Back to Journals » Journal of Inflammation Research » Volume 17
Actives from the Micro-Immunotherapy Medicine 2LMIREG® Reduce the Expression of Cytokines and Immune-Related Markers Including Interleukin-2 and HLA-II While Modulating Oxidative Stress and Mitochondrial Function
Authors Jacques C , Marchand F, Chatelais M, Floris I
Received 3 November 2023
Accepted for publication 13 February 2024
Published 21 February 2024 Volume 2024:17 Pages 1161—1181
DOI https://doi.org/10.2147/JIR.S445053
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tara Strutt
Camille Jacques,1 Flora Marchand,2 Mathias Chatelais,2 Ilaria Floris1
1Preclinical Research Department, Labo’Life France, Pescalis-Les Magnys, Moncoutant-sur-Sevre, 79320, France; 2ProfileHIT, Sainte-Pazanne, 44680, France
Correspondence: Camille Jacques, Preclinical Research Department, Labo’Life France, Pescalis-Les Magnys, Moncoutant-sur-Sevre, 79320, France, Tel +33228444905, Email [email protected]
Introduction: Micro-immunotherapy (MI) is a therapeutic option employing low doses (LD) and ultra-low doses (ULD) of cytokines and immune factors to help the organism at modulating the immune responses. In an overpowering inflammatory context, this strategy may support the restoration of the body’s homeostasis, as the active ingredients of MI medicines’ (MIM) could boost or slow down the physiological functions of the immune cells. The aim of the study is to evaluate for the first time the in vitro anti-inflammatory properties of some actives employed by the MIM of interest in several human immune cell models.
Methods: In the first part of the study, the effects of the actives from the MIM of interest were assessed from a molecular standpoint: the expression of HLA-II, interleukin (IL)-2, and the secretion of several other cytokines were evaluated. In addition, as mitochondrial metabolism is also involved in the inflammatory processes, the second part of the study aimed at assessing the effects of these actives on the mitochondrial reactive oxygen species (ROS) production and on the mitochondrial membrane potential.
Results: We showed that the tested actives decreased the expression of HLA-DR and HLA-DP in IFN-γ-stimulated endothelial cells and in LPS-treated-M1-macrophages. The tested MIM slightly reduced the intracellular expression of IL-2 in CD4+ and CD8+ T-cells isolated from PMA/Iono-stimulated human PBMCs. Additionally, while the secretion of IL-2, IL-10, and IFN-γ was diminished, the treatment increased IL-6, IL-9, and IL-17A, which may correspond to a “Th17-like” secretory pattern. Interestingly, in PMA/Iono-treated PBMCs, we reported that the treatment reduced the ROS production in B-cells. Finally, in PMA/Iono-treated human macrophages, we showed that the treatment slightly protected the cells from early cell death/apoptosis.
Discussion: Overall, these results provide data about the molecular and functional anti-inflammatory effects of several actives contained in the tested MIM in immune-related cells, and their impact on two mitochondria-related processes.
Keywords: micro-immunotherapy, mitochondrial metabolism, cytokines, inflammation, anti-inflammatory, interleukin-2
Introduction
Current medical science reveals that around 90% of chronic diseases are connected to changes in mitochondria. These changes can lead to various types of conditions, such as neurodegenerative, age-related, and metabolic diseases, as well as different forms of cancer.1–3 Therefore, it is essential to properly regulate the function of mitochondria to prevent the development and progression of these diseases and restore organic balance. Mitochondria produces the basic energy for life from food sources like carbohydrates, fats, and proteins. Through the process of oxidative phosphorylation, adenosine triphosphate (ATP) is created, which not only produces energy but also helps with calcium balance, apoptosis regulation, and fatty acid oxidation. However, large numbers of reactive oxygen species (ROS) are produced as by-products, which, despite having a role in cell signaling, can damage mitochondrial function if produced in an uncontrolled and excessive manner.
The oxidative stress derived from the overproduction of ROS can activate several transcription factors like nuclear factor kappa B (NF-kB), leading to the production and activation of pro-inflammatory cytokines, chemokines, and lymphocytes, which, in turn, lead to the production of more ROS.4 This bidirectional self-amplifying relationship between the development of chronic oxidative stress and chronic systemic inflammation is well documented.5,6 It is indeed not surprising that mitochondria can also affect different aspects of the immune response, largely through their involvement in respiratory chain functions.
Within an immunoregulatory perspective, the low/ultra-low-doses-based-approach of immunotherapy micro-immunotherapy (MI), could be an interesting therapeutic option in order to manage (i) inflammation and (ii), mitochondrial function; two physiological features which, when dysregulated, can lead to chronic diseases. MI medicines (MIM) are manufactured as sugar pillules, also called globules, impregnated with active ingredients, packaged into capsules, and are intended to be taken in a fasted state, through oromucosal administration. MI formulations exist either as unitary medicines, including a sole active substance, or they can also be developed as complex formulations, combining, in this case, several ingredients. The panel of actives used in MI includes immune-related cytokines, hormones and growth factors, in association with nucleic acid preparations, either under the form of plant-derived total deoxyribonucleic acid/ribonucleic acid (DNA/RNA) or as specific nucleic acids (SNA®). In MIM, the amount of these active substances, expressed as centesimal Hahnemannian (CH) dilutions, governs the orientation of biological responses either towards an activation or an inhibition, depending on the range of CH employed. Indeed, as previously reported, low doses (LD), ranging from 3 CH to 6 CH were shown to display immune boosting effects,7,8 whereas ultra-low doses (ULD) (from 7 CH up to 30 CH) exert a modulatory/inhibitory effect.9–13 In addition, the efficacy of MI has been documented in various in vitro and in vivo models, from airway allergic diseases to influenza infections, or cancers.14,15 The MIM of interest in the present study is made of ten different capsules (hereafter referred as MIM-1, MIM-2, MIM-3, etc.), each one being intended to be administered in a sequential manner, on ten consecutive days (MIM-1 on day 1, MIM-2 on day 2, MIM-3 on day 3, etc.). The capsules contain the following active substances: human recombinant (hr)-interleukin (IL)-1β, hr-IL-2, hr-IL-5, hr-IL-6, and hr-tumor necrosis factor α (TNF-α), either at 10 CH or 27 CH (hereafter referred as (10–27 CH)), depending on the capsule number; hr-transforming growth factor β (TGF-β) (10–15 CH); prostaglandin E2 (PGE2) (3–10 CH); plant-derived RNA and DNA (10–18 CH); SNA®-HLA-I and SNA®-HLA-II (10–18 CH), specially designed to target human leukocyte antigen (HLA)-I and -II, respectively, thus further referred as SNA-HLA-I and SNA-HLA-II; and SNA®-MIREG® (10–18 CH), specially designed to target human IL-2, further referred as SNA-IL-2. Overall, as cytokines and immune factors are mainly employed at ULD in the tested MIM, it is hypothesized that the in vitro effects of this medicine would rather be oriented towards an immune-modulation/inhibition. The presence of the two active substances IL-1β (27 CH) and TNF-β (27 CH) could exert anti-inflammatory effects as, in the form of unitary MIM, both displayed the capacity to inhibit the secretion of IL-1β and TNF-α, in a model of human primary monocytes as well as in THP-1 cells, after LPS exposure.13 Moreover, when combined together in other complex MIM, both could also play an important role in reducing intestinal, systemic and neuronal inflammation.13 Knowing the strong links and correlations between inflammation and mitochondrial metabolism,1–3,5,6 as well as the effects of several anti-inflammatory medicines commonly used in a large spectrum of inflammatory-mediated diseases such as metformin,16 or non-steroidal anti-inflammatory drugs on mitochondrial health,17 we wanted to assess in this study how and if this ULD-based MIM could affect the mitochondria.
Regarding the complexity of the formulation of the MIM of interest, the present work intends to serve as a pilot study to investigate the effects of some of the capsules making up the complete sequence of this MIM (namely, MIM-2, MIM-5, MIM-7 and MIM-10; see their individual composition in Material and methods, Table 1). The two major aims of this in vitro study were thus: (i) to assess their immune-modulatory effects, and (ii), to analyze their effects on the mitochondrial metabolism. In order to tackle this, several models of inflammation were chosen, all of human origin: the human umbilical vein endothelial cells (HUVEC) cell line, human PBMCs-derived macrophages, and human peripheral blood mononuclear cells (PBMCs).
 |
Table 1 Composition of the Tested Capsules of MIM |
Our results highlighted the immune-modulatory effect of MIM-7, as it reduced the expression of both HLA-DR and HLA-DP, in interferon-γ (IFN-γ)-treated HUVEC cells, and in human lipopolysaccharide (LPS)-treated-PBMCs-derived-M1-macrophages, respectively. In addition, we showed here that MIM-10 reduced the intracellular IL-2 content in the CD4+ and CD8+ T-cells immune sub-populations from human PBMCs inflamed with phorbol myristate acetate/ionomycin (PMA/Iono). These data were confirmed by enzyme-linked immunosorbent assay (ELISA), as not only the secretion of IL-2 was decreased in supernatants (SNs) retrieved from MIM-10-treated-PMA/Iono-PBMCs, but the ones of IL-10 and IFN-γ were reduced too. Interestingly, the secretion of IL-6, IL-9, and IL-17A, in a lesser extent, was found to be increased in such treatment conditions, this pattern possibly suggesting an orientation of the lymphocytes towards a Th17-like phenotype.
