Back to Journals » Neuropsychiatric Disease and Treatment » Volume 18
Assessing the Capabilities of Transcranial Magnetic Stimulation (TMS) to Aid in the Removal of Brain Tumors Affecting the Motor Cortex: A Systematic Review
Authors Schiavao LJV, Neville Ribeiro I , Yukie Hayashi C, Gadelha Figueiredo E, Russowsky Brunoni A , Jacobsen Teixeira M , Pokorny G, Silva Paiva W
Received 3 February 2022
Accepted for publication 17 May 2022
Published 16 June 2022 Volume 2022:18 Pages 1219—1235
DOI https://doi.org/10.2147/NDT.S359855
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Roger Pinder
Lucas Jose Vaz Schiavao,1,2 Iuri Neville Ribeiro,1,2 Cintya Yukie Hayashi,1 Eberval Gadelha Figueiredo,1 Andre Russowsky Brunoni,1 Manoel Jacobsen Teixeira,1 Gabriel Pokorny,3 Wellingson Silva Paiva1
1Neurology, Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo- FMUSP – University of São Paulo, São Paulo, Brazil; 2Neurology, Instituto do Câncer do Estado de São Paulo – ICESP, São Paulo, Brazil; 3Spine, Instituto de Patologia da Coluna, São Paulo, Brazil
Correspondence: Gabriel Pokorny, Tel/Fax +55 11971652026, Email [email protected]
Introduction: The brain tumor is frequently related to severe motor impairment and impacts the quality of life. The corticospinal tract can sometimes be affected depending on the type and size of the neoplasm, so different tools can evaluate motor function and connections. It is essential to organize surgical procedures and plan the approach. Functional motor status is mapped before, during, and after surgery. Studying corticospinal tract status can help map the functional areas, predict postoperative outcomes, and help the decision, reducing neurological deficits, aiming to preserve functional networks, using the concepts of white matters localization and fibbers connections. Nowadays, there are new techniques that provide functional information regarding the motor cortex, such as transcranial magnetic stimulation (TMS), direct cortical stimulation (DCS), and navigated TMS (nTMS). These tools can be used to plan a customized surgical strategy and the role of motor evoked potentials (MEPs) is well described during intra-operative, using intraoperative neuromonitoring. MEPs can help to localize primary motor areas and delineate the cut-off point of resection in real-time, using direct stimulation. In the post-operative, the MEP has increased your function as a predictive marker of permanent or transitory neurological lesion marker.
Methods: Systematic review performed in MEDLINE via PUBMED, EMBASE, and SCOPUS databases regarding the post-operative assessment of MEP in patients with brain tumors. The search strategy included the following terms: ((“Evoked Potentials, Motor”[Mesh]) AND “Neoplasms”[Mesh]) AND “Transcranial Magnetic Stimulation”[Mesh] AND “Brain Tumor”[Mesh]), the analysis followed the PRISMA guidelines for systematic reviews, the review spanned until 06/04/2021, inclusion criteria were studies presenting confirmed diagnosis of brain tumor (primary or metastatic), patients > 18 y/o, using TMS, Navigated TMS, and/or Evoked Potentials as tools in preoperative planning or at the intra-operative helping the evaluation of the neurological status of the motor cortex, articles published in peer-reviewed journals, and written in English or Portuguese.
Results: A total of 38 studies were selected for this review, of which 14 investigated the potential of nTMS to predict the occurrence of motor deficits, while 25 of the articles investigated the capabilities of the nTMS technique in performing pre/intraoperative neuro mapping of the motor cortex.
Conclusion: Further studies regarding motor function assessment are needed and standardized protocols for MEPs also need to be defined.
Keywords: brain tumors, transcranial magnetic resonance, systematic review, neurophysiology, motor cortex mapping
Introduction
Neoplasms involving the central nervous system are a highly relevant topic in medicine, due to their impact on daily activities, high surgery complexity, and the severe impact of the possible complications. And given the complexity of the brain and the multitude of presentations of the neoplasms, the risk of neurological damage is a concern, with emphasis on lesions located in areas near typically motor regions, such as primary motor areas, or even subcortical structures in contact with the corticospinal tract, due to the possibility of partial or structural damage to the motor pathway. Therefore, resection of tumors in the motor area must be widely planned and carefully done.1–3
Several tools might aid in the planning of a brain tumor surgery, such as studying anatomic marks using imaging exams (MRI, CT) or functional exams, including evoked potential and Transcranial magnetic stimulation (TMS).4 Recently, new techniques such as preoperative brain mapping by TMS, and navigated TMS, were validated and well-accepted techniques to perform surgical planning,5–7 allowing a better anatomic and functional correlation, of imaging exams, with anatomical and physiological alterations as well as with physical examination.8
Through TMS, it is possible to assess motor evoked potentials (MEP), which represents the activation of cortical and medullar pathways, seen as graphic responses in the monitor. Allowing the evaluation of the integrity, and functioning of the cerebral motor pathways, by evaluating the motor thresholds in specific pre-chosen peripheral muscles.9–11
In addition to preoperative preparation tools, modern technologies used during the procedure turn brain tumor surgery safer. Neurophysiological monitoring, using electrical stimulation to generate motor evoked potentials in real-time during surgery, provide valuable information to the surgeon about the cortico-spinal tract (CTS) integrity12 Moreover, MEPs can find primary motor areas and help surgeons to delimit the cut-off point of resection in almost real-time, using direct stimulation. Therefore, knowing and making effective use of all these techniques can improve the surgical result, allow safer, and more precise resections.13,14
Although well studied in the literature, few works are compiling the existing evidence of the effectiveness and safety of the neurostimulation procedures in brain tumor surgeries. Therefore, this work aims to perform a systematic review of the literature regarding the use of these techniques to improve the planning and safety of brain tumor surgeries.
