Back to Journals » Nature and Science of Sleep » Volume 15
Mind, Body and Machine: Preliminary Study to Explore Predictors of Treatment Response After a Sleep Robot Intervention for Adults with Insomnia
Authors Støre SJ , Tillfors M, Wästlund E, Angelhoff C, Andersson G, Norell A
Received 23 February 2023
Accepted for publication 23 May 2023
Published 13 July 2023 Volume 2023:15 Pages 567—577
DOI https://doi.org/10.2147/NSS.S408714
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Ahmed BaHammam
Siri Jakobsson Støre,1 Maria Tillfors,1 Erik Wästlund,1 Charlotte Angelhoff,2,3 Gerhard Andersson,3– 5 Annika Norell6,7
1Department of Social and Psychological Studies, Karlstad University, Karlstad, Region Värmland, Sweden; 2Crown Princess Victoria’s Child and Youth Hospital, Linköping University Hospital, Linköping, Region Östergötland, Sweden; 3Department of Biomedical and Clinical Sciences (BKV), Linköping University, Linköping, Region Östergötland, Sweden; 4Department of Behavioural Sciences and Learning, Linköping University, Linköping, Region Östergötland, Sweden; 5Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Region Stockholm, Sweden; 6School of Behavioural, Social and Legal Sciences, Örebro University, Örebro, Region Örebro län, Sweden; 7Faculty of Health Sciences, Kristianstad University, Kristianstad, Region Skåne, Sweden
Correspondence: Siri Jakobsson Støre, Department of Social and Psychological Studies, Karlstad University, Karlstad, SE-651 88, Sweden, Email [email protected]
Introduction: The study aimed to explore characteristics of responders to a sleep robot intervention for adults with insomnia, and the likelihood that participants responded to the intervention.
Methods: Data from the intervention and the control group in a randomized waitlist-controlled trial (n = 44) were pooled together after both had undergone the intervention. A repeated measures ANOVA and Friedman tests were used to explore changes over time. Differences in baseline characteristics between responders (n = 13), defined as a reduction of − 5 on the Insomnia Severity Index from pre- to post-intervention, and non-responders (n = 31) were analyzed with t-tests and chi-square tests. Finally, logistic regression models were estimated.
Results: Baseline anxiety was the only statistically significant difference between responders and non-responders (p = 0.03). A logistic regression model with anxiety and sleep quality as predictors was statistically significant, correctly classifying 83.3% of cases.
Discussion: The results imply that people with lower anxiety and higher sleep quality at baseline are more likely to report clinically significant improvements in insomnia from the sleep robot intervention.
Keywords: anxiety, depression, insomnia, sleep, sleep diary, sleep robot, treatment response
Introduction
Insomnia, or sleeplessness, is the most common sleep disorder in adults,1 and the second most common mental disorder of all.2 The definition of insomnia in the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) involves a self-experienced difficulty with falling asleep or maintaining sleep throughout the night and/or in the early morning, causing significant distress or impairment in, for instance, social or occupational functioning.3 The first-line treatment of insomnia is Cognitive-Behavioral Therapy (CBT-I).4 At least partly due to a shortage of CBT-therapists,5 the most common insomnia treatment is still hypnotic medicine.6 Research on potential treatment options is warranted. Moreover, average treatment effects in studies may mask unrecognized insomnia subtypes, and further, affect successful replication and synthetization of results from individual studies in meta-analyses.7 Hence, it is important to consider the significance of participant characteristics for the effects, or lack thereof, in intervention studies, for more personalized insomnia treatments.