In addition, our analysis of the mitochondria-related biology revealed that MIM-2, MIM-5, and MIM-10 capsules displayed an inhibitory effect towards the ROS production within the B-cells and the neutrophil sub-populations, in a model of PMA/Iono-stimulated human PBMCs. Interestingly, as we found that lymphocytes and neutrophil sub-populations from PBMCs treated with 20 ng/mL of commercially available hr-IL-1β, hr-IL-2, hr-TNF-α and hr-TGF-β, displayed increased ROS levels in comparison with untreated cells, it could be possible that the inhibitory effect of the tested capsules on such ROS production, is attributable, at least partially, to the ULD employ of these above-mentioned cytokines within the formulation of this MIM. Finally, we were able to report here that MIM-2, MIM-5, and MIM-10 also exerted a protective effect towards PMA/Iono-induced cell death, in a model of human macrophages.
Overall, these in vitro results highlighted for the first time all the promising potential of the tested actives from the MIM of interest in regulating some parameters related to immune functions and mitochondria metabolism, within an inflammatory context.
Materials and Methods
Tested Items and Experimental Controls
The tested MIM, 2LMIREG®, is a homeopathic medicinal product consisting of sucrose-lactose globules impregnated with ethanolic preparations of cytokines and SNA®. The total formulation of this medicine encompasses 10 different capsules (MIM-1, MIM-2, MIM-3, MIM-4, etc.) intended to be taken in numerical order. For the investigational purposes of this study, only four capsules out of the 10 were tested, namely: MIM-2, MIM-5, MIM-7 and MIM-10. The composition of the active ingredients of these capsules is listed in Table 1. The tested MIM was manufactured and provided by Labo’Life España, as previously described.7,8
The Veh. control globules used in the study and in previous published studies are produced in order to provide a suitable control for preclinical research.7–11 For the current in vitro tests, the globules of MIM or the Veh. were freshly diluted in 100 mL of culture medium, to reach the final sucrose-lactose concentration of 11 mM.
Evaluation of the Expression of HLA-II
In HUVEC Cells
HUVEC (passage 5, pool from 6 donors, ref: #C-12203, Promocell, Heidelberg, Germany) were plated at the density of 5.000 cells/well on Day 0 (D0), in 96-well plate, in endothelial cell growth medium with supplements (ref: #C-12010, Promocell). Treatment and control were performed in low serum level medium, 2% fetal bovine serum (FBS) (ref: P30-3302, batch number #P170201, PAN-Biotech GmbH, Aidenbach, Germany). The cells were treated on D4 with rh IFN-γ 20 ng/mL (ref: #300-02, batch number #091927, PeproTech, Inc., by Thermo Fischer Scientific, Waltham, MA, USA), w/wo either the Veh., or MIM-7, at the final sucrose-lactose concentration of 11 mM, for the next two days. At the end of the incubation period, the cells were harvested, immune-stained with anti-HLA-II antibody (ref: 307622, BioLegend, San Diego, CA, USA), fixed, and the expression of HLA-DR was assessed by flow cytometry, on a BD FACS Canto II, configuration 4/2/2.
In Human M1-Macrophages
Healthy volunteers were enrolled by the Blood Bank Center (EFS, Pays de Loire, France). All blood samples were approved by the Ethics committee of the EFS Blood Bank Center, with written informed consent obtained for all the donors, in accordance with the Declaration of Helsinki. Briefly, PBMCs were isolated from 2 donors (25- and 30-year-old females); then, on D0, the cells were plated in 96-well plate, at the density of 500.000 cells/well, and cultivated in complete Roswell Park Memorial Institute Medium (RPMI) medium (ref: P04-17500, batch number #7131121, PAN-Biotech GmbH), added with 2% decomplemented human AB serum (ref: #H4522, batch number #SLCC1483, Sigma-Aldrich, Saint-Louis, MO, USA) and 50 ng/mL M-CSF. On D1, the cells were concomitantly treated with r h IFN-γ 20 ng/mL (PeproTech), w/wo either the Veh., or MIM-7, at the final sucrose-lactose concentration of 11 mM. On D4, the media/treatments were renewed, and the cells were challenged with 100 ng/mL LPS (ref: L6529, batch number #059M4103V, Sigma). On D7, the cells were harvested, immune-stained with anti-HLA-DP antibody (ref: 566825, BD Pharmingen), fixed, and the viability as well as the HLA-DP expression levels were assessed by flow cytometry. The intensity of the staining was measured as MFI value.
Intracellular IL-2 Assessment in Human PBMCs
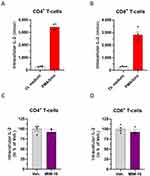
Four healthy volunteers (one 44-year-old male and three 27-, 29-, and 22-year-old females) were enrolled by the Blood Bank Center (EFS, Pays de Loire, France). PBMCs were isolated from the blood of these donors (Ficoll® density of 1.077 g/L) and cultivated in RPMI 1640, added with 2% human serum, 1 mM non-essential amino acids, 1 mM pyruvate, 2 mM L-glutamine, and 10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) buffer. The cells were plated at 200.000 cells/well and treated with either the Veh. or MIM-10 at the final concentration of 11 mM sucrose-lactose during 48 hours. Concomitant treatments with activating stimuli were also done by adding 1X PMA/Iono, the latter being incubated for the last 16 hours of treatment. After 48 hours, the cells were harvested and immune-stained with fluorescent antibodies in order to analyze the intracellular IL-2 content amongst the PBMCs sub-populations, labelled as followed: CD4+ T-cells: CD3+, CD11b−, CD4+, CD8−, CD19−, CD56−, CD14−, CD16−, SSClow; CD8+ T-cells: CD3+, CD11b−, CD4−, CD8+, CD19−, CD56−, CD14−, CD16−, SSClow. The cells were analyzed by flow cytometry on a BD FACS Canto II, configuration 4/2/2.
Assessment of the Cytokine-Secretion Profile in Human PBMCs
The secretion levels of twelve cytokines (IL-2, IL-10, IFN-γ, IL-6, IL-9, IL-17A, IL-4, IL-5, IL-13, IL-17F, IL-22 and TNF-α) were evaluated by ELISA assay, within the SNs, in the treatment conditions described in section “Intracellular IL-2 Assessment in Human PBMCs”.
Evaluation of the ROS Production in Several Sub-Populations Discriminated Amongst Human PBMCs
Evaluation of the Effect of Cytokines and Immune Factors on ROS Production
For this experiment, three healthy volunteers were enrolled by the EFS Pays de Loire. PBMCs were isolated from the blood of these donors and cultivated as described in section “Intracellular IL-2 Assessment in Human PBMCs”. The cells were incubated for 24 hours in the presence of 20 ng/mL of either rh-IL-1β (ref: #200-01B, Peprotech), rh-IL-2 (ref: #200-02, Peprotech), rh-TGF-β (ref: #100-21, Peprotech), or rh-TNF-α (ref: #130-094-014, Miltenyi). The cells were then fixed, stained with the superoxide-sensitive dye MitoROSTM 580 (AAT Bioquest Inc.), and the ROS signal was appraised by flow cytometry. The total lymphocytes and neutrophil sub-populations of interest were discriminated amongst the PBMCs, based on their FSC/SSC.
Evaluation of the Effect of MIM on ROS Production
For this part, four healthy volunteers (31-, 25-, 29- and 24-year-old females) were enrolled by the EFS Pays de Loire. PBMCs were isolated from the blood of these donors and cultivated as described in section “Intracellular IL-2 Assessment in Human PBMCs”. Briefly, on day 0 (D0), the cells were plated at 200.000 cells/well and treated with either the Veh., MIM-2, MIM-5, or MIM-10 capsules, at the final concentration of 11 mM sucrose-lactose. The cells were incubated in these conditions for 48 hours, without media renewal. On day 2 (D2), 1X PMA/Iono was employed as a ROS inducer, and was added, alone, into the culture media for 30 min. Thirty minutes later, the fluorescent probe MitoROSTM 580 (AAT Bioquest Inc., Sunnyvale, CA, USA), which selectively targets mitochondria, was co-incubated during the next 30 minutes. The cells were then harvested and immune-stained with fluorescent antibodies in order to analyze the ROS production amongst the PBMCs sub-populations, labeled as follows: B-cells: CD3−, CD4−, CD8−, CD19+, CD56−, CD14−, SSClow; neutrophils: CD3−, CD4−, CD8−, CD19−, CD56−, CD14±, SSChigh. The cells were analyzed by flow cytometry on a BD FACS Canto II, configuration 4/2/2.
Evaluation of the ROS Production in PMA-Stimulated Neutrophils
Neutrophils isolated from total human peripheral blood, from a 61-year-old female donor were cultivated at 37°C, 5% CO2. After isolation, the cells were pre-incubated for 45 minutes in an assay medium containing DHR fluorescent probe (5 µM), added with the following tested compounds: Veh., MIM-2, or MIM-10 (at the final sucrose-lactose concentration of 11 mM), or the reference compound; BHA, as an inhibitor of the ROS production, at 100 µM. The cells were then stimulated with PMA, at 0.2 µM and incubated for 30 minutes. Control without an inducer (non-stimulated control; Ct.) was performed in parallel. All experimental conditions were done in triplicate. The ROS production assay was evaluated in an assay medium corresponding to PBS/bovine serum albumin (BSA) 0.1%. The analysis was performed by flow cytometry.
