Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 18
Exercise Rehabilitation and Chronic Respiratory Diseases: Effects, Mechanisms, and Therapeutic Benefits
Authors Xiong T , Bai X, Wei X, Wang L, Li F, Shi H , Shi Y
Received 12 February 2023
Accepted for publication 14 June 2023
Published 19 June 2023 Volume 2023:18 Pages 1251—1266
DOI https://doi.org/10.2147/COPD.S408325
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Ting Xiong,1 Xinyue Bai,1 Xingyi Wei,1 Lezheng Wang,1 Fei Li,2 Hui Shi,3 Yue Shi2
1School of Exercise and Health, Shanghai University of Sport, Shanghai, 200438, People’s Republic of China; 2School of Athletic Performance, Shanghai University of Sport, Shanghai, 200438, People’s Republic of China; 3Department of Rheumatology and Immunology, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, 200025, People’s Republic of China
Correspondence: Yue Shi, School of Athletic Performance, Shanghai University of Sport, No. 399, Changhai Road, Shanghai, 200438, People’s Republic of China, Tel +86-21- 18521361033, Fax +86-21-65508050, Email [email protected]
Abstract: Chronic respiratory diseases (CRD), is a group of disorders, primarily chronic obstructive pulmonary disease and asthma, which are characterized by high prevalence and disability, recurrent acute exacerbations, and multiple comorbidities, resulting in exercise limitations and reduced health-related quality of life. Exercise training, an important tool in pulmonary rehabilitation, reduces adverse symptoms in patients by relieving respiratory limitations, increasing gas exchange, increasing central and peripheral hemodynamic forces, and enhancing skeletal muscle function. Aerobic, resistance, and high-intensity intermittent exercises, and other emerging forms such as aquatic exercise and Tai Chi effectively improve exercise capacity, physical fitness, and pulmonary function in patients with CRD. The underlying mechanisms include enhancement of the body’s immune response, better control of the inflammatory response, and acceleration of the interaction between the vagus and sympathetic nerves to improve gas exchange. Here, we reviewed the new evidence of benefits and mechanisms of exercise intervention in the pulmonary rehabilitation of patients with chronic obstructive pulmonary disease, bronchial asthma, bronchiectasis, interstitial lung disease, and lung cancer.
Keywors: chronic respiratory disease, exercise training, chronic obstructive pulmonary disease, asthma, interstitial lung disease, bronchiectasis, lung cancer
Introduction
Chronic respiratory diseases (CRD) are a group of common disorders with lesions primarily occurring in the trachea, bronchi, alveoli, and chest cavity.1 Over the last three decades, the incidence of CRD has been increasing yearly due to various factors such as environmental exposure, poor lifestyle habits, air pollution, occupational carcinogens, smoking, and alcohol consumption. In 2020, the World Health Organization (WHO) released a list of the top 10 deadly diseases worldwide, which include: chronic obstructive pulmonary disease (COPD), lower respiratory tract infections, and tracheal, bronchial, and lung cancers.2 COPD, the third most deadly disease worldwide, accounts for 6% of all deaths. There were approximately 2.2 million cases of tracheal, bronchial and lung cancer worldwide in 2019, affecting 1.52 million men and 737,000 women, which is an increase of 23.3% from 2010.3 Chronic and severe airway pathologies have caused a huge medical burden on countries worldwide, greatly affecting the quality of life of patients and becoming a major disease that plagues humanity.4 Thus, there is an urgent need to find efficient and economical means for the prevention and rehabilitation in CRD and to compensate for the shortcomings in its prevention and control.