Subtypes of insomnia have been proposed, for instance, initial, middle, late, and mixed insomnia, based on people’s most salient sleep complaint.7 Moreover, it has been suggested that non-sleep characteristics may constitute more important subtypes, for instance demographics, mood, affect, personality, and life history.7,8 According to the diagnostic criteria, insomnia does not necessarily entail short sleep duration. Insomnia combined with objectively defined short sleep duration has been found to be a more severe type of insomnia, compared with that of insomnia and adequate sleep duration.9 People with insomnia are often diagnosed with other psychiatric disorders as well, for example anxiety and depression.10,11 Given the nature of the diagnostic criteria, the number of people who meet the criteria, combined with objectively defined short sleep duration versus adequate sleep hours, and numerous possible comorbid conditions, people with insomnia should be viewed as a heterogeneous group of people, likely with different treatment needs (eg, full CBT-I program versus certain components, or evidence-based treatment options). Regarding clinical heterogeneity, or differences in participant characteristics, sex (female), age (older), education (low), and marital status (separated/divorced or widowed) are established predictors of insomnia.12–14 However, these factors have not been shown to be significant predictors of treatment success when it comes to CBT-I.15–17 Higher levels of anxiety symptoms have, on the other hand, been found to predict poorer psychological treatment response in adults with insomnia and chronic hypnotic use.18 Insomnia is by definition more of a sleep quality than a sleep quantity problem. Sleep quality has been found to be an important outcome when it comes to defining treatment response.19,20 It is therefore important to address sleep quality in insomnia intervention studies in general – and specifically when analyzing treatment response.
Consumer sleep technology has been defined as “Non-prescription devices directly marketed to consumers that may make an assertion to perform sleep monitoring, tracking, or sleep-related interventions” by the American Academy of Sleep Medicine.21 Most sleep devices, and research on such devices, concern monitoring and tracking. One line of sleep-related interventions are sleep robots. In a systematic review and network meta-analysis of robots’ effects on nighttime sleep, no statistically significant differences were found between robot interventions, plush toys, or treatment as usual on nighttime sleep in older adults.22 The robot used in all four included studies was a companion robot, ie, not a robot first and foremost meant to be able to treat troubled sleep. An example of a sleep intervention device is the Somnox sleep robot, a sort of pillow with integrated Intelligent Smart Breathing Technology.23 The robot is meant to be held close to the body at bedtime with the aim of synchronized optimal breathing in order to foster relaxation and improve sleep. Relaxation is widely recognized to have positive impacts on both physical health and mental well-being.24,25 In the context of CBT-I, relaxation can take various forms, including progressive muscle relaxation, guided imagery, and breathing techniques.26,27 Hyperarousal is a common feature in the majority of insomnia models, encompassing heightened levels of somatic arousal such as increased heart rate and respiration, cognitive arousal with excessive worry and rumination, and cortical arousal in terms of increased brain activity. The hyperarousal or the neurocognitive model of insomnia – an extension of the 3P behavioral model where the three Ps stand for predisposing, precipitation and perpetuating factors – highlights the importance of all three forms of hyperarousal in insomnia.28,29 It is commonly believed that relaxation can be helpful in reducing somatic arousal and enhance sleep. However, it may also work by calming pre-sleep cognitive activity, ie, by simultaneously reducing worries and increasing positive thoughts about one’s sleep.30
The sleep robot intervention has previously been described in the original randomized waitlist-controlled trial and in a feasibility study.31,32 The aforementioned studies are the only independent studies on the Somnox sleep robot. The conclusion from the feasibility study was that there were mixed results regarding individual effects of the sleep robot intervention, but that the intervention was found both acceptable in terms of adherence, and safe in terms of emotional distress.32 The randomized waitlist-controlled trial focused on somatic arousal as this factor was believed to be the sleep robot’s target, to reduce physiological arousal through breathworks. Group means did not show statistically significant effects of the intervention on any of the outcome measures (insomnia severity, somatic arousal, symptoms of anxiety and depression, sleep variables measured subjectively and objectively). However, looking at the raw scores on an individual level, several participants reported clinically relevant reductions regarding insomnia severity post-intervention that are important to explore. The aims of the current study were to assess the proportions of responders and non-responders in the sleep robot intervention, find out if responders and non-responders differed from each other in terms of baseline demographics and clinical characteristics, and examine the likelihood that participants responded to the intervention based on identified characteristics. The research questions were: (1) How do responders and non-responders differ from each other at baseline; and (2) What is the likelihood that participants are responders? The study is the first to explore possible predictors of treatment response after an intervention with the Somnox sleep robot.
Methods
Study Design
In the original study,31 a pre-registered randomized waitlist-controlled trial (n = 44) was conducted to evaluate the effects of an at-home intervention with a sleep robot (Somnox) for three weeks (n = 22) compared with the waitlist control group (n = 22). The study complied with the Declaration of Helsinki. The Swedish Ethical Review Board approved the study project (DNR 2020–069759), which was conducted in Karlstad, Sweden in 2021. Informed consent was obtained from participants prior to the start of the study. The trial was registered in the ISRCTN registry (ISRCTN35134834). In the current study, data were pooled from both the intervention and the waitlist control group when the control group had also received the robot intervention. Differences between responders and non-responders regarding baseline demographics and clinical characteristics were analyzed, as these variables constitute potential predictors of treatment response.