Evaluation of the Mitochondrial Membrane Potential in PBMCs-Derived Macrophages
The cells were isolated and cultivated as described in section “Evaluation of the effect of MIM on ROS production”. Briefly, on D0, the cells were plated, incubated in a macrophage-differentiation medium, and treated with either the Veh., MIM-2, MIM-5, or MIM-10 capsules, at the final concentration of 11 mM sucrose-lactose. The media and treatments were renewed three times, on D2, D4, and D6. On D7, the cells were stimulated with 1X PMA/Iono and incubated with 1 µL of JC-10 dye-working solution from Cell MeterTM JC-10 Mitochondrion Membrane Potential Assay Kit (ref: #22801; AAT Bioquest, Sunnyvale, CA, USA). In normal cells, JC-10 concentrates in the mitochondrial matrix (polarized mitochondrial membrane), where it forms red fluorescent aggregates. However, in apoptotic and necrotic cells, JC-10 occurs in monomeric form and stains the cells harboring depolarized mitochondrial membranes in green. The cells were incubated at 37°C, 5% CO2, and fluorescence signal measures were performed after 4.5 hours of incubation by Bio-Imaging thanks to the MuviCyteTM microscope (ref: HH40000000, PerkinElmer). For each condition, pictures were taken (10X magnification), in duplicate, to collect enough cells for downstream quantification by MuviCyteTM Analysis software (version 2.0.18). A quantization of the Green: Red ratios was calculated, as a percentage of the fluorescent cells amongst total cells.
Statistical Analysis
The graphs in the figures were performed with GraphPad Prism, Version 10.0.3.275 for Windows (GraphPad Software Inc., San Diego California, USA, updated on 03/10/2023). Authors have followed the recent recommendations of D.L. Vaux that encourages performing descriptive statistics instead of making statistical inferences when the number of independent values is small.18 It is recommended that, if the results of the in vitro studies are derived from only one, or two, or three (n = 1, or n = 2, or n = 3) experiment(s), it is always better to include a full dataset, plotting data points, letting the readers interpret the data for themselves, rather than drawing statistical inferences, showing p values, standard error of the mean (S.E.M.), or results that are not representative. Indeed, no statistical inference has been performed to analyze the results of the studies presented here.
Results
Actives from MIM Reduce the Expression of Human Leukocyte Antigen-II in Endothelial Cells and in Peripheral Blood Mononuclear Cells-Derived M1-Macrophages
Amongst other substances (see Table 1), the capsule of MIM-7 includes SNA-HLA-II at 18 CH in its formulation, which is intended to target and down-regulate the expression of HLA-II. As HLA-II encompasses several alleles, including HLA-DR and HLA-DP, we wanted to assess if MIM-7 could modulate the expression of these alleles in two different cellular models. As a first model of primary cell line, the human umbilical vascular endothelial cells (HUVEC) were chosen. Indeed, regarding the facts that endothelial cells (i) participate in immune modulation through various processes such as cytokine secretion, co-stimulatory and co-inhibitory receptors expression, leukocyte recruitment, and phagocytic capabilities, (ii) are advantageously placed as a first-line defense system to contribute to immune reactions,19 and (iii) express HLA-DR after IFN-γ stimulation,20 they were considered as a relevant model to specifically evaluate the effect of MIM-7 on the expression of this HLA. In order to confirm that those cells were rightly able to express HLA-DR, they were treated with 20 ng/mL IFN-γ alone, or left untreated, to serve as control (Ct.) (Figure 1A). Then, regarding the experimental setting, the cells were treated with 20 ng/mL IFN-γ in concomitance with either the Veh. or MIM-7 (Figure 1B). The MIM-7 capsule was tested twice in the same experiment (#1 and #2 representing two independent capsules tested in parallel). The cells were treated for 48 hours, and the expression of HLA-DR was assessed by flow cytometry. As depicted in Figure 1B, the expression of HLA-DR was reduced by about 12%, when compared with the Veh. condition.
As we then wanted to assess the effect of MIM-7 on the expression of HLA-DP, and as this variant was not expressed by the HUVEC (data not shown), an alternative model of PBMCs-derived macrophages was chosen. As, our previous studies have already reported, in same model, that this long-course treatment allowed to better delineate the effects of other tested MIMs, either in terms of membrane marker expression or cytokine secretion,7,8,15 a similar 6-day incubation with MIM-7 was thus performed. The overall protocol is depicted in Figure 1C, but briefly, human PBMCs were isolated from two donors and cultivated in the presence of 20 ng/mL IFN-γ, in order to make the macrophages differentiate into M1 pro-inflammatory subtype. At the same time, the cells were treated with either the Veh. or MIM-7 for 6 days. The medium/treatment was renewed on day 3 (D3), and the cells were challenged with LPS 24 hours before flow cytometry analysis. The results of cell viability and HLA-DP expression are illustrated in Figure 1D and E, respectively, with the detail of the individual response per donor provided in Supplementary Figure S1. As a 6-day long-course treatment was performed, we wanted to make sure that MIM-7 did not show any toxicity in this cellular model. Our results confirmed the innocuity of MIM-7, as it slightly increased the M1-macrophages’ viability by about 10% compared with the Veh. for the two analyzed donors (Figure 1D). Interestingly, regarding HLA-DP expression, the two donors responded in a similar fashion to a 6-day MIM-7 treatment, as an overall reduction of HLA-DP expression of about 20% was induced, in comparison with the Veh. (Figure 1E).
Actives from MIM Slightly Reduce the Intracellular Expression of Interleukin-2 in Phorbol Myristate Acetate/Ionomycin-Stimulated Human Lymphocytes
Regarding the fact that MIM-10 contains the SNA at 18 CH, especially designed to target IL-2, we then wanted to focus our attention on the effects of this capsule on the intracellular IL-2 content of PBMCs. Briefly, PBMCs were freshly isolated from four healthy donors (donor #A, #B, #C, and #D), and treated for 48 hours with either the Veh. or MIM-10. A 1X PMA/Iono treatment was applied during the last 16 hours of incubation as a pro-inflammatory stimulus in order to trigger IL-2 expression. At the end of the incubation period, the cells were permeabilized, immune-stained with an anti-IL-2 antibody, and analyzed by flow cytometry. In order to validate the inflammatory model, the intracellular IL-2 content was appraised after treatment with/without PMA/Iono alone, and the results are presented in Figure 2A and B, Supplementary Figure S2A-B, within the CD4+ and the CD8+ T-cell sub-populations, respectively. With an overall mean of the median fluorescence intensity (MFI) of about 3400, the intracellular expression of IL-2 drastically increased by about 1100% after PMA/Iono stimulation, in the CD4+ T-cells from the four tested donors (Figure 2A). Similar trends were observed in the CD8+ T-cell, in the same treatment conditions, as the overall mean of the MFI for the four donors was of about 2800, corresponding to an increase of about 900% from the basal level (Figure 2B). Interestingly, cell treatment with the MIM-10 capsule led to a slight reduction in the intracellular levels of IL-2, in both the CD4+ and the CD8+ T-cells (Figure 2C and D, Supplementary Figure S2C-D). Indeed, in these two sub-populations, the overall decrease in the MFI induced by MIM-10 treatment was of about 10% compared with the Veh. condition.
Actives from MIM Reduce Interleukin-2 Secretion and Modulate the Cytokine Secretion Pattern in Phorbol Myristate Acetate/Ionomycin-Stimulated Human Peripheral Blood Mononuclear Cells
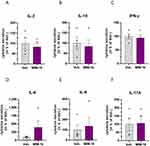
In order to confirm the previous IL-2 results obtained in the lymphocyte population, we wanted to assess if the tested MIM-10 capsule impacted the secretion of this cytokine, in the same PBMCs-treated model as presented in the previous section. At the end of the 48 hours of incubation period, the SN was collected, and the IL-2 content was evaluated by ELISA assay. The mean results obtained for the four analyzed donors are presented in Figure 3A, and the data for each individual donor are provided in Supplementary Figure S3A. Taking into account all the donors, the MIM-10-treatment reduced the overall IL-2 secretion within the SN by an average of about 17%. In addition to IL-2, and taking into account that other tested MIMs were previously reported to modulate cytokine-secretion patterns in vivo,11,14 and in vitro, in a wide range of non-immune,13,21 and immune cells, including PBMCs,7–10,13,15 we wanted to assess how MIM-10 impacted the secretion of a larger panel of cytokines. Thus, the secretion of IL-10, IFN-γ, IL-6, IL-9, IL-17A, IL-4, IL-5, IL-13, IL-17F, IL-22 and TNF-α was also analyzed. For what it concerns IL-10, IFN-γ, IL-6, IL-9, and IL-17A, the results are presented in Figure 3B–F and Supplementary Figure S3. Treatment with MIM-10 induced a global decrease in the secretion of IL-10 and IFN-γ within the four analyzed donors, when compared with the Veh., the average of this reduction being of about 15% for IL-10 (Figure 3B) and of about 6% for IFN-γ (Figure 3C). On the opposite side of the spectrum, with an average increase of about 297% for the four analyzed donors, MIM-10 induced an impressive rise in the IL-6 secretion in the treated cells when compared with the Veh. (Figure 3D), this massive increase being largely driven by one donor, who even reached an individual increase of about 945% (Supplementary Figure S3D). The same trend towards an enhanced cytokine secretion was found for the IL-9 (Figure 3E) and the IL-17A (Figure 3F); with global increments of about 37% and 7%, respectively, when compared with the Veh. condition. Heterogeneous results were obtained regarding the secretion of IL-4, IL-5, IL-13, IL-17F, IL-22, and TNF-α, as the secretion of these cytokines either did not vary or underwent an increase or a decrease, depending on the donor (Supplementary Figure S4).
Overall, regarding the modulations in the cytokine-secretion profile induced by the active ingredients from MIM-10, it seemed that this capsule reduced the secretion of IL-2, IL-10, and IFN-γ, while increasing the ones of IL-6, IL-9, and IL-17A when compared with the Veh. Even if further investigations are still needed to confirm this signature, this first set of evidence could suggest a potential orientation of the lymphocytes towards a “Th17-like” phenotype.