In 2007, the American College of Sports Medicine (ACSM) launched the Exercise is Medicine program, which aims to guide and encourage doctors to evaluate the exercise ability of patients when designing treatment plans and to promote the treatment and prevention of chronic diseases through scientific exercise. Exercise can promote health and combat diseases, by changing the abundance of biomolecules in the body and triggering functional changes in the body’s tissues and organs.5,6 Exercise reportedly regulates the body’s immune response, among other things.7 A large body of research data on sports medicine provides a scientific basis for formulating exercise programs in patients with respiratory diseases.8 The typical characteristics of CRD are exertional dyspnea and exercise intolerance. Its physiological mechanisms include respiratory limitation, inadequate gas exchange, central and peripheral hemodynamic restriction, and decreased skeletal muscle function. In 2006, the American Thoracic Society (ATS) and European Respiratory Society (ERS) stated that active pulmonary rehabilitation can reduce the adverse symptoms of patients with CRD to a certain extent, effectively prevent exacerbations, and improve pulmonary function, exercise endurance and quality of life.9 Exercise training is not only the cornerstone of lung rehabilitation, but also an economic and easy means of preventing and rehabilitating the diseases.10,11
Recently, the effectiveness of exercise interventions in improving COPD, interstitial lung disease, asthma, and pulmonary fibrosis has been confirmed. In 2013, the ATS and ERS published an official exercise rehabilitation program guideline for people with CRD: endurance training 3–5 times/week of 20–60 min duration each, with gradually increasing intensity and a target of > 70% of the expected maximum heart rate.12 The British Thoracic Society (BTS) also provides guidelines for resistance training programs: resistance/strength training of 2–4 sets/session, with 10–15 reps/set and 30–60 min/session, and a recommended interval of at least 48 h between training sessions.13 Additionally, individualized exercise programs should be developed according to the patient’s specific situation. For patients with severe diseases, high-intensity interval training (HIIT) can be used as an alternative because of their ability to perform high-intensity exercises for a short period with sufficient rest in between.14
In this study we have reviewed the rehabilitative effects of exercise on COPD, bronchial asthma, bronchiectasis, interstitial lung disease, and lung cancer, and elucidated the mechanisms underlying the pathophysiological changes. We hope that this study will provide guidance for the application and practice of exercise rehabilitation in chronic lung diseases, as well as for the in-depth exploration of the pathological mechanism of exercise in improving lung diseases in the future. Furthermore, we aim to raise public awareness of pulmonary rehabilitation and facilitate the promotion and application of pulmonary rehabilitation methods.
Exercise and COPD
COPD is a common condition characterized by persistent airflow limitation and a series of clinical manifestations such as progressive decline in lung function, including chronic cough, sputum, and shortness of breath, and skeletal muscle dysfunction. It can progress to severe pulmonary heart disease or respiratory failure, with high mortality.15 A large number of randomized controlled trials have recently provided evidence regarding the efficacy of exercise training interventions in patients with COPD. Resistance training can improve patients’ exercise tolerance, muscle strength, and arm function, while aerobic exercises can improve patients’ maximum oxygen consumption, neurological control of heart rate, and quality of life. The following table summarizes the researches on exercise interventions to improve COPD patients in the last decade (Table 1).
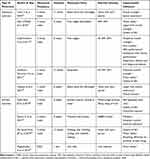 |
Table 1 Studies Related to the Rehabilitative Effects of Exercise Interventions in Patients with COPD |
The body of patients with COPD is reportedly in a chronic inflammatory state with impaired intrinsic immunity.25 This disease often worsens due to airway infections, with 22–40% of patients with COPD experiencing at least one moderate or severe exacerbation each year. Furthermore, the mortality rate is > 15% within 3 months of hospitalization for acute exacerbations.26 Regular exercise can reportedly enhance the immune response of patients and control the body’s inflammatory response. In animal studies, aerobic exercise was found to prevent the increase in macrophage and neutrophil count in mice with COPD;27 a similar trend was found in population trials, with a significant reduction in eosinophil count in vivo after 6 weeks of endurance and strength training.28 The benefits of exercise training on the innate immunity were demonstrated by Fernandes 2018 et al29 who identified a significant increase in CD4+ T-cells, improved immune response, and a reduction in exacerbations and hospitalizations after 12 weeks of exercise training in patients with COPD.29 Thus, we hypothesized that the exercise-induced improvement of the intrinsic immune response would subsequently lead to the activation of the adaptive immune response. Wang et al determined that aerobic exercises upregulated interleukin (IL)-10 and chemokine (CXCL)-1 levels in bronchoalveolar lavage fluid (BALF), downregulated transforming growth factor (TGF)-β, IL-1β and tumor necrosis factor (TNF)-α levels in BALF, upregulated IL-10 levels in serum, and activated Sirt1 expression. These in turn suppressed the inflammatory responses and attenuated the oxidative stress in mice.27 In a population trial, aerobic exercise reduced the serum expression of TNF-α, IL-4, IL-6 and C-reactive protein (CRP).30 These results suggest that exercise training is an effective strategy for reducing pulmonary and systemic inflammation, alleviating symptoms, and preventing disease progression in patients with COPD.