Participants and Procedures
Participants were screened for eligibility in two stages: firstly with the Insomnia Severity Index (ISI),33 and the Pre-Sleep Arousal Scale (PSAS).34 In the next stage, we administered the Duke Structured Interview for Sleeping Disorders (DSISD),35 and the Mini International Neuropsychiatric Interview (M.I.N.I.).36 The screening was conducted by phone by first author SJS. Inclusion criteria were that participants had to speak Swedish fluently, be over 18 years of age, and meet the DSM-5 diagnostic criteria of insomnia. Exclusion criteria were to meet the diagnostic criteria of another current psychiatric disorder, or untreated sleep disorder (hypersomnia, narcolepsy, sleep apnea, circadian rhythm sleep disorder, parasomnia, restless legs). Of the 54 people that were screened, 44 fulfilled the requirements.31
Intervention and Control Conditions
The intervention consisted of at-home usage of the Somnox sleep robot for three consecutive weeks. The robot had different settings but was set on the 30-minute program called “sleeping” for all participants, with the possibility to speed up or slow down the breathing manually to some degree. Meanwhile, the control group remained on a waitlist – that is, no intervention during the experimental stage of the study.31 The control group received the intervention after the waiting period, and completed an additional post-intervention measurement, enabling the pooling of data from both groups to assess, with a larger sample size, predictors of treatment response. See Figure 1 for the study’s flow chart.
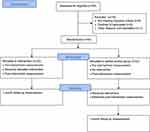 |
Figure 1 Study’s flow chart. |
Measures
Information about participants was collected in terms of gender identity, marital status, children, level of education, employment, and country of birth (in or outside Sweden). Effects of the intervention were assessed in multiple ways. Questionnaires were administered pre-intervention, mid-intervention (main outcome measure only), post-intervention, and at 1-month follow-up. Sleep variables from the Consensus Sleep Diary and wrist actigraphy were assessed the week before the intervention and the last intervention week (weekly means).37,38 Questionnaires and sleep diaries were completed on the Iterapi online platform.39 Additionally, participants completed three daily questions on treatment adherence throughout the intervention.
The primary outcome measure was the Insomnia Severity Index (ISI: 7 items, score range 0–28, cut-off 11). The criteria for a positive treatment response was −5 points on the ISI, as −4.7 is widely considered a slight clinical improvement.40 Arousal was considered the probable mechanism in case of a beneficial treatment response. Arousal was measured with the Pre-Sleep Arousal Scale (PSAS), which consists of two scales (cognitive and somatic), of which only the somatic scale was used (8 items, score range 8–40). Higher scores indicate hyperarousal. Ten was used as a cut-off of hyperarousal, in line with previous studies.41 Symptoms of anxiety and depression (emotional distress) were measured with the Hospital Anxiety and Depression Scale (HADS),42 which consists of 14 items (7 items in each, score range 0–21). Higher scores indicate clinical levels of anxiety and depression (cut-off 8). For the anxiety scale, a change of 1.3 represents a slight improvement, whereas a change of 1.4 represents a slight improvement for the depression scale.43
Sleep onset latency (SOL), wake after sleep onset (WASO), total sleep time (TST), and sleep efficiency (SE) were measured both subjectively with the Consensus Diary, and objectively with wrist actigraphy. The sleep diary included several other variables that were not analyzed in the original study, for instance sleep quality (SQ). The sleep quality question was framed as How did you sleep? With five possible answers: (1) very poorly, (2) pretty poorly, (3) neither good nor bad, (4) pretty good, and (5) very good. The current study focused on diary-defined SOL, WASO, and TST (not SE as its calculation is based on TST), in addition to SQ.
Statistical Approach
We used the pooled data from both the intervention group and the control group when the latter group had also received the intervention (with an additional post-intervention assessment consisting of the ISI, the PSAS and the HADS), that is, after the randomized phase of the study. This enabled analyses of the 1-month follow-up data, in line with the published study protocol.44 The power analysis, described in the study protocol, was calculated on the main outcome measure only, why the results in the current study should be interpreted with caution.