Commercially Available Human Recombinant Interleukin-1β, Interleukin-2, Transforming Growth Factor-β, and Tumor Necrosis Factor-α Trigger the Mitochondria-Related Production of Reactive Oxygen Species in Lymphocytes and Neutrophils Sub-Populations from Human Peripheral Blood Mononuclear Cells
The previous parts of this study report the results of our investigations on the immune-modulating effects of the tested MIM from a molecular standpoint, while the next sections are dedicated to providing a preliminary body of data about the effects of this MIM on the mitochondrial function.
In particular, as the formulation of MIM encompasses ULD of IL-1β, IL-2, TGF-β, and TNF-α, either employed at 10 CH, 15 CH, or 27 CH, depending on the capsules (see Table 1) and knowing that the above-mentioned cytokines were implicated in the ROS production in various cellular models,22–25 we firstly appraise their ability to trigger the mitochondria-related production of ROS in a model of human PBMCs, when provided as commercially available rh form, and used at high doses (20 ng/mL).
Briefly, PBMCs were isolated from 3 healthy donors and incubated for 24 hours in the presence of 20 ng/mL of either hr- IL-1β, IL-2, TGF-β, or TNF-α. The cells were stained with MitoROSTM 580 dye, then fixed, and the ROS signal was appraised by flow cytometry. In order to better define the cellular origin of the mitochondrial-ROS production, the lymphocytes and the neutrophil sub-populations were discriminated amongst the total PBMCs, based on their forward scatter/side scatter (FSC/SSC). Overall, our results showed that, for all the three assessed donors and at the tested concentration, IL-1β, IL-2, TGF-β, and TNF-α were able to increase the mitochondrial-ROS signal by about two times in the lymphocyte sub-population, compared with the untreated Ct. cells (Figure 4A). The results obtained in the neutrophil sub-population were more heterogeneous, depending on the donor, but still highlighted a slight mitochondrial-ROS-inducing effect of IL-1β, TGF-β, and TNF-α when compared with the Ct. (Figure 4B).
As our previous publications reported that the ULD employed in MI displayed inhibitory effects,9–14,26,27 these data provided us a rationale for the selection of the MIM capsules which encompass ULD of IL-1β, IL-2, TGF-β, and TNF-α, either alone or combined together, in order to further test their abilities to reduce the mitochondrial-ROS production.
Actives from MIM Reduce the Mitochondria-Specific Production of Reactive Oxygen Species in B-Cells and Neutrophils Sub-Populations from Human Peripheral Blood Mononuclear Cells
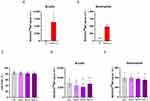
Considering the effects of IL-1β, IL-2, TGF-β, and TNF-α on the mitochondrial-ROS production (see previous section) and as MIM-2, MIM-5 and MIM-10 contain these cytokines at ULD (see Table 1), these three capsules were selected for the study of the mitochondrial function. In an attempt to be even more precise regarding the targeted cells compared with the preliminary data obtained in the previous section, a particular emphasis was made on the B-cells and the neutrophils, as the metabolism of these cells is known to be particularly sensitive to ROS.28–33 Thus, in order to discriminate the levels of ROS in B-cells and in neutrophils, human PBMCs from four donors were treated with PMA/Iono for one hour, stained with the mitochondria-specific MitoROSTM 580 dye, and incubated with a panel of antibodies allowing for the discrimination of B-cells and neutrophils. The ROS induction was appraised by flow cytometry, and the results are presented in Figure 5A and B. Overall, the PMA/Iono induced a strong increase in ROS production in the two analyzed PBMC’s sub-populations. With an average MFI of about 6.400 and about 500, respectively, the ROS expression was higher in B-cells than in neutrophils (Figure 5A and B). Although in B-cells, two donors out of the four displayed a particularly pronounced increase in ROS production compared with the others (Figure 5A), the data repartition collected between the four donors was more homogeneous in the neutrophils (Figure 5B). Considering that these results allowed to validate the PMA/Iono as a ROS-inducer in this model, the cells were then treated with either the Veh., MIM-2, MIM-5 or MIM-10 for 48 hours, before PMA/Iono stimulation and MitoROSTM 580 staining. The cell viability and the production of ROS were appraised by flow cytometry. As illustrated in Figure 5C, none of the tested capsules affected the cellular viability of the whole PBMCs’ population when compared with the Veh. Figure 5D and E displays the ROS production within the above-mentioned cell sub-populations. Interestingly, the results showed that all the assessed capsules of MIM had an inhibitory effect on ROS production within B-cells (Figure 5D). Of note, with respective decreases in the ROS levels of about 20% and 30% in comparison with the Veh., MIM-2 and MIM-5 appeared to have the strongest ROS-inhibitory effects in those cells. In addition, when assessed in neutrophils, while MIM-2 did not seem to have an impact on the mitochondrial-ROS level, MIM-5 and MIM-10 both displayed slight inhibitions of about 5 and 10%, respectively (Figure 5E). Altogether, these data highlighted an interesting inhibitory effect of the components of the tested medicine towards the mitochondria-targeted ROS production, especially within the B-cells, in our experimental conditions.
As neutrophils are particularly prone to ROS production during phagocytosis and in response to stimulation with soluble agonists,34 we wanted to test if better results could be obtained in another neutrophil model. Thus, a second experiment was designed, thanks to a dihydrorhodamine (DHR) fluorescent probe, which is a well-known tool to measure cellular ROS without mitochondria-origin’s specificity. Briefly, the cells were pre-incubated for 45 min with 5 µM of DHR probe alone, or in concomitance with either 100 µM butylated hydroxyanisole ([BHA], an inhibitor of ROS production), Veh., MIM-2, or MIM-10. The cells were then stimulated with 0.2 µM PMA for 30 minutes as a trigger for ROS production. The fluorescence was measured by flow cytometry after a total incubation duration of 75 min. As represented in Supplementary Figure S5A, PMA treatment induced an increase in ROS production by about 50 times compared with the unstimulated control condition (Ct.); an increase that was reduced by about 3.7 times by BHA treatment. However, in PMA-stimulated conditions, none of the tested capsules displayed any effect on the ROS production compared with the Veh. (Supplementary Figure S5B). These data seem to suggest that the tested actives from the assessed MIM do not affect the ROS-induced production associated with the phagocytosis, while reducing mitochondria-targeted ROS production, especially within the B-cells, in our experimental conditions.
Actives from MIM Modulate the Mitochondrial Membrane Potential of Phorbol Myristate Acetate/Ionomycin-Stimulated Human Macrophages, Slightly Protecting Them from Apoptosis
As another way to assess the mitochondrial function, measures of the mitochondrial membrane potential (mtMP) were done in a model of human PBMCs-derived macrophages. The polarization status of such membranes is indeed considered a good indicator of cellular health, as metabolically active living cells are characterized by a polarized mitochondrial membrane, while dying/apoptotic cells display a depolarized one.35 Based on this statement, a bio-imaging technique was employed, after staining the cells with the cyanine dye 5.6-dichloro-1,1’,3,3’-tetraethylimidacarbocyanine iodide (JC-10).36 This probe allows to discriminate the mitochondrial membrane polarization level: while its monomeric green form is found in dying/apoptotic cells, it forms characteristic red aggregates in mitochondrial membrane-polarized-living cells. In an attempt to assess if the actives from MIM could display an early anti-apoptotic effect in immune cells, human PBMCs-derived macrophages isolated from two donors were cultivated during 7 days in the presence of either the Veh., MIM-2, MIM-5 or MIM-10 capsules, the medium/treatment being renewed every other day. PMA/Iono and JC-10 probe were then co-incubated for 4.5 hours, and representative pictures of the fluorescence were taken for each condition. Representative pictures obtained from one donor out of the two analyzed are illustrated in Figure 6A, where the green signal at wavelength 520 nm represents unhealthy cells, and the red signal at wavelength 570 nm represents live cells. The Green: Red fluorescence ratios, calculated based on the percentage of green (or red) cells amongst the total cells, are displayed in Figure 6B. Overall, and with an effect of about 30% in comparison with the Veh., MIM-10 seemed to be the only capsule out of the three tested ones, which decreased the JC-10 Green: Red ratio.
The first set of results presented here seemed to suggest that MIM-10 could act on the mtMP in a manner that could be interpreted as a protective effect from PMA/Iono-induced cell death in a model of human primary macrophages.
Discussion
The MIM of interest in this study is composed of ten capsules, each one displaying a unique composition made of a combination of LD/ULD of cytokines and other immune regulators as active ingredients. As a complex medicine, and considering that the effects of MIM were appraised for the first time in a preclinical setting, only four capsules of the complete sequence were chosen in this study (ie, MIM-2; −5; −7 and −10). In addition, it is also worth precising that the capsules’ choice for each molecular and cellular effects measured here has been motivated by the specific LD or ULD at which the actives are employed, thus bringing more knowledge, not directly on some of the actives from the tested MIM itself, but also about how LD/ULD work in MI. As both the immunity-side and the mitochondria-related-side aspects were covered by the current work, the discussion has been divided accordingly.
In the first part of this study, the effect of some of the actives from MIM (specifically referred as MIM-7 and MIM-10) was uncovered, from an immune perspective.
