection at Hawassa University Teaching and Referral Hospital, Southern Ethiopia: a cross-sectional study. J Health Sci. 2017;27(3):283. doi:10.4314/ejhs.v27i3.10
18. CDC/NHSN surveillance definitions for specific types of infections. Atlanta (GA): Centers for Disease Control and Prevention; 2017. Available from: https://www.cdc.gov/nhsn/pdfs/pscmanual/17ps.cnosinfdef_current.pdf.
19. Laloto TL, Gemeda DH, Abdella SH. Incidence and predictors of surgical site infection in Ethiopia: prospective cohort. BMC Infect Dis. 2017;17:119. doi:10.1186/s12879-016-2167-x
20. World Health Organization. Protocol for Surgical Site Infection Surveillance with a Focus on Settings with Limited Resources. Geneva: World Health Organization; 2018. Licence: CC BY-NC-SA 3.0 IGO.
21. Steel KJ, Barrow G, Feltham R. Cowan and steel’s Manual for the Identification of Medical Bacteria. Cambridge: Cambridge University Press; 1993.
22. Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing.
23. Fisha K, Azage M, Mulat G, et al. The prevalence and root causes of surgical site infections in public versus private hospitals in Ethiopia: a retrospective observational cohort study. Patient Saf Surg. 2019;13:26. doi:10.1186/s13037-019-0206-4
24. Ayala D, Tolossa T, Markos J, Yilma MT. Magnitude and factors associated with surgical site infection among mothers underwent cesarean delivery in Nekemte town public hospitals, western Ethiopia. PLoS One. 2021;16(4):e0250736. doi:10.1371/journal.pone.0250736
25. Alemye T, Oljira L, Fekadu G, Mengesha MM. Post cesarean section surgical site infection and associated factors among women who delivered in public hospitals in Harar city, Eastern Ethiopia: a hospital-based analytic cross-sectional study. PLoS One. 2021;16(6):e0253194. doi:10.1371/journal.pone.0253194
26. Mukagendaneza MJ, Munyaneza E, Muhawenayo E, et al. Incidence, root causes, and outcomes of surgical site infections in a tertiary care hospital in Rwanda: a prospective observational cohort study. Patient Saf Surg. 2019;13:10. doi:10.1186/s13037-019-0190-8
27. Akhter MS, Verma R, Madhukar KP, Vaishampayan AR. Incidence of surgical site infection in postoperative patients at a tertiary care centre in India. Wound Care. 2016;25(4):214–217. doi:10.12968/jowc.2016.25.4.210
28. World Health Organization. Report on the Burden of Endemic Health Care-Associated Infection Worldwide. A Systematic Review of the Literature. Geneva: World Health Organization; 2011.
29. Misha G, Chelkeba L, Melaku T. Bacterial profile and antimicrobial susceptibility patterns of isolates among patients diagnosed with surgical site infection at a tertiary teaching hospital in Ethiopia: a prospective cohort study. Ann Clin Microbiol Antimicrob. 2021;20(1):33. doi:10.1186/s12941-021-00440-z
30. Gebissa T, Bude B, Yasir M, et al. Bacterial isolates and their antibiotic sensitivity pattern of surgical site infections among the surgical ward patients of Asella Referral and Teaching Hospital. Futur J Pharm Sci. 2021;7:100. doi:10.1186/s43094-021-00255-x
31. Mezemir R, Seid A, Gishu T, Demas T, Gize A. Prevalence and root causes of surgical site infections at an academic trauma and burn center in Ethiopia: a cross-sectional study. Patient Saf Surg. 2020;14:1–7. doi:10.1186/s13037-019-0229-x
32. Kefale B, Tegegne GT, Degu A, Molla M, Kefale Y. Surgical site infections and prophylaxis antibiotic use in the surgical ward of public hospital in Western Ethiopia: a hospital-based retrospective cross-sectional study. Infect Drug Resist. 2020;15(13):3627–3635. doi:10.2147/IDR.S281097
33. European Center for Disease Prevention and Control. Surveillance of Surgical Site Infections in Europe 2010–2011. Stockholm: ECDC; 2013.
34. National and state healthcare-associated infections progress report. Atlanta (GA): National Center for Emerging and Zoonotic Infectious Diseases, Centers for Disease Control and Prevention; 2016. Available from: http://www.cdc.gov/HAI/pdfs/progressreport/hai-progress-report.pdf.
35. Darge A, Kahsay AG, Hailekiros H, Niguse S, Abdulkader M. Bacterial contamination and antimicrobial susceptibility patterns of intensive care units medical equipment and inanimate surfaces at Ayder Comprehensive Specialized Hospital, Mekelle, Northern Ethiopia. BMC Res Notes. 2019;12:621. doi:10.1186/s13104-019-4658-5
36. Shiferaw T, Beyene G, Kassa T, Sewunet T. Bacterial contamination, bacterial profile and antimicrobial susceptibility pattern of isolates from stethoscopes at Jimma University Specialized Hospital. Ann Clin Microbiol Antimicrob. 2013;12:39. doi:10.1186/1476-0711-12-39
37. Ndu IK, Asinobi IN, Ekwochi U, et al. The bacterial profile and antibiotic sensitivity of the isolated pathogens from medical equipment and surfaces in the children’s emergency room of a Nigerian hospital. Med Sci Discov. 2019;6(9):192–197. doi:10.36472/msd.v6i9.291
38. Tadesse S, Alemayehu H, Tenna A, et al. Antimicrobial resistance profile of Staphylococcus aureus isolated from patients with infection at Tikur Anbessa Specialized Hospital, Addis Ababa, Ethiopia. BMC Pharmacol Toxicol. 2018;19(1):24. doi:10.1186/s40360-018-0210-9
39. Asres GS, Legese MH, Woldearegay GM. Prevalence of multdrug resistant bacteria in post-operative wound infections at Tikur Anbessa Specialized Hospital, Addis Ababa, Ethiopia. Arch Med. 2017;9(4):12. doi:10.21767/1989-5216.1000233
40. Manyahi J, Matee MI, Majigo M, et al. Predominance of multi-drug resistant bacteria pathogens causing surgical site infections in Muhimbili national hospital, Tanzania. BMC Res Notes. 2014;7:500. doi:10.1186/1756-0500-7-500
41. European Center for Disease Prevention and Control. Point Prevalence Survey of Healthcare Associated Infections and Antimicrobial Use in European Acute Care Hospitals. Stockholm: ECDC; 2013.
42. Holmes AH, Moore LSP, Sundsfjord A, et al. “Understanding the mechanisms and drivers of antimicrobial resistance,” (in eng). Lancet. 2016;387:10014, 176–187. doi:10.1016/S0140-6736(15)00473-0
43. World Health Organization. WHO Report on Surveillance of Antibiotic Consumption: 2016–2018; Early Implementations. Geneva: World Health Organization; 2018. Licence: CC BY-NC-SA 3.0 IGO.
44. Ayele Y, Taye H. Antibiotic utilization pattern for surgical site infection prophylaxis at Dil Chora Referral Hospital Surgical Ward, Dire Dawa, Eastern Ethiopia. BMC Res Notes. 2018;11:537. doi:10.1186/s13104-018-3629-6
45. Halawi E, Assefa T, Hussen S. Pattern of antibiotics use, incidence and predictors of surgical site infections in a Tertiary Care Teaching Hospital. BMC Res Notes. 2018;11(1):538. doi:10.1186/s13104-018-3643-8
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.