Methods
Systematic scoping review following the Preferred Reporting Items for Systematic Reviews (PRISMA) recommendations.15 The article was not registered in any systematic review protocol database.
Inclusion Criteria
Studies eligible for inclusion were studies presenting confirmed diagnosis of brain tumor (primary or metastatic), patients >18 y/o, using TMS, Navigated TMS, and/or Evoked Potentials as tools in preoperative planning or at the intra-operative helping the evaluation of the neurological status of the motor cortex and pyramidal tract, during the pre-operative moment and the intra-operative, that underwent the procedure under general anesthesia. Also, articles published in peer-reviewed journals, and written in English or Portuguese language were included.
Exclusion Criteria
Studies presenting only neoplasms outside the encephalus (ie, on the spinal cord), articles not related to surgery, or studies that TMS was used as a clinical treatment. Also, articles that could not be retrieved as full articles through institutional database access or by asking the involved authors were excluded.
Searched Bases & Search Mechanism
Through MEDLINE via PubMed (US National Library of Medicine National Institutes of Health), EMBASE, and SCOPUS, with no time limit until 06/04/21. The search was limited only to English and Portuguese Languages and human subjects. Using the following search mechanism ((“Evoked Potentials, Motor”[Mesh]) AND “Neoplasms”[Mesh]) AND “Transcranial Magnetic Stimulation”[Mesh] AND “Brain Tumor”[Mesh]).
Study Selection
Ninety-six studies were retrieved after a database search. The titles were screened for relevance to our research question and duplicate records. Following the full-text examination, irrelevant or not related articles were excluded. Finally, 38 full texts that met the final eligibility criteria were selected for this review (Figure 1).
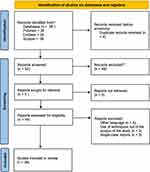 |
Figure 1 PRISMA flowchart. Notes: PRISMA figure adapted from Moher D, Liberati A, Altman D, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Journal of clinical epidemiology. 2009;62(10). Creative Commons.76 |
Data Extraction
From each included study, the following data were extracted: authors, year of publication, study design, type of neurophysiological technique, type of tumor, TMS stimulation intensity, use of direct cortical stimulation [Yes/No], and the direct cortical stimulation set-up, number of patients included, presence of control group, the mean age of the patients, complications occurred during the stimulation, motor deficit prediction, preoperative mapping general findings.
Results
Characteristics, Population, and Database
The total included studies were 38, presenting 20 prospective,9 retrospective case series, 7 cross-sectional, 1 Randomized Clinical Trial, and 1 Nonrandomized Clinical Trial. A total of 1827 participants were analyzed with ages varying from 18 to 83 years old, no gender differences were emphasized.
Primary tumors of the central nervous system were the most evaluated in the articles, such as Glioblastoma, Astrocytoma, Glioma, and Oligodendroma.14 articles presented Brain Metastasis, while 5 evaluated Meningiomas, 3 articles classified the tumors by localization, 7 did not quote. One article cited benign tumors without further information.
TMS and DCS Technique and Evaluation Parameters
Of the included articles, 29 used the neuronavigated TMS technique (nTMS), one article reported using a navigated-repeated TMS (nrTMS),16 four other studies used a transcranial electric stimulation (TES),17–20 one article reported using standard TMS (TMS),21 three other articles did not report the technique description with enough details.22–24 All articles reporting details of the stimulation coil (32/38) used a figure of eight stimulation coil instruments. The resting motor thresholds (RMT) were the most utilized TMS parameter presented in all, but one, of the articles that reported any parameters (25/38). Thirteen of the articles also used the amplitude, while 10 used the latency of MEP signals as parameters to assess the motor areas activation. As for the TMS thresholds, a wide variety of ranges for resting motor potential were reported, with 140% being the highest and 70% the lowest of the stimulation intensity threshold (Table 1).
 |  |  |  |  |  |
Table 1 Table Containing the Included Articles and the Extracted Data |
Of the 38 included studies, 17 included the use of DCS which 11 (65%) used monopolar electrodes for stimulation, 5 (29%) used bipolar electrodes, and 1 (6%) did not report the electrode polarity.
Concerning TMS and nTMS, in all articles found, the coil in the format of 8 was used. No other type of coil was mentioned in the studies presented. Cortical mapping navigated by TMS was executed by the Nexstim eXimia NBS system (only FDA-approved brand to perform primary motor cortex mapping), ranging from version 3.2 or 4.3. There were few citations from other systems like BrainLab and Brainsight TMS version 1.7, Canada. The review showed that mapping was performed by localizing the motor hotspot using a stimulation that was performed after the measurement of the resting motor threshold (rMT).8 The range of increment was 105–130% of the RMT. nTMS motor mapping in patients with brain tumors is commonly based on single-pulse nTMS (sp-nTMS) in the clinical setting; however, paired-pulse nTMS (pp-nTMS) combined with biphasic pulse waveforms have been made available just recently.25,26 No significant difference between methods regarding motor map volumes, motor hotspot locations as described although pp-nTMS works with considerably lower stimulation intensities.27
The main muscles used for the electromyography evaluation captured employing surface electrodes were the abductor pollicis brevis muscle (APB), abductor digiti minimi muscle (ADM), flexor carpi radial muscle (FCR), biceps brachii muscle (BCS), tibialis anterior muscle (TA), and gastrocnemius muscle (GCN); however, those choices might change depending on the tumor location.
Resting Motor Threshold (RMT), Amplitude, and Latency of MEPs were the main parameters of cortical excitability evaluated.