The assumptions of normality and sphericity were met for the Insomnia Severity Index (ISI), but not for the Pre-Sleep Arousal Scale (PSAS) and the Hospital Anxiety and Depression Scale (HADS). A one-way repeated measures analysis of variance (ANOVA) was conducted on the ISI, and Friedman tests on the PSAS and the HADS, to assess the short- and long-term time effects of the sleep robot intervention. Regarding missing data, cases were excluded listwise (n = 7, 15.9%).
After defining responders as those with a −5-point reduction on the ISI from pre- to post-intervention, t-tests and chi-square tests were conducted to compare the baseline demographics and clinical characteristics of responders versus non-responders. This was done in order to form hypotheses about possible subgroups that might benefit from the sleep robot intervention. Predictors of treatment response were investigated with logistic regression models, with a categorical responder/non-responder outcome variable. The following predictor variables were initially added as predictors in Model A: symptoms of insomnia, arousal, anxiety and depression, measured with the Insomnia Severity Index, the Pre-Sleep Arousal Scale, and the Hospital Anxiety and Depression Scale, respectively; diary defined sleep onset latency, wake after sleep onset, total sleep time, and sleep quality. These were selected based on previous findings in the literature.45 In Model B, we only added anxiety and sleep quality as predictors. This second part of the study was not described in the study protocol, meaning that these are exploratory analyses.
Results
Insomnia Severity
For the Insomnia Severity Index, Shapiro–Wilk tests were not statistically significant for pre-intervention (p = 0.26), post-intervention (p = 0.19), or 1-month follow-up (p = 0.47), suggesting that the assumption of normality was not violated. Thus, a one-way repeated measures ANOVA was conducted, which resulted in a statistically significant beneficial time effect, F (2, 35) = 7.22, p = 0.00, η2 = 0.29. The Least Significance Difference post hoc test (default in SPSS) revealed statistically significant differences between pre- and post-intervention (p = 0.01) and between pre-intervention and 1-month follow-up (p < 0.001), but not between post-intervention and 1-month follow-up (p = 0.20). See Table 1 for means and standard deviations for each time point.
 |
Table 1 Means and Standard Deviations for Each Outcome Measure at Each Time Point, and Pairwise Comparisons of Time Points |
Somatic Arousal
For the Pre-Sleep Arousal Scale, Shapiro–Wilk tests were statistically significant for pre-intervention (p = 0.01) and post-intervention (p = 0.00), but not for the 1-month follow-up (p = 0.38), indicating that the assumption of normality was violated on the first two time points. Therefore, the Friedman test was conducted, which rendered a statistically significant beneficial time effect, χ2 (2, N = 37) = 11.82, p = 0.00. The Wilcoxon Signed Ranks post-hoc test (default in SPSS) showed a statistically significant difference between pre- and post-intervention (p = 0.01), but no statistically significant differences between pre-intervention and 1-month follow-up (p = 0.13) or between post-intervention and 1-month follow up (p = 0.48).
Emotional Distress
Regarding the anxiety part of the Hospital Anxiety and Depression Scale, Shapiro–Wilk tests were not statistically significant for pre-intervention (p = 0.07) or 1-month follow-up (p = 0.25) but was statistically significant for post-intervention (p = 0.02). The Friedman test was not statistically significant, χ2 (2, N = 37) = 4.27, p = 0.12, that is, there were no statistically significant short- or long-term time effects regarding symptoms of anxiety. Regarding symptoms of depression, Shapiro–Wilk tests suggested that the depression part of the Hospital Anxiety and Depression Scale was normally distributed at pre-intervention (p = 0.07), but not normally distributed post-intervention (p = 0.02) nor at the follow-up (p = 0.02), which is why the Friedman test was conducted here as well, with a non-statistically significant result, χ2 (2, N = 37) = 3.77, p = 0.15.
Baseline Characteristics
Responders were defined as those with a −5 change on the ISI from pre- to post-intervention. Of the 44 participants, 13 met this criterion and 31 did not. Baseline characteristics of responders versus non-responders are depicted in Table 2. The only statistically significant difference between responders and non-responders on the 0.05 level was baseline anxiety symptoms according to the Hospital Anxiety and Depression Scale, t(42) = −2.21, p = 0.03. Sleep quality was marginally significant on the less stringent 0.10 (p = 0.09).