Thus, concerning MIM-7, an analysis of its impact on the expression of HLA-II molecules was done. Our results delineated for the first time the ability of MIM-7 to reduce the expression of two HLA-II variants; namely, HLA-DR and HLA-DP, in IFN-γ-treated HUVEC cells (Figure 1B) and in LPS-treated PBMC-derived M1 macrophages isolated from two donors, respectively (Figure 1E). These results could be attributable to the presence of SNA-HLA-II at 18 CH, specially designed to target both HLA-DR and HLA-DP, as an active ingredient, not only included in the formulation of MIM-7 but also into a wide panel of other MIMs.11,12,14,15,26 Interestingly, a recent publication also reported the same HLA-II inhibitory effects of another MIM, intended to be used in the context of EBV-related diseases.37 These data could resonate with the fact that, in certain pathological contexts related to inflammation and mitochondria deregulation, such as allergies or autoimmune diseases, endothelial cells can contribute to an amplification of Th2 responses, through the expression/production of Th2-related cytokines.38–41 On the other side, when Th1 cells are the predominant effectors during the inflammatory process, endothelial cells preferentially express cytokines and markers that will help in the recruitment of Th1 over Th2 cells, such as C-X-C motif ligand 10 (CXCL10) and E-selectin for instance.42,43 In parallel, for what it concerns macrophages, the communication between those cells and lymphocytes, which is mediated by HLA-II molecules, takes place in several different inflammatory scenarios, for example in cases of obesity. Macrophages in adipose tissue, which become active in response to stressed adipocytes, are responsible for activating CD4+ T-cells.44,45 In such inflammatory environments, it is thus possible that the actives from MIM could help in reducing both Th1 and Th2 responses, through its mediating action towards a reduction in the expression of HLA-II molecules, like HLA-DR and HLA-DP.
These data about the immune-modulatory properties of the MIM-7 capsule were enriched by the results about the effect of MIM-10 on the intracellular expression of IL-2 within the CD4+ and the CD8+ T-cells of four donors, after stimulation with PMA/Ionomycin (Figure 2C and D). As resting immune cells only produce minimal amounts of cytokines that are essential for their basic needs, cytokine profiles obtained from inactive PBMC samples may not accurately represent the immune system’s functional status. Thus, a PMA/Ionomycin treatment was chosen as a PBMC stimulation method as it has previously been shown to induce both IL-2 mRNA expression and mitochondrial ROS production in CD4+ T-cells.46 The tested treatment could not only reduce the intracellular expression of IL-2 but also its secretion, as the levels of IL-2 that we found within the SN decreased compared with the Veh. condition (Figure 3A). As MIM-10 contains SNA-IL-2 at 18 CH (which is intended to target IL-2), this active substance may have directly played a role in reducing the IL-2 expression in the treated lymphocytes. Furthermore, MIM-10 also encompasses an inhibitory dose of TGF-β (used at 15 CH) in its formulation, which could also have helped in reducing the IL-2 secretion. Indeed, Yang et al previously reported that TGF-β up-regulated IL-2 expression and secretion in intra-tumoral CD4+ T-cells recovered from B-cells non-Hodgkin lymphomas.47 While more investigations are needed, specifically to assess the effect of the actives SNA-IL-2 at 18 CH and TGF-β at 15 CH one by one, the overall results of this study added more knowledge about the possible mode of action of ULD-based MIM and their inhibitory effects on their targets.
Regarding the results of the cytokine secretion, while the magnitude of the effects is small, it seemed that MIM-10 induced an overall trend of a reduction in the secretion of IL-10 and IFN-γ, accompanied by an increase in the IL-6, IL-9, and IL-17A secretion, when compared with the Veh., within the four analyzed donors (Figure 3B–F). In general, the polarization of the lymphocytes is determined by the cytokines present in the cellular microenvironment. Indeed, cytokines can direct lymphocytes towards an immune response of Th1, Th2, Th9, Th17, or Treg type, depending on the needs of the organism. In the specific case of MIM-10 treatment, the overall reduced secretion of IL-2, IL-10, and IFN-γ and the increased secretion of IL-6, IL-9, and IL-17A that have been observed could remind us of the cytokine pattern found in the case of an orientation towards a Th17 immune response. Indeed, while the expression of IL-6 is often associated with polarization of lymphocytes toward the Th17 pathway,48 IFN-γ and IL-10 are typically associated with Th1 and Th2 polarization status, respectively.49,50 In addition, it is worth mentioning that IL-2 is important for the survival and proliferation of T-cells, contributes to both Th1 and Th2 fates in antigen receptor-activated CD4+ T-cells,51 and also displays an inhibitory role in Th17 differentiation.52 The effects mediated by the activation of the Th17 pathway are pleiotropic; it has been described that Th17 cells possess both pro-inflammatory and anti-inflammatory IL-10-mediated functions, provided that they are generated independently of IL-1β.53
Returning to the interpretation of our experiments and taken as a whole, the results of the first part of the study could thus conceivably support the overall anti-inflammatory properties of some of the actives from the tested MIM formulation, already evoked by the effect of the actives from MIM-7 on HLA-II expression (Figure 1), in a way that could (i) attenuate Th1 and Th2 responses, without abolishing them completely, as the effects observed are quite small, and (ii), favorize a possible orientation towards Th17 immune responses.
The second part of the present study was intended to complement the previous set of results by bringing more information about the capability of some of the actives from MIM to regulate several mitochondria-related processes. Indeed, in addition to conduct oxidative phosphorylation, mitochondria also intervene in other essential cellular functions such as proliferation, programmed cell death/apoptosis, metabolism regulation, calcium homeostasis, lipids, amino acids and nucleotide synthesis, as well as antiviral response and immune cell-related biological functions.54,55 Thus, these cellular organelles are now acknowledged as pivotal centers that regulate innate immunity and inflammatory responses.56,57 In particular, mitochondria have been shown to be crucial for the proper regulation of the neutrophils and the macrophages innate immune sub-populations.58,59 In addition, mitochondria also have significant roles in B-cell development, activation, and differentiation that ensure adjustments to phenotypic and environmental deviations encountered during its lifespan, including inflammatory-related events.60
Cytokines can influence the mitochondrial ROS production and, in particular, IL-1β, IL-2, TGF-β and TNF-α trigger an increase of ROS production in immune cells, as such effect has been demonstrated in various cellular models.22–25 Our results (Figure 4) confirmed those findings and, interestingly, highlighted that the neutrophil sub-population was less sensitive to the presence of the tested cytokines than the lymphocytes. In addition, it is also worth mentioning that our data tended to show a higher heterogeneity between donors regarding those cells’ response (Figure 5A and B). This heterogeneity may be due to the density of the Ficoll® used in this experiment, which may have favored the isolation of T-cells, B-cells, NK cells, and monocytes. Neutrophils are the most common type of granulocytes in human blood, which contain distinctive cytoplasmic granules and a nucleus that is divided into three segments. They are the first cells to arrive at the site of infection and play a key role in innate immunity against bacterial infection.61 Neutrophils are equipped with several mechanisms to destroy foreign microorganisms such as bacteria, including phagocytosis, degranulation, formation of neutrophil extracellular traps, and generation of ROS including superoxide which are further converted into H2O2 by superoxide dismutase. Phagocytosis of bacteria by neutrophils triggers a respiratory burst which generates ROS that can act in concert with the release of toxic granular agents to kill the ingested microorganisms.
In this part of the study, the capsules −2, and −5 of MIM were analyzed, in addition to MIM-10. MIM-2 was selected because of the presence of IL-1β and TNF-α at 27 CH in its composition, while MIM-5 was also included because it was having IL-2 at 27 CH. Thus, the effect of these three capsules on viability and mitochondrial-ROS production was assessed in B-cells and neutrophils discriminated from human PBMCs treated with PMA/Iono. In addition, the impact of these MIMs on the mtMP of PBMCs-derived macrophages was finally evaluated, as a direct reflection of the ability of the actives from these MIMs to protect the cells from PMA/Iono-induced cell death.
For what it concerned the PMA/Iono-induced mitochondrial-ROS production, our results showed that a drastic increase in the MitoROSTM 580 signal was induced in the B-cells and the neutrophils after only one hour of PMA/Iono stimulation (Figure 5A and B). The magnitude of the B-cells response was higher than the one of neutrophils and one of the possible interpretations of this discrepancy between those cells could be because neutrophils possess an efficient NADPH oxidase system to carry ROS production for microbicidal purposes,62 which could make them less prone to mitochondria-specific-ROS production. Nonetheless, we were able to show here that a 48-hour MIM treatment reduced the PMA/Iono-induced mitochondrial-ROS production, especially in the B-cells (Figure 5D and E).
The ROS generation within the immune cells can be linked with the immune response, as those species are implicated in the processes related to the expression of cytokines/chemokines such as IFN-γ, or in the phagocytosis for instance, which occurs in anti-bacterial or anti–viral responses.63 It is also known that Toll-like receptor (TLR) signaling augments macrophage bactericidal activity through mitochondrial ROS.64 Thus, in the context of this study, a reduced production of those oxygen species could mean a decreased activation of the immune cells, which are known to be often over-activated by chronic inflammation in metabolic-related diseases.65 Collectively, those data elegantly complement the first part of the study, as, interestingly enough, the actives from MIM both reduced the mitochondrial-ROS production, especially in B-cells (Figure 5D) while also impacting the expression of HLA-II (Figure 1B and E) and IL-2 (Figures 2C, D and 3A), in endothelial cells and in other immune-related cell types. It is thus highly possible that the effects of MIM on ROS and the ones observed on the HLA-II expression and IL-2 intracellular expression/secretion are linked together. Indeed, a study showed that the TCR-triggered ROS generation by complex I was indispensable for activation-induced IL-2 and −4 expression and secretion in resting and pre-activated human T-cells.66 Another study demonstrated that autologous and allogeneic mixed lymphocyte reactions performed in the presence of monoclonal anti-HLA-DR antibodies led to a reduction in the IL-2 production.67 In addition, while the dose-dependent effects of IL-2 in up-regulating the expression of HLA-DR have been studied in CD4+ and CD8+ T-cells,68 in vitro stimulation of neutrophils with IL-2 also led to an increased expression of HLA-II.69 Altogether, these pieces of evidence sustain the fact that the concomitant presence of ULD of IL-2, SNA-HLA-II, and SNA-IL-2 (the latter being specially designed to target IL-2) could have synergistically led to the reduction of both the expression of HLA-DR/HLA-DP and the expression/production of IL-2 in the cellular models tested here. Moreover, it is worth noticing that the MIM-10 capsule, which displayed a consistent slight inhibitory effect on ROS production both in B-cells and in neutrophils, contains TGF-β at 15 CH. As it was reported that TGF-β led to redox imbalances through its capability to increase ROS production and to suppress antioxidant enzymes,70 it is not excluded that the presence of ULD of TGF-β in MIM formulation, and especially within MIM-10 capsule, may have played a particular role in down-regulating the mitochondrial-ROS levels.