Neurophysiological Tests and Postoperative Motor Deficits
In total fourteen articles (14/38) investigated the potential of TMS to predict or avoid postoperative motor deficits (Table 1).
Two articles demonstrated that the intraoperative transcranial electric stimulation (TES) might hold an excellent value in predicting true postoperative motor deficits approaching 100% detection power; however, its power depends much on the selected parameters.19,20 Also, a study showed that using lower MEP amperages might help reduce the rate of false-negative findings (when the MEP shows no decrease and the patient presents motor deficit).22
Further, another study showed that the TMS technique presented 100% accuracy in true positive deficit detection and that the two factors that showed significance to predict the occurrence of deficit were the RMT value and the presence of the arachnoidal cleavage plane.28 Finally, a study by Seidel et al, 2019 showed that the use of immediate postoperative nTMS provides useful information on whether the patient will recover from a motor deficit. The authors found that if MEPs are elicited in postoperative nTMS there are great chances of recovering from the motor deficit, similarly when no MEPs are elicited the study found a 100% chance of not recovering from the deficit in one month. Also, the study showed that the postoperative nTMS might help clarify the intraoperative MEP signal loss, in those scenarios 2 of 3 patients with intraoperative irreversible loss and postoperative nTMS MEP signal presence presented good motor recovery.29
Moreover, another three studies pointed out that MEP signal loss during the procedures also bore great insights into possible new motor deficits. One of the articles showed that a reduction of intraoperative MEPs higher than 50% is indicative of prolonged motor deficits,6 while another study demonstrated that an irreversible MEP loss or an irreversible MEP reduction inside the surgery was a strong predictor of postoperative motor deficits, with 12 of 15 patients presenting postoperative deficits in those conditions.18 Finally, the last article pointed out that adding the analysis of the MEP stimulation intensity together with the amplitude variations might increase both true positive and true negative deficits detection.23
Furthermore, two articles demonstrated that patients with tumors closer to motor areas mapped by TMS, such as motor hotspot (the site eliciting maximum MEP) or corticospinal tract, are prone to present from motor deficits compared to patients with lesions located father to these areas.30,31 Also, another article demonstrated that a ratio of intraoperative RMTs higher than 100% between affected and unaffected hemispheres might indicate imminently decadence of motor functions.32
Further, another article pointed out that resection of tumors with positive nTMS signal in pre-rolandic gyri might be a risk of developing new motor deficits, as 10 of 13 cases presented postoperative deficits.33 Additionally, another study showed that patients receiving a multimodal approach (nTMS + sodium fluoride marker) had significantly better motor outcomes compared to those receiving only nTMS.34
Only one article compared the predictive power between DCS and TES, showing that MEP signal alterations in DCS served better to detect postoperative motor deficits.17
TMS Parameters and Preoperative Mapping
Twenty-five (25/38) articles focused on the capacities of TMS to accurately perform the pre/intraoperative mapping of the motor regions (Table 1).
Two of them investigated the implications of performing a paired-pulse TMS compared to a single-pulse TMS. They showed that the paired-pulse elicited signals in 100% of the patients, without increasing the patient discomfort, as this TMS modality has a lower stimulation intensity.27,35 Another study showed that the use of a 500 micro-volts threshold could reduce the presence of artifacts compared to using a 50 micro-volts threshold.36
Others focused on the capability of the TMS to accurately identify the motor cortex. Kantelhardt et al, 2009 demonstrated that the usage of robot-assisted image-guided TMS could identify 100% of the areas that elicited responses on the fMRI finger-tapping examen.37 Further, a study compared the DCS and nTMS, showing that both had similar capabilities to identify the safety margin of tumor resection.7 Another article showed that the TMS technique had been concordant with the Intraoperative neuromonitoring regarding the location of the tumor area in 94.6% of the cases.28 One showed that the technique had the potential to localize the structures related to the upper limbs,38 while the other one showed that patients receiving TMS + radiosurgery received fewer Gy than patients receiving the standard radiosurgery protocol.39 Moreover, pointed out that the use of electric-field navigated TMS detected a higher number of positive signals (MEP responses with amplitudes of at least 50 micro-volts in one or more muscles according to EMG recording were regarded as a motor-positive response) and a higher rate of positive/total stimulus compared to the line-navigated method.40
Moreover, a study showed that the nTMS could detect signals from non-primary motor-related areas (NPMA); however, in its majority, the MEP signals seemed to be derived from M1 areas.41 Also, a study found that mapping the precentral gyrus of patients with tumors in that region elicited more MEPs counts (a count is an area that elicited signal after MEP stimulation) in this region than patients with a lesion in other regions. A similar response was seen in patients with postcentral gyrus (PoG) tumors with those patients presenting a higher number of MEPs counts in the PoG region compared to other patients.38 Similarly, another study suggested that the plasticity of the brain could be even higher than previously imaged by showing that the primary motor areas and polysynaptic areas drastically change their position according to the tumor location.42 Further, a study showed that hemispheres with tumors had different motor excitability patterns than the healthy hemisphere; it also reported that the excitability of hemispheres with glioblastomas was different from hemispheres with other types of tumors.43 Also, a study found that patients with high tumor grades had the excitability pattern of the hemisphere different from the low tumor grades patients.44
A study demonstrated that patients with tumors located on the brainstem or in the cortical motor area had higher rMT than healthy control subjects.21 Further, another article showed that the RMTs of patients with brain tumors and apparent paresis were significantly higher than in healthy subjects or patients without apparent paresis.45 Following the same line, another study found that with higher grades of tumors the higher was the latency and lower were the RMTs of the lower limbs in the patients.44
Additionally, a study pointed out that using an electric field model would generate more accurate predictions of motor areas, compared to the standard point-cloud model.46 Similarly, an article from 2020 showed that motor positive areas predicted by a computer model based on a preoperative TMS had 80% concordance with intraoperative direct electrical stimulation (DES).47 Still following the application of machine learning/artificial intelligence algorithms, a study pointed out that the use of the nLM algorithm found the ideal rMT threshold of the patients with fewer pulses than the R-R (Rossini-Roswell) method.48
Two studies reported the impact of a preoperative nTMS in surgery decision-making. One study showed that the preop nTMS led to a 10% rate of surgical planning modification,49 and one article showed that 27% suffered planning alterations given the results obtained in the nTMS.50
Complications of the TMS Mapping
Of the included articles, thirteen mentioned adverse event analysis, with 11 mentioning that any adverse event occurred, one reporting the occurrence of minor headaches or small scalp pinch,43 and one reporting that 7 of 12 patients had complained about strong facial contractions51 (Table 1).