Exercise and Bronchial Asthma
The Global Initiative for Asthma31 guidelines defines bronchial asthma as a heterogeneous disease characterized by chronic airway inflammation and hyperresponsiveness with varying degrees of airflow limitation, including cough, wheezing, chest tightness, dyspnea, and other clinical manifestations. It is one of the most common and serious CRDs affecting human health worldwide.32
Reduction or even elimination of physical activity is advised in patients with asthma to avoid symptom deterioration or exercise-induced bronchoconstriction. However, the reduction in physical activity leads to decreased fitness and exercise tolerance,33,34 making asthmatics more prone to fatigue and breathing difficulties during exercise; ultimately, this leads to exercise avoidance.35 In addition, steroid used to treat asthma can also lead to a decrease in muscle endurance.36 The primary goals of asthma treatment proposed by the GINA are to control symptoms, reduce future risks, and improve the quality of life.32 Current common clinical treatments include the use of bronchodilators and anti-inflammatory drugs; however, their efficacy is not satisfactorily adequate. Therefore, it is necessary to find an active and effective non-pharmacological treatment option.37 As an important part of pulmonary rehabilitation, exercise training is a new non-pharmacological therapy used in some clinical studies. The following table summarizes the researches on exercise interventions to improve patients of bronchial asthma in the last decade (Table 2).
 |
Table 2 Studies Related to the Rehabilitative Effects of Exercise Interventions in Patients with Bronchial Asthma |
Asthmatics are capable of physical activity, and moderate physical activity can reportedly improve their health status.47 The limitation of exercise capacity is sometimes more due to skeletal muscle dysfunction than due to airflow limitation. A large number of population-based trials have shown that aerobic exercise is beneficial in patients with asthma; the lung function is enhanced by improving the forced vital capacity (FVC), forced expiratory volume of 1st second (FEV1), peak expiratory flow (PEF), and other indicators.48–50 Furthermore, it helps better control the asthmatic symptoms 51–53 and improves bronchial hyperresponsiveness,54–56 aerobic capacity, quality of life, anxiety, and depression.52,57–60 Physical activity and conventional therapy can effectively improve the quality of life and asthma control in patients with nocturnal deterioration.61 Several epidemiological studies have shown an association between asthma and obesity; weight loss improves asthma control in overweight and obese patients.62 However, exercise regimen formulations differ in exercise-induced asthma; it is necessary to consider the safety, feasibility, scientific nature, and focus of the regimen.