Even though these results tend to support the potential anti-inflammatory properties of some of the actives from the tested MIM, an assessment of its ability to protect the cells from inflammation-induced toxicity is also of paramount importance in the context of balanced immune-responses. As variations in the mtMP and permeability reflect depolarization of the transmembrane potential, a mechanism that is associated with the release of apoptotic factors and subsequent cell death, the final part of this study aimed to assess if this medicine could impact the mtMP in a model of inflamed macrophages, thanks to bio-imaging techniques. By using the JC-10 probe, which has been reported as a powerful method to assess mitochondria-related metabolism and cellular stress,71 our last set of results suggested that the actives from MIM-10 could have an impact on the mtMP, as they reduced Green: Red ratios, potentially attesting here of an ability to decrease the accumulation of unhealthy mitochondria, and the potential to protect macrophages from PMA/Iono-induced cell death (Figure 6). Moreover, besides the putative apoptosis-protective role of MIM-10, our JC-10 probes’ results could also be interpretated as another evidence of the anti-inflammatory effect of MIM-10. Indeed, in the context of viral-related inflammation, it has already been shown that reduced mtMP was correlated with a reduced antiviral response,72 thus potentially illustrating here that our JC-10 results could also reflect a reduced level of immune activation in response to an inflammatory trigger such as PMA/Iono. Finally, our choice to only make a short 4.5-hour-PMA/Iono incubation as an inflammatory stimulus in our last experimental protocol, completely resonates with the fact that the mitochondria-related processes of inflammation are mediated very quickly after the threat-detection by the immune cells, as previous studies have shown that ROS are generated within 15 minutes of TCR cross-linking.73
Even if these results still need to be confirmed, it appeared that, out of the four tested capsules, MIM-10 was the one that seemed to display the most protective effect towards inflammation-induced cell death. Such results could be attributable to the concomitant presence of TGF-β at 15 CH and the IL-2-targeting SNA at 18 CH, as several evidence reported the physiological impact of these immune factors in mtMP disturbance. Indeed, while IL-2 has been shown to induce an increase in mitochondrial mass, mtMP, and PGC-1α expression in human NK cells,74 Abe et al, found that TGF-β acted as an enhancer of the oxidative phosphorylation, the glycolysis and the ROS production in transformed mouse podocytes.75 Additionally, the latter group reported that TGF-β did not show any effect on cell or mitochondria number, mitochondria mass or gene expression, but that it increased MM potential in a dose-dependent manner.
In the intricate domain of immune system modulation, the inhibition of ROS production within immune cells heralds a significant therapeutic potential. As previously mentioned, accumulating evidence underscores the dualistic nature of ROS as both indispensable signaling molecules and deleterious agents precipitating oxidative stress when in excess.4 Controlling ROS levels through tailored inhibition can alleviate the latter’s adverse impact on cellular components, thus safeguarding immune cells from oxidative damage. In this context, not only the homeostatic regulation which may be restored by MIM could enhance the longevity and functional integrity of immune cells but it could also attenuate the inflammatory response, a benefit poised to ameliorate chronic inflammation-related pathologies. When the levels of ROS are chronically elevated, the effector functions of immune cells are compromised.76 Just to give an example, the chronic antigen stimulation and accumulation of ROS results in T-cells being more prone to cell death.77 Dysregulated levels of ROS in T-cells have been described in the context of auto-immune diseases or cancer. All this body of knowledge indicates that controlling ROS production and avoiding its over-production is critical for the maintenance of an efficient T-cell-mediated immunity, opening novel perspectives to develop therapies targeting ROS to improve patient outcomes in these diseases.78 Neuroinflammatory disorders, such as multiple sclerosis, also have ROS implicated in the initiation and progression of the diseases.79 In line with these pieces of evidence, our results, while preliminary, are relevant and position MIM as a potential medicine in the context of immune-mediated diseases in which chronic inflammation and high levels of ROS are strongly related to pathogenesis.
Moreover, the selective inhibition of ROS in immune cells may reduce the risk of propagated injury to adjacent tissues, thus preserving overall organismal health. Nonetheless, cautions against over-suppression of ROS are warranted, given their essential physiological roles. Future investigations and axes of research about MIM should aim to delineate the nuanced balance between ROS as cellular messengers and their potential for harm and in which extent MIM treatment could help in optimizing strategies to (i) bolster host defense while (ii) averting the pathogenesis associated with excess oxidative stress. In order to do so, in-depth studies assessing more specifically activities of mitochondrial enzymes involved in oxidative phosphorylation and the tricarboxylic acid cycle, such as citrate synthase and complex I-V of the electron transport chain for instance, or even techniques involving real-time measurement of the oxygen consumption rate and extracellular acidification rate in live immune cell subpopulations, could provide insights on how MIM regulate mitochondrial respiration and glycolysis.
Conclusion
Overall, our results provide for the first-time preclinical information about the effect of the actives from the MIM of interest on two intrinsically linked sides of biology; the immune-related responses and the mitochondria-related metabolism regulation. As the tested MIM is a medicine encompassing ten different capsules impregnated with unique combinations of LD/ULD of cytokines, immune factors, and nucleic acids, this study aimed at firstly evaluating the anti-inflammatory and the metabolism-related effects of some of its capsules. Here, we thus reported that the actives from MIM-7 slightly reduced the expression of HLA-DR and HLA-DP in IFN-γ-stimulated HUVEC cells and LPS-stimulated human PBMCs-derived-M1 macrophages, respectively. Moreover, in PMA/Iono stimulated human PBMCs, the actives from MIM-10 were shown to slightly reduce the intracellular expression of IL-2 within the CD4+ T-cells and CD8+ T-cells sub-populations, which also reflected at the secretion level, when analyzed by ELISA assays. In addition, in the assessed experimental conditions, this capsule also impacted the cytokine secretion at a larger scale, as it concomitantly reduced the secretion of IL-10 and IFN-γ, while increasing the one of IL-6, IL-9 and IL-17A, which could be attesting of a Th17-like signature. In the same model of PMA/Iono-stimulated human PBMCs, while none of the MIM capsules −2; −5; and −10 affected the cell viability, they slightly reduced the mitochondrial-ROS production, especially within the B-cells sub-population. Finally, the effects of these three particular capsules were appraised on the mitochondrial membrane potential changes, as a witness of the induction of an early cell death/apoptosis. Our experiment revealed that MIM-10 could slightly reduce the mitochondrial damages induced by the PMA/Iono and could thus protect the cells from its toxicity.
Taken together, our results delineated for the first time the potential anti-inflammatory effect of some of the actives from the tested MIM, and suggest its underlined capability to improve mitochondrial functions that can be dysregulated under chronic inflammatory conditions.
Abbreviations
ATP, adenosine triphosphate; BHA, butylated hydroxyanisole; BSA, bovine serum albumin; CH, centesimal Hahnemannian; CXCL10, C-X-C motif ligand 10; DHR, dihydrorhodamine; DNA, deoxyribonucleic acid; ECBM, endothelial cell growth medium; EFS, Etablissement Français du Sang; ELISA, enzyme-linked immunosorbent assay; FBS, fetal bovine serum; FSC, forward scatter; HEPES, 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid; HLA, human leukocyte antigen; hr, human recombinant; HUVEC, human umbilical vein endothelial cells; GMFI, geometric mean fluorescence intensity; IFN-γ, interferon-γ; IL, interleukin; Iono, Ionomycin; JC-10, 5.6-dichloro-1,1’,3,3’-tetraethylimidacarbocyanine iodide; LD, low doses; LPS, lipopolysaccharide; M-CSF, macrophage colony-stimulating factor; MFI, median fluorescence intensity; MI, micro-immunotherapy; MIM, micro-immunotherapy medicine; mtMP, mitochondrial membrane potential; PBMCs, peripheral blood mononuclear cells; PBS, phosphate-buffered saline; PGE2, prostaglandin E2; PMA, phorbol myristate acetate; RNA, ribonucleic acid; ROS, reactive oxygen species; RPMI, Roswell Park Memorial Institute medium; S.D., standard deviation; S.E.M., standard error of the mean; SNA®, specific nucleic acids; SNs, supernatants; SSC, side scatter; TGF-β, tumor growth factor-β; TLR, Toll-like receptor; TNF-α, tumor necrosis factor-α; ULD, ultra-low doses; Veh., vehicle.
Acknowledgments
The authors are grateful to the staff of QIMA Sciences, especially Adrien Brulefert for their work in performing the experiments of the in vitro study on PMA-stimulated granulocytes, and Laura Garcia-Sureda, from Labo’Life Spain, for having managed this part of the project. The authors would like to thank Miquel Ensenyat and his team for having prepared, provided and sent the Veh. and the tested MIMs to ProfileHIT and QIMA facilities. The authors thank Sofia Frau for the insightful comments that improved the final version of the manuscript. Finally, the authors thank Servier Medical Art (https://smart.servier.com/, accessed on the 02/06/2023) for the items used in the figures.