Discussion
The use of transcranial navigated techniques to aid surgeons performing brain tumor surgeries is increasing in the last decade, with the improvement of both TMS equipment and complimentary software/hardware, however, the true impact of the TMS in improving surgical and clinical outcomes is still in debate. Raffa et al, 2013 performed a systematic review and meta-analysis where they showed that the use of TMS in brain surgery resulted in an increased odds of obtaining gross total resection (GTR) and a reduced craniotomy extent.52
The Population of the Studies
The evaluation of the articles was conducted in two blocks. The articles addressed the theme of neurophysiological testing, and the articles evaluated the motor outcomes. Most texts addressed the relationship of neurophysiological tests without preoperative or post-operative assessment of motor status. It is a consensus that the loss of intraoperative motor evoked potentials is associated with postoperative motor deficits.18,53
The number of evaluated patients in the studies was variable, with studies presenting a vast number of patients22,48 most studies had an average of around 20 participants. In 6 studies, there was a healthy control group. The age of patients in the studies ranged from 18-to 83.
Despite the major differences in the content of the articles, the study population studies were quite homogeneous concerning inclusion criteria, even including different countries and cultures. The fact that they have brain tumors, and adult age, creates a reliable group to identify the use of surgical planning and follow-up techniques as well as their effectiveness. It let identify the use of navigated TMS, direct cortical stimulation in different centers, showing excellent accuracy for motor mapping with significant improvement in surgical results.6,8,10,13,34,44,50,54–57
Neurophysiological Test Specificities and Protocols
In patients with injuries, the motor functions are not exercised solely by typical motor areas.61,62 One of the roles of nTMS is to study, even in the preoperative period, the topographical and functional repercussions resulting from the tumor, as the role of the motor supplemental area. Evidence showed that it is possible to obtain motor responses outside of typical motor areas.63,64 The location of functional areas in the human brain is not static, motor topography representation is a dynamic process caused by the plastic reshaping of cortical and subcortical functions.59,65 It is important for the maintenance of motor functions when there is the involvement of motor areas.62 Almost 40% of MEPs in patients with brain tumors were elicited during stimulation out of other gyri beyond the precentral gyrus, like tumors or anatomical variations (for healthy subjects) might push some motor areas to unexpected locations. As there is usually not enough time for extensive motor mapping during surgery, 40% of motor function would remain undetected if relying only on DCS.38
Given those possible variations, anatomical or tumor-driven, and the new developments occurring in the informatics area, the protocols for TMS can vary widely across institutions and time, as shown in our study. Results that are aligned with two recent literature revision focusing in the TMS protocols, both studies depicted that the protocols of TMS are improving very quickly with the advance of technology, however they are still very heterogeneous in some of its key precedents, such as intensity of stimulation or coil position.58,59,65–67
TMS and Preoperative Brain Mapping
The transcranial magnetic resonance is a valuable tool to investigate the brain pathways prior surgery adding unprecedented knowledge to the surgeons. Lefechauer and Pitch, 2016 in a narrative revision brings out several positive points of using the preoperative TMS. They show that one of most interesting point of using the TMS preoperatively is to have a better understanding of how the tumors in entangled in the motor cortex, allowing the surgeon to visualize the areas of the tumor that cannot be removed without causing permanent deficit.68
Navigated TMS mapping provides results consistent with DCS findings. Besides, being considered a good complementary method to DCS, nTMS is an effective tool able to perform before surgery, proposing to map supplementary motor areas, association motor fibers, and evaluate the change in cortical topography representation promoted by tumors.58,59,65 In a study comparing the use of TMS with the gold-standard mapping method of the direct cortical stimulation, it showed that both techniques had strong agreement regarding the hotspots (4.70 mm of difference), pointing out that TMS might be an effective tool to perform the preoperative brain mapping.69 Similarly, another study found no significant difference between the use hotspots for DCS and TMS. The average difference in motor cortex location between nTMS and subdural stimulation was 11 mm for the hand and 16 mm for the arm.60
In a study involving 400 patients, Frey et al, 2014, showed that the addition of the TMS to the preoperative planning led to an increase in the tumor-free survival time, in the number of gross total resection cases, while adding crucial information that led to changes in the pre-conceive surgical plan in 63.5% of the cases.70 Moreover, a study comparing the use of intraoperative monitoring only and preop TMS + intraoperative monitoring, showed that using the TMS the surgeons achieved higher rates of GTR.71 In the same line, an article published in 2021 showed that use of TMS for excision of gliomas affecting the motor cortex promote a higher rate of GTR (72.3% vs 53.2%, p = 0.04); however, there was no difference in postoperative deficits occurrence, nor the TMS was associated with better tumor-free survival time.72