At present, it is widely accepted that bronchial asthma is closely related to inflammation, immunity, genetics, and the environment. The airway inflammatory response is the central link in triggering bronchial asthma, which is dominated by eosinophil and mast cell infiltration and an enhanced T helper cell 2 (Th2)-type response.63,64 Exercises may reportedly have a protective effect by reducing airway inflammation and increasing the bronchial patency.65,66 In animal models, appropriate aerobic exercise training downregulated IgE and IgG levels in the early stages and reduced the inflammatory factor release, which alleviated the symptoms of acute allergic asthma.67 Recently, aerobic exercise has been found to effectively reduce airway eosinophilic expression, which in turn reduces the inflammation, inhaled glucocorticoid (ICS) dosage, and acute exacerbations, under the premise of standardizing and optimizing ICS medication.68 Aerobic training can also positively modulate airway inflammation and remodeling mediators. Patient’s FeNO and sputum eosinophil counts were reduced with aerobic exercise interventions, which was more pronounced in patients with higher levels of inflammation.52,55,69 Together, these findings suggest that aerobic training can be an effective adjunct to medication use in patients with asthma.
Exercise and Bronchiectasis
Bronchiectasis is a recurrent suppurative infection caused by various factors. Small- and medium-sized bronchi are repeatedly damaged and blocked, which destroys the wall structure and results in bronchial abnormalities and persistent dilation. The clinical manifestations include chronic cough, massive expectoration, and intermittent hemoptysis. If not treated promptly, it can lead to pulmonary heart disease and respiratory failure.70 Secondary problems such as decreased peripheral muscular endurance and activity also cause significant damage to a patient’s personal and social life.71 Current clinical treatments focus on the acute exacerbation phase and are based on the principles of suppressing acute and chronic bronchial infections, improving mucociliary clearance, reducing the impact of structural lung disease, preventing deterioration, reducing symptoms, and improving the quality of life.72
Bronchiectasis is not an uncontrollable or unpreventable respiratory disease, and the risk of acute exacerbation can be reduced by preventive interventions and increased awareness of self-management during the stable phase.73 Several population-based trials have demonstrated the benefits of exercise interventions in patients with bronchiectasis. The following table summarizes recent studies on exercise interventions that have improved the condition of patients with bronchiectasis (Table 3). The findings indicate that resistance training and aerobic exercises of the upper and lower extremities can increase exercise capacity and endurance, enhance peripheral and respiratory muscle strength, improve lung function, reduce dyspnea, and raise the quality of life.74–76 However, maintaining these benefits is challenging; as exercise cycles increase, patient compliance decreases, and the positive cumulative effect decreases accordingly.77,78 A great deal of experimentation and research is still warranted to reach a consensus on how long exercise training can maintain the improvement and what type of exercise training is easy for patients to adhere to.
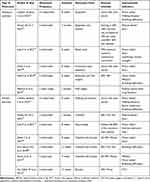 |
Table 3 Studies Related to the Rehabilitative Effects of Exercise Interventions in Patients with Bronchiectasis |
The inefficient clearance of mucus and microorganisms and inflammation progression are the main causes of irreversible lesions in bronchiectasis. Regular exercise training can alter the autonomic balance of mucociliary clearance and accelerate vagal and sympathetic interactions for the recovery of gas exchange capacity.83 Inflammatory progression results in a large cellular infiltration in the airway epithelium.84 Neutrophil-mediated immune responses, which secrete excessive amounts of matrix metalloproteinases 8 and 9 (MMP-8 and MMP-9), lead to continuous airway destruction.85 Furthermore, the patient’s serum, bronchoalveolar lavage fluid, and lung tissue showed increased levels of chemokines and pro-inflammatory cytokines, such as IL-8 and IL-17.86,87 Currently, there is a lack of investigation into the underlying mechanism by which exercise improves bronchiectasis; however, numerous studies have confirmed that exercise training can reduce the levels of inflammatory markers in the body88 and inhibit neutrophil hyperactivation.89 Therefore, it can be hypothesized that exercise prevents or inhibits disease progression by reducing airway inflammation and modulating the functional activity of immune cells. However, further studies are needed to clarify these mechanisms.