Disclosure
The authors declared the following conflicts of interest with respect to the research, authorship, and/or publication of this article: Camille Jacques and Ilaria Floris work for Labo’Life France, the company service provider of Labo’Life, specialized in preclinical research and regulatory affairs. This professional relationship does not imply any misconduct on the part of the authors. Mathias Chatelais and Flora Marchand work for ProfileHIT, an innovative profiling company involved in vascular and immunology crosstalk research field in human. This study was entirely funded by Labolife France. The authors report no other conflicts of interest in this work.
References
1. Pagano G, Aiello talamanca A, Castello G, et al. Oxidative stress and mitochondrial dysfunction across broad-ranging pathologies: toward mitochondria-targeted clinical strategies. Oxid Med Cell Longev. 2014;2014:e541230. doi:10.1155/2014/541230
2. Wallace DC. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a Dawn for evolutionary medicine. Annu Rev Genet. 2005;39:359–407. doi:10.1146/annurev.genet.39.110304.095751
3. Hill S, Van Remmen H. Mitochondrial stress signaling in longevity: a new role for mitochondrial function in aging. Redox Biol. 2014;2:936–944. doi:10.1016/j.redox.2014.07.005
4. Vaamonde-García C, Riveiro‐Naveira RR, Valcárcel‐Ares MN, et al. Mitochondrial dysfunction increases inflammatory responsiveness to cytokines in normal human chondrocytes. Arthritis Rheum. 2012;64:2927–2936. doi:10.1002/art.34508
5. López-Armada MJ, Riveiro-Naveira RR, Vaamonde-García C, Valcárcel-Ares MN. Mitochondrial dysfunction and the inflammatory response. Mitochondrion. 2013;13:106–118.
6. Bouhamida E, Morciano G, Perrone M, et al. The interplay of hypoxia signaling on mitochondrial dysfunction and inflammation in cardiovascular diseases and cancer: from molecular mechanisms to therapeutic approaches. Biology. 2022;11:300. doi:10.3390/biology11020300
7. Jacques C, Chatelais M, Fekir K, Brulefert A, Floris I. The unitary micro-immunotherapy medicine interferon-γ (4 CH) displays similar immunostimulatory and immunomodulatory effects than those of biologically active human interferon-γ on various cell types. Int J Mol Sci. 2022;23:2314. doi:10.3390/ijms23042314
8. Jacques C, Chatelais M, Fekir K, et al. The micro-immunotherapy medicine 2LEID exhibits an immunostimulant effect by boosting both innate and adaptive immune responses. Int J Mol Sci. 2021;23:110. doi:10.3390/ijms23010110
9. Floris I, et al. Pro-inflammatory cytokines at ultra-low dose exert anti-inflammatory effect in vitro: a possible mode of action involving sub-micron particles? Dose-Response Publ Int Hormesis Soc. 2020;18:1559325820961723.
10. Floris I, Appel K, Rose T, Lejeune B. 2LARTH®, a micro-immunotherapy medicine, exerts anti-inflammatory effects in vitro and reduces TNF-α and IL-1β secretion. J Inflamm Res. 2018;11:397–405. doi:10.2147/JIR.S174326
11. Floris I, García-González V, Palomares B, Appel K, Lejeune B. The micro-immunotherapy medicine 2LARTH® reduces inflammation and symptoms of rheumatoid arthritis in vivo. Int J Rheumatol. 2020;2020:1594573. doi:10.1155/2020/1594573
12. Jacques C, Floris I, Lejeune B. Ultra-low dose cytokines in rheumatoid arthritis, three birds with one stone as the rationale of the 2LARTH® Micro-immunotherapy treatment. Int J Mol Sci. 2021;22:6717. doi:10.3390/ijms22136717
13. Jacques C, Floris I. Special focus on the cellular anti-inflammatory effects of several micro-immunotherapy formulations: considerations regarding intestinal-, immune-axis-related- and neuronal-inflammation contexts. J Inflamm Res. 2022;15:6695–6717. doi:10.2147/JIR.S389614
14. Floris I, Chenuet P, Togbe D, Volteau C, Lejeune B. Potential role of the micro-immunotherapy medicine 2LALERG in the treatment of pollen-induced allergic inflammation. Dose-Response Publ Int Hormesis Soc. 2020;18:1559325820914092.
15. Jacques C, Marchesi I, Fiorentino FP, et al. A micro-immunotherapy sequential medicine mim-seq displays immunomodulatory effects on human macrophages and anti-tumor properties towards in vitro 2D and 3D models of colon carcinoma and in an in vivo subcutaneous xenograft colon carcinoma model. Int J Mol Sci. 2022;23:6059. doi:10.3390/ijms23116059
16. Ji D, Yin J-Y, Li D-F, et al. Effects of inflammatory and anti-inflammatory environments on the macrophage mitochondrial function. Sci Rep. 2020;10:20324. doi:10.1038/s41598-020-77370-x
17. Watanabe T, Tanigawa T, Nadatani Y, et al. Mitochondrial disorders in NSAIDs-induced small bowel injury. J Clin Biochem Nutr. 2011;48:117–121. doi:10.3164/jcbn.10-73
18. Vaux DL. Research methods: know when your numbers are significant. Nature. 2012;492:180–181. doi:10.1038/492180a
19. Amersfoort J, Eelen G, Carmeliet P. Immunomodulation by endothelial cells — partnering up with the immune system? Nat Rev Immunol. 2022;22:576–588. doi:10.1038/s41577-022-00694-4
20. Maenaka A, Kenta I, Ota A, et al. Interferon-γ-induced HLA Class II expression on endothelial cells is decreased by inhibition of mTOR and HMG-CoA reductase. FEBS Open Bio. 2020;10:927–936. doi:10.1002/2211-5463.12854
21. Ferrà-Cañellas MDM, Munar‐Bestard M, Garcia‐Sureda L, et al. BMP4 micro-immunotherapy increases collagen deposition and reduces PGE2 release in human gingival fibroblasts and increases tissue viability of engineered 3D gingiva under inflammatory conditions. J Periodontol. 2021;92:1448–1459. doi:10.1002/JPER.20-0552
22. Ansari MY, Khan NM, Ahmad I, Haqqi TM. Parkin clearance of dysfunctional mitochondria regulates ROS levels and increases survival of human chondrocytes. Osteoarthritis Cartilage. 2018;26:1087–1097. doi:10.1016/j.joca.2017.07.020
23. Yang D, Elner SG, Bian Z-M, et al. Pro-inflammatory cytokines increase reactive oxygen species through mitochondria and NADPH oxidase in cultured RPE cells. Exp Eye Res. 2007;85:462–472. doi:10.1016/j.exer.2007.06.013
24. Akkaya B. Increased mitochondrial biogenesis and ROS production accompany prolonged CD4+ T cell activation. J Immunol. 2018;201:3294–3306.
25. Krstić J, Trivanović D, Mojsilović S, Santibanez JF. Transforming growth factor-beta and oxidative stress interplay: implications in tumorigenesis and cancer progression. Oxid Med Cell Longev. 2015;2015:654594. doi:10.1155/2015/654594
26. Jacques C, Floris I. How an immune-factor-based formulation of micro-immunotherapy could interfere with the physiological processes involved in the atopic march. Int J Mol Sci. 2023;24:1483. doi:10.3390/ijms24021483
27. Ferrà-Cañellas MDM, Munar-Bestard M, Floris I, et al. A sequential micro-immunotherapy medicine increases collagen deposition in human gingival fibroblasts and in an engineered 3D gingival model under inflammatory conditions. Int J Mol Sci. 2023;24:10484. doi:10.3390/ijms241310484
28. Zhang H, Wang L, Chu Y. Reactive oxygen species: the signal regulator of B cell. Free Radic Biol Med. 2019;142:16–22. doi:10.1016/j.freeradbiomed.2019.06.004
29. Bystrom J, Taher TE, Henson SM, Gould DJ, Mageed RA. Metabolic requirements of Th17 cells and of B cells: regulation and defects in health and in inflammatory diseases. Front Immunol. 2022;13:990794. doi:10.3389/fimmu.2022.990794
30. Vené R, Delfino L, Castellani P, et al. Redox remodeling allows and controls B-cell activation and differentiation. Antioxid Redox Signal. 2010;13:1145–1155. doi:10.1089/ars.2009.3078
31. Bertolotti M, Yim SH, Garcia-Manteiga JM, et al. B- to plasma-cell terminal differentiation entails oxidative stress and profound reshaping of the antioxidant responses. Antioxid Redox Signal. 2010;13:1133–1144. doi:10.1089/ars.2009.3079
32. Winterbourn CC, Kettle AJ, Hampton MB. Reactive oxygen species and neutrophil function. Annu Rev Biochem. 2016;85:765–792.
33. Vorobjeva N, Prikhodko A, Galkin I, et al. Mitochondrial reactive oxygen species are involved in chemoattractant-induced oxidative burst and degranulation of human neutrophils in vitro. Eur J Cell Biol. 2017;96:254–265. doi:10.1016/j.ejcb.2017.03.003
34. Chen Y, Junger WG. Measurement of oxidative burst in neutrophils. Methods Mol Biol Clifton NJ. 2012;844:115–124.
35. Matsuyama S, Reed JC. Mitochondria-dependent apoptosis and cellular pH regulation. Cell Death Differ. 2000;7:1155–1165. doi:10.1038/sj.cdd.4400779
36. Donaghy L, Kraffe E, Le Goïc N, et al. Reactive oxygen species in unstimulated hemocytes of the pacific oyster Crassostrea gigas: a mitochondrial involvement. PLoS One. 2012;7:e46594. doi:10.1371/journal.pone.0046594
37. Jacques C, Marchand F, Chatelais M, Brulefert A, Floris I. Understanding the mode of action of a micro-immunotherapy formulation: pre-clinical evidence from the study of 2LEBV® active ingredients. Life Basel Switz. 2024;14:102.