Finally, a revision performed in 2019 showed that the use of TMS to map motor regions of the brain allowed for reduced occurrence of postoperative motor deficits. Further, the study demonstrated that the use of TMS led to a reduced size of craniotomy and to a higher total gross resection rates compared to controls.52
TMS and Motor Deficits
Studies show that the use of TMS could lead to reduced occurrence of postoperative motor deficits. An article published in 2018 investigating the use of DTI-fiber tracking system alongside with the TMS technology showed that by using this new approach or using only the TMS there was better motor postoperative motor performance compared with patients not using the TMS approach.73 Further, two studies investigating the use of sodium fluorescein to help guide the TMS showed that using this multimodal approach could promote significant reduction in the occurrence of postoperative motor deficits when compared to the use of just intraoperative monitoring.34,74
Another interesting application of the TMS could be in the prediction of motor deficits recovery after the brain tumor surgery. A study published in 2017 showed that cases where there was pathological intercortex excitability, the chances of occurring a motor deficit were higher, and further when the RMT threshold was higher in tumorous hemisphere and a deficit occurred, this deficit tended to not present any improvement with time.75 Moreover, a study comparing the deficit recovery of patients showing TMS positive MEP one-week after surgery and those presenting TMS negative MEP showed that the positive group showed better motor deficit recovery in one, three and twelve weeks.31 Finally, an article published in 2019 showed that patients presenting TMS positive MEP signs after surgery had 90% chances of presenting full recovery of motor status, while patients presenting TMS negative MEP after surgery had 100% negative predictive value of presenting motor deficit full-recover.29
Conclusion
Transcranial magnetic stimulation is a valuable tool to enhance the safety and effectiveness of brain tumor resection, by performing a high accurate preoperative mapping of the motor area and its relationship with the tumor. Also, intra/postoperative TMS is a valuable tool to predict the occurrence or duration of motor deficits, helping the surgeon to better align the postoperative recovery expectation with the patient.
Novel studies and new evaluation protocols of the corticospinal tract and white fibers need to be developed, as well as standardized protocols for MEP need to be defined.
Ethical Approval
Hospital Ethics Committee and Internal Review Board approval were not needed for this study.
Funding
There was no funding for this research.
Disclosure
None of the authors states any conflict of interest.
References
1. Sanai N, Berger MS. Intraoperative stimulation techniques for functional pathway preservation and glioma resection. Neurosurg Focus. 2010;28(2):E1. doi:10.3171/2009.12.FOCUS09266
2. Krivosheya D, Prabhu SS, Weinberg JS, Sawaya R. Technical principles in glioma surgery and preoperative considerations. J Neurooncol. 2016;130(2):243–252. doi:10.1007/s11060-016-2171-4
3. Hervey-Jumper SL, Berger MS. Role of surgical resection in low- and high-grade gliomas. Curr Treat Options Neurol. 2014;16(4):1–19. doi:10.1007/s11940-014-0284-7
4. Tarapore PE, Tate MC, Findlay AM, et al. Preoperative multimodal motor mapping: a comparison of magnetoencephalography imaging, navigated transcranial magnetic stimulation, and direct cortical stimulation. J Neurosurg. 2012;117(2):354. doi:10.3171/2012.5.JNS112124
5. Julkunen P, Säisänen L, Danner N, et al. Comparison of navigated and non-navigated transcranial magnetic stimulation for motor cortex mapping, motor threshold and motor evoked potentials. Neuroimage. 2009;44(3):790–795. doi:10.1016/j.neuroimage.2008.09.040
6. Krieg SM, Shiban E, Buchmann N, et al. Utility of presurgical navigated transcranial magnetic brain stimulation for the resection of tumors in eloquent motor areas: clinical article. J Neurosurg. 2012;116(5):994–1001. doi:10.3171/2011.12.JNS111524
7. Picht T, Mularski S, Kuehn B, Vajkoczy P, Kombos T, Suess O. Navigated transcranial magnetic stimulation for preoperative functional diagnostics in brain tumor surgery technique and assessment; 2009 [cited October 23, 2021]; Available from: www.neurosurgery-online.com.
8. Raffa G, Quattropani MC, Germanò A. When imaging meets neurophysiology: the value of navigated transcranial magnetic stimulation for preoperative neurophysiological mapping prior to brain tumor surgery. Neurosurg Focus. 2019;47(6):E10. doi:10.3171/2019.9.FOCUS19640
9. Raffa G, Conti A, Scibilia A, et al. The impact of diffusion tensor imaging fiber Tracking of the corticospinal tract based on navigated transcranial magnetic stimulation on surgery of motor-eloquent brain lesions. Neurosurgery. 2018;83(4):768–782. doi:10.1093/neuros/nyx554
10. Rossini rome P, Barker Sheffield A, Berardelli Rome A, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee; 1994.;
11. Rossini PM, Burke D, Chen R, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clin Neurophysiol. 2015;126(6):1071. doi:10.1016/j.clinph.2015.02.001
12. MacDonald DB, Skinner S, Shils J, Yingling C. Intraoperative motor evoked potential monitoring – a position statement by the American Society of Neurophysiological Monitoring. Clin Neurophysiol. 2013;124(12):2291–2316. doi:10.1016/j.clinph.2013.07.025
13. Kombos T, Suess O, Ciklatekerlio Ö, Brock M. Monitoring of intraoperative motor evoked potentials to increase the safety of surgery in and around the motor cortex. J Neurosurg. 2001;95(4):608–614. doi:10.3171/jns.2001.95.4.0608
14. Berger MS, Rostomily RC. Low grade gliomas: functional mapping resection strategies, extent of resection, and outcome. J Neuro Oncol. 1997;34(1):85–101. doi:10.1023/A:1005715405413
15. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1–e34.