Exercise and Interstitial Lung Disease
Interstitial lung disease (ILD) is a diverse group of CRDs characterized by dyspnea, exercise-induced hypoxemia,90 and exercise intolerance.91,92 It can severely limit the ability of patients to maintain even moderate levels of functional physical activity, including those of daily living and employment.93
ILD differs from other respiratory diseases because it causes significant EIH, which often makes it difficult for patients to achieve adequate exercise intensity.90 The standard exercise program for COPD is effective for ILD. After aerobic exercise training, the 6-minute walking distance (6MWD) of patients with ILD increased significantly, and the clinical cardiopulmonary function improved conspicuously.94–96 An increase in 6MWD reportedly coincides with a decrease in patient-reported fatigue,97 which subsequently improves a patient’s health-related quality of life (HRQoL).98 During exercise training, transcutaneous oxygen saturation (SpO2) and degree of dyspnea should be monitored in real time. If EIH or dyspnea is difficult to control, the following training strategies or methods should be considered: interval training, supplemental oxygen, transnasal high-flow oxygen therapy, noninvasive ventilation, alternative exercises (Nordic walking or downhill training), and the use of energy-saving techniques and equipment. Owing to the difficulty in implementing an exercise program in the late stages of uncontrolled symptoms, all patients with ILD should be started on exercise training as early as possible.91 The intervention plans and results of exercise intervention on patients of ILD are summarized in the following table (Table 4).
 |
Table 4 Studies Related to the Rehabilitative Effects of Exercise Interventions in Patients with ILD |
The main aim when treating interstitial pneumonia is to control alveolar inflammation. This can be achieved with glucocorticoids, which have strong anti-inflammatory effects and can induce lymphocyte apoptosis. These immunosuppressive effects prevent the lethality of excessive inflammation and increases the risk of infection and cancer. In addition, long-term use negatively affects the immune system and leads to secondary infections.107 Studies on the effects of exercise training on the patient’s immune system in patients with ILD are limited. Exercise is reportedly effective in improving the function of the body’s immune system.108 Perhaps, exercise could possibly reduce lung inflammation and glucocorticoid-induced damage to the immune system.
Exercise and Lung Cancer
Lung cancer is one of the most common malignancies worldwide and the leading cause of cancer-related deaths.109 The WHO classifies lung cancer into two broad histological subtypes: non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC). NSCLC accounts for about 85–88% of the cases and SCLC accounts for about 12–15%.110 Long-term smoking, presence of excessive carcinogens in the work environment, ionizing radiation, lack of physical activity, genetics, and previous chronic lung infections are strongly associated with the development of lung cancer.111 Patients often present with symptoms such as cough, hemoptysis, fever, chest pain, shortness of breath, enlarged supraclavicular lymph nodes, and hoarseness of voice. The mainstay of clinical treatments include surgery, drug therapy, chemotherapy, targeted therapy, radiation therapy, interventional therapy and Chinese medicine.112–115
Currently, pneumonectomy is the most effective treatment for stages I, II, and IIIA of NSCLC and offers the best prospects for long-term survival.112 Compared with healthy individuals, the physical activity level of patients with NSCLC is lower and further declines within 6 months of diagnosis.116 In particular, reduced lung function increases the risk of surgery in patients with operable diseases.117 Some patients were excluded from surgical treatment because of poor preoperative evaluations.118 We conducted a study of patients who underwent pneumonectomy. Preoperative rehabilitation for patients undergoing pneumonectomy became a landmark study. After four weeks of aerobic exercise and respiratory training, the lung function improved in patients who could not undergo surgery due to poor pulmonary function tests; this greatly increased their chances of undergoing surgery.119 A study conducted by the University of California, determined that HIIT for two to six weeks may be the best perioperative exercise program; however, there is heterogeneity in the intensity and duration.120 In some population-based trials, exercise interventions benefited patients both preoperatively and postoperatively, with improved muscle mass, strength, and sleep quality after resistance training.121,122 Aerobic exercise improves exercise tolerance and cardiorespiratory fitness and reduces postoperative respiratory morbidity, length of hospital stay, cancer fatigue, anxiety, and depression;123–126 both are beneficial for lung function, exercise capacity, cancer pain reduction, quality of life, and life extension.127–129 The summary of exercise intervention to improve various indicators of lung cancer patients is shown in Table 5.