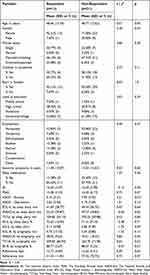 |
Table 2 Baseline Demographic and Clinical Characteristics of Responders (n = 13) versus Non-Responders (n = 31). Statistical Significance Tested with t-Tests and Chi-Square Tests |
Likelihood of Treatment Response
To assess the likelihood that participants would respond to the intervention, binary logistic regression analyses were performed, firstly with the following baseline variables as predictors: the ISI, the PSAS, the HADS anxiety scale, the HADS depression scale, in addition to diary defined SOL, WASO, TST, and SQ. The result was statistically significant, χ2 (8, N = 39) = 24.28, p = 0.00, R2N = 0.65, indicating that the model was able to distinguish between responders and non-responders. Moreover, the overall percentage of correctly classified cases was 89.7%. Only HADS-anxiety (p = 0.03) and sleep quality (p = 0.02) made unique statistically significant contributions to the model, which is why a new logistic regression analysis was conducted with these two variables as the only predictors, see Table 3. The second model was statistically significant, χ2 (2, N = 42) = 10.21, p = 0.01, R2N = 0.30, as were the unique contributions of anxiety (p = 0.02) and sleep quality (p = 0.04). The latter model correctly classified 83.3% of cases.
 |
Table 3 Binary Logistic Regression Models Predicting Likelihood of Treatment Response, Defined as a −5 reduction on the Insomnia Severity Index from Pre- to Post-Intervention |
Adherence
Responders did not use the sleep robot for more days than non-responders did; 14.77 days (SD 4.69) for responders compared with 15.62 days (SD 5.41) for non-responders, t (40) = −0.49, p = 0.63. Responders used the robot for an average of 41.53 minutes (SD 11.82), whereas non-responders used the robot for an average of 57.32 minutes (SD 70.73) each night, t (38) = 0.47, p = 0.47.
Discussion
The study demonstrated a statistically significant time effect on the Insomnia Severity Index, for all three measurement points (pre-intervention, post-intervention, and 1-month follow-up, albeit no difference between the latter two), indicating that the sleep robot might lead to persistent changes regarding level of insomnia symptoms weeks after a person stops using it. It should be noted that the average effect was small and that post-treatment insomnia severity did not end up under the cut-off threshold for insomnia. For the Pre-Sleep Arousal Scale, the time effect was only statistically significant in the short term from pre- to post-intervention, suggesting that the sleep robot only affects physiological arousal while it is actively used. Finally, the time effects on anxiety and depression symptoms as measured with the Hospital Anxiety and Depression Scale were not statistically significant.
Both anxiety and sleep quality predicted the likelihood that participants would respond to the sleep robot intervention for insomnia. Responders (n = 13) had lower baseline anxiety compared with non-responders (n = 31) (ie, non-responders had higher baseline anxiety), and the difference was statistically significant. This is in line with previous research showing that people with higher levels of anxiety respond less well to insomnia treatments,18 even when the symptoms do not meet the diagnostic criteria of an anxiety disorder. As Tramonti et al (p. 309) point out,46
anxiety and depressive symptoms are still interpreted in terms of comorbidity, and the criteria for identifying insomnia and psychopathological conditions still rely on threshold measures, whose limitations and risks of neglecting the clinical relevance of subthreshold presentations have been underscored.
The higher level of anxiety symptoms in non-responders may also be more associated with cognitive arousal, unfortunately not captured in the current study as we only included the somatic scale from the Pre-Sleep Arousal Scale. Perhaps those with elevated levels of anxiety would have benefited more from a cognitive-behavioral intervention rather than a sleep robot whose main target can be said to be somatic arousal.