16. Rohde V, Mayfrank L, Weinzierl M, Krings T, Gilsbach JM. Focused high frequency repetitive transcranial magnetic stimulation for localisation of the unexposed primary motor cortex during brain tumour surgery. J Neurol Neurosurg Psychiatry. 2003;74(9):1283–1287. doi:10.1136/jnnp.74.9.1283
17. Moiyadi A, Velayutham P, Shetty P, et al. Combined motor evoked potential monitoring and subcortical dynamic mapping in motor eloquent tumors allows safer and extended resections. World Neurosurg. 2018;120:e259–68. doi:10.1016/j.wneu.2018.08.046
18. Szelényi A, Hattingen E, Weidauer S, Seifert V, Ziemann U. Intraoperative motor evoked potential alteration in intracranial tumor surgery and its relation to signal alteration in postoperative magnetic resonance imaging. Neurosurgery. 2010;67(2):302–313. doi:10.1227/01.NEU.0000371973.46234.46
19. Majchrzak K, Bobek-Billewicz B, Hebda A, Adamczyk P, Majchrzak H, Ładziński P. Surgical treatment of adult patients with thalamic tumors with the aid of tractography, fMRI, transcranial electrical stimulation and direct electrical stimulation of the subcortical white matter. Neurol Neurochir Pol. 2018;52(6):720–730. doi:10.1016/j.pjnns.2018.07.001
20. Abboud T, Schaper M, Dührsen L, et al. A novel threshold criterion in transcranial motor evoked potentials during surgery for gliomas close to the motor pathway. J Neurosurg. 2016;125(4):795–802. doi:10.3171/2015.8.JNS151439
21. Gimranov RF. Study of thresholds of motor evoked responses during transcranial magnetic stimulation in healthy subjects and patients with brain tumors. Hum Physiol. 2002;28(4):413–416. doi:10.1023/A:1016573713772
22. Umemura T, Nishizawa S, Nakano Y, et al. Intraoperative monitoring of motor-evoked potential for parenchymal brain tumor removal: an analysis of false-negative cases. J Clin Neurosci. 2018;57:105–110. doi:10.1016/j.jocn.2018.08.019
23. Krammer MJ, Wolf S, Schul DB, Gerstner W, Lumenta CB. Significance of intraoperative motor function monitoring using transcranial electrical motor evoked potentials (MEP) in patients with spinal and cranial lesions near the motor pathways. Br J Neurosurg. 2009;23(1):48–55. doi:10.1080/02688690802563349
24. Szelényi A, Gasser T, Seifert V. Technique and assessment intraoperative neurophysiological monitoring in an open low-field magnetic resonance imaging system: clinical experience and technical considerations tumor. Oper Neurosurg. 2008;63:ONS268–ONS276.
25. Mohammadi A, Ebrahimi M, Kaartinen S, Jarnefelt G, Karhu J, Julkunen P. Individual characterization of fast intracortical facilitation with paired biphasic-wave transcranial magnetic stimulation. IEEE Trans Neural Syst Rehabil Eng. 2018;26(9):1710–1716. doi:10.1109/TNSRE.2018.2864311
26. Julkunen P, Järnefelt G, Savolainen P, Laine J, Karhu J. Facilitatory effect of paired-pulse stimulation by transcranial magnetic stimulation with biphasic wave-form. Med Eng Phys. 2016;38(8):813–817. doi:10.1016/j.medengphy.2016.04.025
27. Sollmann N, Zhang H, Kelm A, et al. Paired-pulse navigated TMS is more effective than single-pulse navigated TMS for mapping upper extremity muscles in brain tumor patients. Clin Neurophysiol. 2020;131(12):2887–2898. doi:10.1016/j.clinph.2020.09.025
28. Raffa G, Picht T, Scibilia A, et al. Surgical treatment of meningiomas located in the rolandic area: the role of navigated transcranial magnetic stimulation for preoperative planning, surgical strategy, and prediction of arachnoidal cleavage and motor outcome. J Neurosurg. 2019;133(1):107–118. doi:10.3171/2019.3.JNS183411
29. Seidel K, Häni L, Lutz K, et al. Postoperative navigated transcranial magnetic stimulation to predict motor recovery after surgery of tumors in motor eloquent areas. Clin Neurophysiol. 2019;130(6):952–959. doi:10.1016/j.clinph.2019.03.015
30. Sollmann N, Wildschuetz N, Kelm A, et al. Associations between clinical outcome and navigated transcranial magnetic stimulation characteristics in patients with motor-eloquent brain lesions: a combined navigated transcranial magnetic stimulation–diffusion tensor imaging fiber tracking approach. J Neurosurg. 2017;128(3):800–810. doi:10.3171/2016.11.JNS162322
31. Takakura T, Muragaki Y, Tamura M, et al. Navigated transcranial magnetic stimulation for glioma removal: prognostic value in motor function recovery from postsurgical neurological deficits. J Neurosurg. 2017;127(4):877–891. doi:10.3171/2016.8.JNS16442
32. Picht T, Strack V, Schulz J, et al. Assessing the functional status of the motor system in brain tumor patients using transcranial magnetic stimulation. Acta Neurochir. 2012;154(11):2075–2081. doi:10.1007/s00701-012-1494-y
33. Moser T, Bulubas L, Sabih J, et al. Resection of navigated transcranial magnetic stimulation-positive prerolandic motor areas causes permanent impairment of motor function. Neurosurgery. 2017;81(1):99–110. doi:10.1093/neuros/nyw169
34. Raffa G, Scibilia A, Conti A, et al. Multimodal surgical treatment of high-grade gliomas in the motor area: the impact of the combination of navigated Transcranial magnetic stimulation and fluorescein-guided resection. World Neurosurg. 2019;128:e378–90. doi:10.1016/j.wneu.2019.04.158