 |
Table 5 Studies Related to the Rehabilitative Effects of Exercise Interventions in Patients with Lung Cancer |
In recent years, immunotherapy has rapidly developed as an effective clinical strategy in cancer treatment. It is based on the tumor escape mechanism by manipulating the immune system to reactivate the anti-tumor immune response and overcome immune escape.138 However, the antitumor mechanisms of exercise may be related to immune regulation. In a high-intensity training model of rats, the toxicity and activity of natural killer (NK) cells in rats increased.139 Pedersen et al found that the tumor volume and pro-inflammatory cytokines (IL-1a and iNOS) in Lewis lung cancer (I)mice running voluntarily decreased significantly, and that the NK and T cell activity markers were upregulated.140 Similar effects were observed in other populations.141 Owing to the important role of NK cells in antitumor immunity,142 the ultimate benefits of exercise training may have clinical significance in cancer treatment. In several prospective randomized studies on postoperative patients with NSCLC, 16 weeks of Tai Chi training significantly promoted the proliferation and cytotoxicity of peripheral blood mononuclear cells and maintained stable T1 to T2 ratios and cortisol levels.143,144 More and more results of studies related to exercise immunity and anti-cancer progression suggest that exercise is an effective adjunct to existing anti-cancer therapies.
Conclusions
Exercise training-based pulmonary rehabilitation is effective in alleviating the symptoms of several CRDs, improving cardiovascular and muscle function, enhancing tolerance to physical activity, and improving the quality of life. Moderate-intensity aerobic exercise, resistance training, and HIIT are the most common forms of pulmonary rehabilitation exercises. Tai chi, yoga, aquatic exercise, and whole-body vibration training are also emerging forms of exercise that are gradually being used in the development of individualized pulmonary rehabilitation exercise programs. Although some patients may not respond adequately or respond inconsistently to specific training programs, published guidelines emphasize that pulmonary rehabilitation can benefit patients with stable respiratory disease symptoms. High-quality randomized controlled trials are required to further evaluate individualized training modalities in patients with comorbidities. More in-depth studies are needed to investigate the pathophysiological mechanisms by which different forms of exercise improve CRD and determine alternatives to pulmonary rehabilitation in patients with exercise limitations.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the National Natural Science Foundation of China [Grant No.32000831], the Shanghai Key Lab of Human Performance (Shanghai University of Sport) [Grant numbers 11DZ2261100], and the Shanghai Sailing Program (Fund number: 20YF1446500).
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Soriano JB, Kendrick PJ, Paulso KR, et al. Prevalence and attributable health burden of chronic respiratory diseases, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Respir Med. 2020;8:585–596. doi:10.1016/s2213-2600(20)30105-3
2. WHO. World Health Statistics 2020: Monitoring Health for the Sdgs, Sustainable Development Goals. Geneva: World Health Organization; 2020.
3. Ebrahimi H, Aryan Z, Sahar Saeedi Moghaddam CB. Global, regional, and national burden of respiratory tract cancers and associated risk factors from 1990 to 2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Respir Med. 2021;9:1030–1049. doi:10.1016/s2213-2600(21)00164-8
4. Perez-Padilla R, Marks G, Wong G, Bateman E, Jarvis D. Chronic lower respiratory tract diseases. Cardiovascular Respiratory Related Disorders. 2017;1:263–285.