Responders also had higher baseline sleep quality than non-responders (who had lower baseline sleep quality), albeit not a statistically significant difference on the 0.05 level. Sleep quality is not easily defined. In the current study, a single-item rating scale was used from the sleep diary. In contrast, the widely used and well-validated Pittsburgh Sleep Quality Index (PSQI) contains 19 items (including questions about sleep onset latency and sleep duration).47 The scale of the single item was 1–5, where 3 in the middle represents neither good nor bad. Qualitative research has found that people both with and without insomnia use several criteria when they judge their own sleep quality, and that people with insomnia have more criteria compared with people without insomnia,48 which is why the single item in the current study might be flawed. Additionally, the question How did you sleep? is vague and open for interpretation (eg, sleep satisfaction or depth of sleep), a common problem in sleep quality studies.48 Lastly, the results were not due to a dose-response effect of the sleep robot, as adherence data showed that responders did not use the sleep robot more than non-responders did.
Limitations
The study has limitations and the first is that we analyzed data after the experimental stage of the study, that is, when the randomization was broken. These secondary analyses were only partly described in the study protocol and are therefore partly exploratory (ie, higher risk of bias). Furthermore, we only calculated a power analysis for the main outcome measure in the original study, and no power analyses for the current study. Adding to the limitations, only 13 participants were classified as responders, meaning that only large differences between responders and non-responders turn out statistically significant (ie, risk of ignoring smaller but meaningful differences between responders and non-responders). Yet another limitation was that tests of normality showed that three out of four outcome measures for the time effects analyses were not normally distributed, which is why the less powerful non-parametric Friedman test was conducted instead of repeated measures ANOVAs on these variables (the PSAS, the HADS – anxiety scale, the HADS – depression scale). The first logistic regression model included more predictors than what was sensible with a sample of 44 participants, and the results of the second logistic regression model with anxiety and sleep quality as predictors must be interpreted with caution as well. A final limitation is that we only included somatic arousal from the Pre-Sleep Arousal Scale, and that a cognitive scale might have yielded important information considering the higher anxiety levels and lower sleep-quality ratings for non-responders.
Concluding Remarks
This study implies that people with less anxiety and higher sleep quality are more likely to benefit from the sleep robot intervention, compared to more anxious people, and/or people reporting poorer sleep quality prior to treatment. This is important information that was concealed when we only focused on group effects in the original study.31 A strength with our study is the thorough screening of participants including a wide number of potentially relevant demographics and clinical characteristics. Commercial products are rarely investigated independently from the producers, why another strength is that the study goes in depth and offers impartial guidance regarding for whom the Somnox sleep robot might be associated with a clinically meaningful improvement of insomnia symptoms. These results must be confirmed in more studies before clinicians recommend the Somnox sleep robot as a stand-alone treatment of insomnia. In the larger picture, the study adds to the conversation about different types of insomnia and individually tailored treatments.
Data Sharing Statement
Data are available from the corresponding author upon reasonable request.
Acknowledgments
The research group would like to thank the participants for their time.
Disclosure
The authors have no conflicts of interest to declare.
References
1. Grandner MA. Epidemiology of insufficient sleep and poor sleep quality. In: Grandner MA, editor. Sleep and Health. London: Academic Press; 2019:11–20.
2. Wittchen HU, Rehm FJJ, Gustavsson A, et al. The size and burden of mental disorders and other disorders of the brain in Europe 2010. Eur Neuropsychopharmacol. 2011;21(9):655–679. doi:10.1016/j.euroneuro.2011.07.018
3. American Psychiatric Association. The Diagnostic and Statistical Manual of Mental Disorders (DSM-5). Arlington, VA: American Psychiatric Association; 2013.
4. Riemann D, Baglioni C, Bassetti C, et al. European guideline for the diagnosis and treatment of insomnia. J Sleep Res. 2017;26(6):675–700. doi:10.1111/jsr.12594
5. Watson NF, Lawlor C, Raymann RJEM. Will consumer sleep technologies change the way we practice sleep medicine? JCSM. 2019;15(1):159–161. doi:10.5664/jcsm.7596
6. Aebischer G, Rieder PA. Awaking insomnia: sleepless in the 19th century through medical literature. Med Humanit. 2020;46:340–347. doi:10.1136/medhum-2019-011683
7. Benjamins JS, Migliorati F, Dekker K, et al. Insomnia heterogeneity: characteristics to consider for data-driven multivariate subtyping. Sleep Med Rev. 2017;36:71–81.