35. Zhang H, Julkunen P, Schröder A, et al. Short-interval intracortical facilitation improves efficacy in nTMS motor mapping of lower extremity muscle representations in patients with supra-tentorial brain tumors. Cancers. 2020;12(11):1–18. doi:10.3390/cancers12113233
36. Lam S, Lucente G, Schneider H, Picht T. TMS motor mapping in brain tumor patients: more robust maps with an increased resting motor threshold. Acta Neurochir. 2019;161(5):995–1002. doi:10.1007/s00701-019-03883-8
37. Kantelhardt SR, Fadini T, Finke M, et al. Robot-assisted image-guided transcranial magnetic stimulation for somatotopic mapping of the motor cortex: a clinical pilot study. Acta Neurochir. 2010;152(2):333. doi:10.1007/s00701-009-0565-1
38. Bulubas L, Sollmann N, Tanigawa N, Zimmer C, Meyer B, Krieg SM. Reorganization of motor representations in patients with brain lesions: a navigated transcranial magnetic stimulation study. Brain Topogr. 2017;31(2):288–299. doi:10.1007/s10548-017-0589-4
39. Tokarev AS, Rak VA, Sinkin MV, et al. Appliance of navigated transcranial magnetic stimulation in radiosurgery for brain metastases. J Clin Neurophysiol. 2020;37(1):50–55. doi:10.1097/WNP.0000000000000621
40. Sollmann N, Goblirsch-Kolb MF, Ille S, et al. Comparison between electric-field-navigated and line-navigated TMS for cortical motor mapping in patients with brain tumors. Acta Neurochir. 2016;158(12):2277–2289. doi:10.1007/s00701-016-2970-6
41. Mirbagheri A, Schneider H, Zdunczyk A, Vajkoczy P, Picht T. NTMS mapping of non-primary motor areas in brain tumour patients and healthy volunteers. Acta Neurochir. 2019;162(2):407–416. doi:10.1007/s00701-019-04086-x
42. Bulubas L, Sabih J, Wohlschlaeger A, et al. Motor areas of the frontal cortex in patients with motor eloquent brain lesions. J Neurosurg. 2016;125(6):1431–1442. doi:10.3171/2015.11.JNS152103
43. Neville IS, Santos Dos AG, Almeida CC, et al. Evaluation of changes in preoperative cortical excitability by navigated transcranial magnetic stimulation in patients with brain tumor. Front Neurol. 2020;11:582262. doi:10.3389/fneur.2020.582262
44. Lavrador JP, Gioti I, Hoppe S, et al. Altered motor excitability in patients with diffuse gliomas involving motor eloquent areas: the impact of tumor grading. Neurosurgery. 2020;88(1):183–192. doi:10.1093/neuros/nyaa354
45. Machetanz K, Gallotti AL, Tatagiba MTL, et al. Time-frequency representation of motor evoked potentials in brain tumor patients. Front Neurol. 2020;11:633224. doi:10.3389/fneur.2020.633224
46. Seynaeve L, Haeck T, Gramer M, Maes F, Vleeschouwer de S, Paesschen van W. Optimized preoperative motor cortex mapping in brain tumors using advanced processing of transcranial magnetic stimulation data. Neuroimage. 2019;21. doi:10.1016/j.nicl.2019.101657
47. Opitz A, Zafar N, Bockermann V, Rohde V, Paulus W. Validating computationally predicted TMS stimulation areas using direct electrical stimulation in patients with brain tumors near precentral regions. Neuroimage. 2014;4:500. doi:10.1016/j.nicl.2014.03.004
48. Engelhardt M, Schneider H, Gast T, Picht T. Estimation of the resting motor threshold (RMT) in transcranial magnetic stimulation using relative-frequency and threshold-hunting methods in brain tumor patients. Acta Neurochir. 2019;161(9):1845–1851. doi:10.1007/s00701-019-03997-z
49. Jung J, Lavrador JP, Patel S, et al. First United Kingdom experience of navigated transcranial magnetic stimulation in preoperative mapping of brain tumors. World Neurosurg. 2019;122:e1578–87. doi:10.1016/j.wneu.2018.11.114
50. Picht T, Schulz J, Hanna M, Schmidt S, Suess O, Vajkoczy P. Assessment of the influence of navigated transcranial magnetic stimulation on surgical planning for tumors in or near the motor cortex. Neurosurgery. 2012;70(5):1248–1257. doi:10.1227/NEU.0b013e318243881e
51. Freigang S, Fresnoza S, Ali KM, et al. Impact of priming on effectiveness of TMS in detecting language-eloquent brain areas in tumor patients. J Neurol Surg a Cent Eur Neurosurg. 2020;81(02):111–129. doi:10.1055/s-0039-1698382
52. Raffa G, Scibilia A, Conti A, et al. The role of navigated transcranial magnetic stimulation for surgery of motor-eloquent brain tumors: a systematic review and meta-analysis. Clin Neurol Neurosurg. 2019;180:7–17. doi:10.1016/j.clineuro.2019.03.003
53. Fujiki M, Furukawa Y, Kamida T, et al. Intraoperative corticomuscular motor evoked potentials for evaluation of motor function: a comparison with corticospinal D and I waves. J Neurosurg. 2006;104(1):85–92. doi:10.3171/jns.2006.104.1.85
54. Plans G, Fernández-Conejero I, Rifà-Ros X, Fernández-Coello A, Rosselló A, Gabarrós A. Evaluation of the high-frequency monopolar stimulation technique for mapping and monitoring the corticospinal tract in patients with supratentorial gliomas. a proposal for intraoperative management based on neurophysiological data analysis in a series of 92 patients. Neurosurgery. 2017;81(4):585–594. doi:10.1093/neuros/nyw087
55. Obermueller T, Schaeffner M, Shiban E, et al. Intraoperative neuromonitoring for function-guided resection differs for supratentorial motor eloquent gliomas and metastases. BMC Neurol. 2015;15(1). doi:10.1186/s12883-015-0476-0.