5. Contrepois K, Wu S, Moneghetti KJ, et al. Molecular choreography of acute exercise. Cell. 2020;181:1112–1130.e1116. doi:10.1016/j.cell.2020.04.043
6. Horowitz AM, Fan X, Bieri G, et al. Blood factors transfer beneficial effects of exercise on neurogenesis and cognition to the aged brain. Science. 2020;369:167–173. doi:10.1126/science.aaw2622
7. De Araújo AL, Silva LCR, Fernandes JR, Benard G. Preventing or reversing immunosenescence: can exercise be an immunotherapy? Immunotherapy. 2013;5:879–893. doi:10.2217/imt.13.77
8. Pedersen BK, Saltin B. Exercise as medicine – evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand J Med Sci Sports. 2015;25:1–72. doi:10.1111/sms.12581
9. Nici L, Donner C, Wouters E, et al. American Thoracic Society/European Respiratory Society statement on pulmonary rehabilitation. Am J Respir Crit Care Med. 2006;173:1390–1413. doi:10.1164/rccm.200508-1211ST
10. Armstrong M, Vogiatzis I. Personalized exercise training in chronic lung diseases. Respirology. 2019;24:854–862. doi:10.1111/resp.13639
11. Spruit MA, Singh SJ, Garvey C, et al. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188:E13–64. doi:10.1164/rccm.201309-1634ST
12. Maltais F, Decramer M, Casaburi R, et al. An official American Thoracic Society/European Respiratory Society statement: update on limb muscle dysfunction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2014;189:e15–62. doi:10.1164/rccm.201402-0373ST
13. Bolton CE, Bevan-Smith EF, Blakey JD, et al. British Thoracic Society guideline on pulmonary rehabilitation in adults: accredited by NICE. Thorax. 2013;68:ii1. doi:10.1136/thoraxjnl-2013-203808
14. Ries AL, Bauldoff GS, Carlin BW, et al. Pulmonary Rehabilitation: joint ACCP/AACVPR Evidence-Based Clinical Practice Guidelines. Chest. 2007;131:4S–42S. doi:10.1378/chest.06-2418
15. Singh D, Agusti A, Anzueto A, et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease: the GOLD science committee report 2019. Eur Respir J. 2019;53:1900164. doi:10.1183/13993003.00164-2019
16. Chen Y, Niu M, Zhang X, Qian H, Xie A, Wang X. Effects of home-based lower limb resistance training on muscle strength and functional status in stable Chronic obstructive pulmonary disease patients. J Clin Nurs. 2018;27:e1022–e1037. doi:10.1111/jocn.14131
17. Silva C, Gomes Neto M, Saquetto MB, Conceição CSD, Souza-Machado A. Effects of upper limb resistance exercise on aerobic capacity, muscle strength, and quality of life in COPD patients: a randomized controlled trial. Clin Rehabil. 2018;32:1636–1644. doi:10.1177/0269215518787338
18. Calik-Kutukcu E, Arikan H, Saglam M, et al. Arm strength training improves activities of daily living and occupational performance in patients with COPD. Clin Respir J. 2017;11:820–832. doi:10.1111/crj.12422
19. Zambom-Ferraresi F, Cebollero P, Gorostiaga EM, et al. Effects of Combined Resistance and Endurance Training Versus Resistance Training Alone on Strength, Exercise Capacity, and Quality of Life in Patients With COPD. J Cardiopulm Rehabil. 2015;35:446–453. doi:10.1097/hcr.0000000000000132
20. Nyberg A, Lindström B, Rickenlund A, Wadell K. Low-load/high-repetition elastic band resistance training in patients with COPD: a randomized, controlled, multicenter trial. Clin Respir J. 2015;9:278–288. doi:10.1111/crj.12141
21. Gallo-Silva B, Cerezer-Silva V, Ferreira DG, et al. Effects of Water-Based Aerobic Interval Training in Patients With COPD: a RANDOMIZED CONTROLLED TRIAL. J Cardiopulm Rehabil. 2019;39:105–111. doi:10.1097/hcr.0000000000000352