38. Moussion C, Ortega N, Girard J-P. The IL-1-like cytokine IL-33 is constitutively expressed in the nucleus of endothelial cells and epithelial cells in vivo: a novel ‘alarmin’? PLoS One. 2008;3:e3331. doi:10.1371/journal.pone.0003331
39. Schmitz J, Owyang A, Oldham E, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. doi:10.1016/j.immuni.2005.09.015
40. Guo L, Wei G, Zhu J, et al. IL-1 family members and STAT activators induce cytokine production by Th2, Th17, and Th1 cells. Proc Natl Acad Sci U S A. 2009;106:13463–13468. doi:10.1073/pnas.0906988106
41. Paul WE, Zhu J. How are TH2-type immune responses initiated and amplified? Nat Rev Immunol. 2010;10:225–235. doi:10.1038/nri2735
42. Mai J, Virtue A, Shen J, Wang H, Yang X-F. An evolving new paradigm: endothelial cells – conditional innate immune cells. J Hematol Oncol. 2013;6:61.
43. Austrup F, Vestweber D, Borges E, et al. P- and E-selectin mediate recruitment of T-helper-1 but not T-helper-2 cells into inflamed tissues. Nature. 1997;385:81–83. doi:10.1038/385081a0
44. Cho KW, Morris D, DelProposto J, et al. An MHC II-dependent activation loop between adipose tissue macrophages and CD4+ T cells controls obesity-induced inflammation. Cell Rep. 2014;9:605–617. doi:10.1016/j.celrep.2014.09.004
45. Morris DL, Cho KW, DelProposto JL, et al. Adipose tissue macrophages function as antigen-presenting cells and regulate adipose tissue CD4+ T cells in mice. Diabetes. 2013;62:2762–2772. doi:10.2337/db12-1404
46. Sena LA, Li S, Jairaman A, et al. Mitochondria are required for antigen-specific T cell activation through reactive oxygen species signaling. Immunity. 2013;38:225–236. doi:10.1016/j.immuni.2012.10.020
47. Yang Z-Z, Grote DM, Ziesmer SC, et al. Soluble and membrane-bound TGF-β-mediated regulation of intratumoral T cell differentiation and function in B-cell non-Hodgkin lymphoma. PLoS One. 2013;8:e59456.
48. Kimura A, Kishimoto T. IL-6: regulator of Treg/Th17 balance. Eur J Immunol. 2010;40:1830–1835.
49. Tau GZ, Von der Weid T, Lu B, et al. Interferon γ signaling alters the function of T helper type 1 cells. J Exp Med. 2000;192:977–986. doi:10.1084/jem.192.7.977
50. Laouini D, Alenius H, Bryce P, et al. IL-10 is critical for Th2 responses in a murine model of allergic dermatitis. J Clin Invest. 2003;112:1058–1066.
51. Ross SH, Cantrell DA. Signaling and function of interleukin-2 in T lymphocytes. Annu Rev Immunol. 2018;36:411–433. doi:10.1146/annurev-immunol-042617-053352
52. Laurence A, Tato CM, Davidson TS, et al. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26:371–381. doi:10.1016/j.immuni.2007.02.009
53. Noster R, de Koning H, Sallusto F, Zielinski C. Two types of human Th17 cells with pro- and anti-inflammatory properties and distinct roles in autoinflammation. Pediatr Rheumatol Online J. 2015;13:O49. doi:10.1186/1546-0096-13-S1-O49
54. McBride HM, Neuspiel M, Wasiak S. Mitochondria: more than just a powerhouse. Curr Biol CB. 2006;16:R551–560.
55. Mills EL, Kelly B, O’Neill LAJ. Mitochondria are the powerhouses of immunity. Nat Immunol. 2017;18:488–498. doi:10.1038/ni.3704
56. Missiroli S, Genovese I, Perrone M, et al. The role of mitochondria in inflammation: from cancer to neurodegenerative disorders. J Clin Med. 2020;9:740. doi:10.3390/jcm9030740
57. Mohanty A, Tiwari-Pandey R, Pandey NR. Mitochondria: the indispensable players in innate immunity and guardians of the inflammatory response. J Cell Commun Signal. 2019;13:303–318. doi:10.1007/s12079-019-00507-9
58. Cao Z, Zhao M, Sun H, et al. Roles of mitochondria in neutrophils. Front Immunol. 2022;13:934444. doi:10.3389/fimmu.2022.934444
59. Wang Y, Li N, Zhang X, Horng T. Mitochondrial metabolism regulates macrophage biology. J Biol Chem. 2021;297:100904. doi:10.1016/j.jbc.2021.100904
60. Sandoval H, Kodali S, Wang J. Regulation of B cell fate, survival, and function by mitochondria and autophagy. Mitochondrion. 2018;41:58–65. doi:10.1016/j.mito.2017.11.005
61. Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13:159–175. doi:10.1038/nri3399
62. Kuwabara WMT, Zhang L, Schuiki I, et al. NADPH oxidase-dependent production of reactive oxygen species induces endoplasmatic reticulum stress in neutrophil-like HL60 cells. PLoS One. 2015;10:e0116410. doi:10.1371/journal.pone.0116410
63. Yang Y, Bazhin AV, Werner J, Karakhanova S. Reactive oxygen species in the immune system. Int Rev Immunol. 2013;32:249–270. doi:10.3109/08830185.2012.755176
64. West AP, Brodsky IE, Rahner C, et al. TLR signalling augments macrophage bactericidal activity through mitochondrial ROS. Nature. 2011;472:476–480.
65. Ganeshan K, Chawla A. Metabolic regulation of immune responses. Annu Rev Immunol. 2014;32:609–634. doi:10.1146/annurev-immunol-032713-120236
66. Kaminski MM, Sauer SW, Klemke CD, et al. Mitochondrial reactive oxygen species control T cell activation by regulating IL-2 and IL-4 expression: mechanism of ciprofloxacin-mediated immunosuppression. J Immunol. 2010;184:4827–4841.
67. Palacios R. HLA-DR antigens render interleukin-2-producer T lymphocytes sensitive to interleukin-1. Scand J Immunol. 1981;14:321–326. doi:10.1111/j.1365-3083.1981.tb00571.x
68. Salgado FJ, Lojo J, Fernández‐Alonso CM, et al. Interleukin-dependent modulation of HLA-DR expression on CD4 and CD8 activated T cells. Immunol Cell Biol. 2002;80:138–147. doi:10.1046/j.1440-1711.2002.01055.x
69. Abdel-Salam BKA-H. Comparing effects of interleukin-2 and interleukin-4 on the expression of MHC class II, CD80 and CD86 on polymorphonuclear neutrophils. Egypt J Med Hum Genet. 2010;11:115–120. doi:10.1016/j.ejmhg.2010.05.002
70. Liu R-M, Desai LP. Reciprocal regulation of TGF-β and reactive oxygen species: a perverse cycle for fibrosis. Redox Biol. 2015;6:565–577. doi:10.1016/j.redox.2015.09.009
71. Younes N, Alsahan BS, Al-Mesaifri AJ, et al. JC-10 probe as a novel method for analyzing the mitochondrial membrane potential and cell stress in whole zebrafish embryos. Toxicol Res. 2022;11:77–87. doi:10.1093/toxres/tfab114
72. Koshiba T, Yasukawa K, Yanagi Y, Kawabata S. Mitochondrial membrane potential is required for MAVS-mediated antiviral signaling. Sci Signal. 2011;4:ra7. doi:10.1126/scisignal.2001147
73. Devadas S, Zaritskaya L, Rhee SG, Oberley L, Williams MS. Discrete generation of superoxide and hydrogen peroxide by T cell receptor stimulation: selective regulation of mitogen-activated protein kinase activation and fas ligand expression. J Exp Med. 2002;195:59–70. doi:10.1084/jem.20010659
74. Miranda D, Jara C, Ibañez J, et al. PGC-1 α -dependent mitochondrial adaptation is necessary to sustain IL-2-induced activities in human NK cells. Mediators Inflamm. 2016;2016:9605253. doi:10.1155/2016/9605253
75. Abe Y, Sakairi T, Beeson C, Kopp JB. TGF-β1 stimulates mitochondrial oxidative phosphorylation and generation of reactive oxygen species in cultured mouse podocytes, mediated in part by the mTOR pathway. Am J Physiol - Ren Physiol. 2013;305:F1477–F1490. doi:10.1152/ajprenal.00182.2013
76. Morris G, Gevezova M, Sarafian V, Maes M. Redox regulation of the immune response. Cell Mol Immunol. 2022;19:1079–1101. doi:10.1038/s41423-022-00902-0
77. Bettonville M, d’Aria S, Weatherly K, et al. Long-term antigen exposure irreversibly modifies metabolic requirements for T cell function. eLife. 2018;7:e30938. doi:10.7554/eLife.30938
78. Yarosz EL, Chang C-H. The role of reactive oxygen species in regulating T cell-mediated immunity and disease. Immune Netw. 2018;18:e14. doi:10.4110/in.2018.18.e14
79. Tavassolifar MJ, Vodjgani M, Salehi Z, Izad M. The influence of reactive oxygen species in the immune system and pathogenesis of multiple sclerosis. Autoimmune Dis. 2020;2020:5793817. doi:10.1155/2020/5793817
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.