8. Blanken TF, Benjamins JS, Borsboom D, et al. Insomnia disorder subtypes derived from life history and traits of affect and personality. Lancet Psychiatry. 2019;6:151–163. doi:10.1016/S2215-0366(18)30464-4
9. Vgontzas AN, Fernandez-Mendoza J, Liao D, et al. Insomnia with objective short sleep duration: the most biologically severe phenotype of the disorder. Sleep Med Rev. 2013;17(4):241–254. doi:10.1016/j.smrv.2012.09.005
10. Johnson EO, Roth T, Breslau N. The association of insomnia with anxiety disorders and depression: exploration of the direction of risk. J Psychiatr Res. 2006;40:700–708. doi:10.1016/j.jpsychires.2006.07.008
11. Wilson SJ, Nutt DJ, Alford C, et al. British Association for Psychopharmacology consensus statement on evidence-based treatment of insomnia, parasomnias and circadian rhythm disorders. J Psychopharmacol. 2010;24(11):1577–1601. doi:10.1177/0269881110379307
12. Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 2002;6:97–111. doi:10.1053/smrv.2002.0186
13. Zhang B, Wing YK. Sex differences in insomnia: a meta-analysis. Sleep. 2006;29:85–93. doi:10.1093/sleep/29.1.85
14. Taylor DJ, Lichstein KL, Durrence HH, et al. Epidemiology of insomnia, depression and anxiety. Sleep. 2005;28:1457–1464. doi:10.1093/sleep/28.11.1457
15. Espie CA, Inglis SJ, Harvey L. Predicting clinically significant response to cognitive behavior therapy for chronic insomnia in general medical practice: analysis of outcome data at 12 months posttreatment. J Consult Clin Psychol. 2001;69:58–66. doi:10.1037/0022-006X.69.1.58
16. Gagné A, Morin CM. Predicting treatment response in older adults with insomnia. J Clin Geropsychol. 2001;7:131–143. doi:10.1023/A:1009537722740
17. Morin CM, Culbert JP, Schwartz SM. Nonpharmacological interventions for insomnia: a meta-analysis of treatment efficacy. Am J Psychiatry. 1994;151:1172–1180.
18. Morgan K, Thompson J, Dixon S, et al. Predicting longer-term outcomes following psychological treatment for hypnotic-dependent chronic insomnia. J Psychosom Res. 2003;54(1):21–29. doi:10.1016/S0022-3999(02)00569-X
19. Wells G, Li T, Maxwell L, et al. Responsiveness of patient reported outcomes including fatigue, sleep quality, activity limitation, and quality of life following treatment with Abatacept for rheumatoid arthritis. Ann Rheum Dis. 2008;67(2):260–265. doi:10.1136/ard.2007.069690
20. Ramlee F, Sanborn AN, Tang NKY. What sways people´s judgment of sleep quality? A quantitative choice-making study with good and poor sleepers. Sleep. 2017;40(7):1–13.
21. Khosla S, Deak MC, Gault D, et al. Consumer sleep technology: an American Academy of Sleep Medicine position statement. JCSM. 2018;14(5):877–880. doi:10.5664/jcsm.7128
22. Støre SJ, Beckman L, Jakobsson N. The effect of robot interventions on sleep in adults: a systematic review and network meta-analysis. JCSM. 2022;18(7):1877–1884. doi:10.5664/jcsm.10022
23. Somnox. Breathe, relax, sleep. Available from: https://somnox.com.
24. Stetter F, Kupper S. Autogenic training: a meta-analysis of clinical outcome studies. Appl Psychophysiol Biofeedback. 2002;27(1):45–98. doi:10.1023/A:1014576505223
25. Manzoni GM, Pagnini F, Castelnuovo G, et al. Relaxation training for anxiety: a ten-years systematic review with meta-analysis. BMC Psychiatry. 2008;8(41):1–12. doi:10.1186/1471-244X-8-41
26. Buysse DJ. Chronic insomnia. Am J Psychiatry. 2008;165(6):678–686. doi:10.1176/appi.ajp.2008.08010129
27. Cheung JMY, Jarrin DC, Ballot O, et al. A systematic review of cognitive behavioral therapy for insomnia implemented in primary care and community settings. Sleep Med Rev. 2018;44:23–36. doi:10.1016/j.smrv.2018.11.001
28. Riemann D, Spiegelhalder K, Feige B, et al. The hyperarousal model of insomnia: a review of the concept and its evidence. Sleep Med Rev. 2010;14(1):19–31. doi:10.1016/j.smrv.2009.04.002
29. Spielman A, Caruso L, Glovinsky P. A behavioral perspective on insomnia treatment. Psychiatr Clin North Am. 1987;10:541–553. doi:10.1016/S0193-953X(18)30532-X
30. Harvey AG, Tang NKY, Browning L. Cognitive approaches to insomnia. Clin Psychol Rev. 2005;25:593–611. doi:10.1016/j.cpr.2005.04.005
31. Støre SJ, Tillfors M, Wästlund E, et al. The effects of a sleep robot intervention on sleep, depression and anxiety in adults with insomnia – a randomized waitlist-controlled trial. J Sleep Res. 2022;2022:1–11.