56. Krings T, Chiappa KH, Foltys H, Reinges MH, Cosgrove RG, Thron A. Introducing navigated transcranial magnetic stimulation as a refined brain mapping methodology. Neurosurg Rev. 2001;24(4):171–179. doi:10.1007/s101430100151
57. Quail DF, Joyce JA. The microenvironmental landscape of brain tumors. Cancer Cell. 2017;31(3):326. doi:10.1016/j.ccell.2017.02.009
58. Duffau H. Introduction: surgery of gliomas in eloquent areas: from brain hodotopy and plasticity to functional neurooncology. Neurosurg Focus. 2010;28(2). doi: 10.3171/2009.12.FOCUS.FEB2010.INTRO
59. Herbet G, Maheu M, Costi E, Lafargue G, Duffau H. Mapping neuroplastic potential in brain-damaged patients. Brain. 2016;139(3):829–844. doi:10.1093/brain/awv394
60. Krieg SM, Lioumis P, Mäkelä JP, et al. Protocol for motor and language mapping by navigated TMS in patients and healthy volunteers; workshop report. Acta Neurochir. 2017;159(7):1187–1195. doi:10.1007/s00701-017-3187-z
61. Seitz RJ, Höflich P, Binkofski F, Tellmann L, Herzog H, Freund HJ. Role of the premotor cortex in recovery from middle cerebral artery infarction. Arch Neurol. 1998;55(8):1081–1088. doi:10.1001/archneur.55.8.1081
62. Fridman EA, Hanakawa T, Chung M, Hummel F, Leiguarda RC, Cohen LG. Reorganization of the human ipsilesional premotor cortex after stroke. Brain. 2004;127(4):747–758. doi:10.1093/brain/awh082
63. Duffau H. Brain plasticity: from pathophysiological mechanisms to therapeutic applications. J Clin Neurosci. 2006;13(9):885–897. doi:10.1016/j.jocn.2005.11.045
64. Uematsu S, Lesser R, Fisher RS, et al. Motor and sensory cortex in humans topography studied with chronic subdural stimulation. Neurosurgery. 1992;31(1):59–72. doi:10.1227/00006123-199207000-00009
65. Duffau H. The huge plastic potential of adult brain and the role of connectomics: new insights provided by serial mappings in glioma surgery. Cortex. 2014;58:325–337. doi:10.1016/j.cortex.2013.08.005
66. Sollmann N, Krieg SM, Säisänen L, Julkunen P. Mapping of motor function with neuronavigated transcranial magnetic stimulation: a review on clinical application in brain tumors and methods for ensuring feasible accuracy. Brain Sci. 2021;11(7):897. doi:10.3390/brainsci11070897
67. Sondergaard RE, Martino D, Kiss ZHT, Condliffe EG. TMS motor mapping methodology and reliability: a structured review. Front Neurosci. 2021;15. doi:10.3389/fnins.2021.709368
68. Lefaucheur JP, Picht T. The value of preoperative functional cortical mapping using navigated TMS. Neurophysiol Clin. 2016;46(2):125–133. doi:10.1016/j.neucli.2016.05.001
69. Picht T, Schmidt S, Brandt S, et al. Preoperative functional mapping for rolandic brain tumor surgery: comparison of navigated transcranial magnetic stimulation to direct cortical stimulation. Neurosurgery. 2011;69(3):581–588. doi:10.1227/NEU.0b013e3182181b89
70. Frey D, Schilt S, Strack V, et al. Navigated transcranial magnetic stimulation improves the treatment outcome in patients with brain tumors in motor eloquent locations. Neuro Oncol. 2014;16(10):1365–1372.
71. Picht T, Frey D, Thieme S, Kliesch S, Vajkoczy P. Presurgical navigated TMS motor cortex mapping improves outcome in glioblastoma surgery: a controlled observational study. J Neurooncol. 2016;126(3):535–543. doi:10.1007/s11060-015-1993-9
72. Hendrix P, Dzierma Y, Burkhardt BW, et al. Preoperative navigated transcranial magnetic stimulation improves gross total resection rates in patients with motor-eloquent high-grade gliomas: a matched cohort study. Neurosurgery. 2021;88(3):627–636. doi:10.1093/neuros/nyaa486
73. Raffa G, Conti A, Scibilia A, et al. The impact of diffusion tensor imaging fiber tracking of the corticospinal tract based on navigated transcranial magnetic stimulation on surgery of motor-eloquent brain lesions. Neurosurgery. 2018;83(4):768–782.
74. Raffa G, Picht T, Angileri FF, et al. Surgery of malignant motor-eloquent gliomas guided by sodium-fluorescein and navigated transcranial magnetic stimulation: a novel technique to increase the maximal safe resection. J Neurosurg Sci. 2019;63(6):670–678. doi:10.23736/S0390-5616.19.04710-6
75. Rosenstock T, Grittner U, Acker G, et al. Risk stratification in motor area-related glioma surgery based on navigated transcranial magnetic stimulation data. J Neurosurg. 2017;126(4):1227–1237. doi:10.3171/2016.4.JNS152896
76. Moher D, Liberati A, Altman D, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Journal of clinical epidemiology. 2009;62(10)
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