32. Støre SJ, Tillfors M, Wästlund E, Angelhoff C, Clarke AN. Technically sleeping? A clinical single-case study of a commercial sleep robot. Front Psychol. 2022;13(919023):1–11. doi:10.3389/fpsyg.2022.919023
33. Bastien CH, Vallières A, Morin CM. Validation of the insomnia severity index as an outcome measure for insomnia research. Sleep Med. 2001;2(4):297–307. doi:10.1016/S1389-9457(00)00065-4
34. Nicassio PM, Mendlowitz DR, Fussell JJ, et al. The phenomenology of the pre-sleep state: the development of the pre-sleep arousal scale. Behav Res and Ther. 1985;23(3):263–271. doi:10.1016/0005-7967(85)90004-X
35. Carney CE, Ulmer C, Edinger JD, et al. Assessing depression symptoms in those with insomnia: an examination of the beck depression inventory second edition (BDI-II). J Psychiatr Res. 2009;43(5):576–582. doi:10.1016/j.jpsychires.2008.09.002
36. Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. JCP. 1998;59(Suppl 20):22–33.
37. Carney CE, Buysse DJ, Ancoli-Israel S, et al. The consensus sleep diary: standardizing prospective sleep self-monitoring. Sleep. 2012;35(2):287–302. doi:10.5665/sleep.1642
38. ActiGraph GT9X Link. ActiGraph GT9X Link. Available from: http://actigraphcorp.com/actigraph-link/.
39. Vlaescu G, Alasjö A, Miloff A, et al. Features and functionality of the Iterapi platform for internet-based psychological treatment. Internet Interv. 2016;6:107–114. doi:10.1016/j.invent.2016.09.006
40. Morin CM, Belleville G, Bélanger L, et al. The insomnia severity index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34(5):601–608. doi:10.1093/sleep/34.5.601
41. Jansson-Fröjmark M, Norell-Clarke A. Psychometric properties of the pre-sleep arousal scale in a large community sample. J Psychosom Res. 2012;72(2):103–110. doi:10.1016/j.jpsychores.2011.10.005
42. Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67(6):361–370. doi:10.1111/j.1600-0447.1983.tb09716.x
43. Puhan MA, Frey M, Büchi S, et al. The minimal important difference of the hospital anxiety and depression scale in patients with chronic obstructive pulmonary disease. Health Qual Life Outcomes. 2008;6(46):1–6. doi:10.1186/1477-7525-6-46
44. Støre SJ, Tillfors M, Wästlund E, et al. The effects of a sleep robot intervention on sleep, depression and anxiety in adults with insomnia – study protocol of a randomized waitlist-controlled trial. Contemp Clin Trials. 2021;110:106588. doi:10.1016/j.cct.2021.106588
45. Bothelius K, Kyhle K, Broman J-E, et al. Initial sleep time predicts success in manual-guided cognitive behavioral therapy for insomnia. Behav Sleep Med. 2016;14(4):378–388.
46. Tramonti F, Maestri M, Gronchi A, et al. Psychological well-being of patients with insomnia and its relationship with anxiety and depression. Psychol Health Med. 2015;21(3):309–316. doi:10.1080/13548506.2015.1069856
47. Buysse DJ, Reynolds CF, Monk TH, et al. The Pittsburgh sleep quality index – a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213.
48. Harvey AG, Stinson K, Whitaker KL, et al. The subjective meaning of sleep quality: a comparison of individuals with and without insomnia. Sleep. 2008;31(3):383–393.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