22. Santos C, Rodrigues F, Santos J, Morais L, Bárbara C. Pulmonary Rehabilitation in COPD: effect of 2 Aerobic Exercise Intensities on Subject-Centered Outcomes—A Randomized Controlled Trial. Respir Care. 2015;60(11):1603–1609. doi:10.4187/respcare.03663
23. de Sousa Pinto JM, Martín-Nogueras AM, Calvo-Arenillas JI, Ramos-González J. Clinical benefits of home-based pulmonary rehabilitation in patients with chronic obstructive pulmonary disease. J Cardiopulm Rehabil. 2014;34:355–359. doi:10.1097/hcr.0000000000000061
24. Pleguezuelos E, Pérez ME, Guirao L, et al. Improving physical activity in patients with COPD with urban walking circuits. Respir Med. 2013;107:1948–1956. doi:10.1016/j.rmed.2013.07.008
25. Fan VS, Gharib SA, Martin TR, Wurfel MM. COPD disease severity and innate immune response to pathogen-associated molecular patterns. Int J Chron Obstruct Pulmon Dis. 2016;11:467–477. doi:10.2147/copd.S94410
26. Hastie AT, Martinez FJ, Curtis JL, et al. Association of sputum and blood eosinophil concentrations with clinical measures of COPD severity: an analysis of the SPIROMICS cohort. Lancet Respir Med. 2017;5:956–967. doi:10.1016/s2213-2600(17)30432-0
27. Wang X, Wang Z, Tang D. Aerobic Exercise Alleviates Inflammation, Oxidative Stress, and Apoptosis in Mice with Chronic Obstructive Pulmonary Disease. Int J Chron Obstruct Pulmon Dis. 2021;16:1369–1379. doi:10.2147/COPD.S309041
28. Neunhäuserer D, Patti A, Niederseer D, et al. Systemic Inflammation, Vascular Function, and Endothelial Progenitor Cells after an Exercise Training Intervention in COPD. Am J Med. 2021;134:e171–e180. doi:10.1016/j.amjmed.2020.07.004
29. Fernandes JR, Marques da Silva CCB, da Silva AG, et al. Effect of an Exercise Program on Lymphocyte Proliferative Responses of COPD Patients. Lung. 2018;196:271–276. doi:10.1007/s00408-018-0107-9
30. Abd El-Kader SM, Al-Jiffri OH. Exercise alleviates depression related systemic inflammation in chronic obstructive pulmonary disease patients. Afr Health Sci. 2016;16:1078–1088. doi:10.4314/ahs.v16i4.25
31. Wesley ACR, Regina GF, Breanne MK, et al. Aerobic Exercise Reduces Asthma Phenotype by Modulation of the Leukotriene Pathway. Front Immunol. 2016;7:65.
32. Reddel HK, Bacharier LB, Bateman ED, et al. Global Initiative for Asthma Strategy 2021: executive Summary and Rationale for Key Changes. Am J Respir Crit Care Med. 2022;205:17–35. doi:10.1164/rccm.202109-2205PP
33. Avallone KM, McLeish AC. Asthma and Aerobic Exercise: a Review of the Empirical Literature. J Asthma. 2013;50:109–116. doi:10.3109/02770903.2012.759963
34. Carson KV, Chandratilleke MG, Picot J, Brinn MP, Esterman AJ, Smith BJ. Physical training for asthma. Cochrane Database Syst Rev. 2013. doi:10.1002/14651858.CD001116.pub4
35. Williams B, Powell A, Hoskins G, Neville R. Exploring and explaining low participation in physical activity among children and young people with asthma: a review. BMC Fam Pract. 2008;9:40. doi:10.1186/1471-2296-9-40
36. Türk Y, van Huisstede A, Franssen FME, et al. Effect of an outpatient pulmonary rehabilitation program on exercise tolerance and asthma control in obese asthma patients. J Cardiopulm Rehabil. 2017;37:214–222. doi:10.1097/hcr.0000000000000249
37. Peters MC, Wenzel SE. Intersection of biology and therapeutics: type 2 targeted therapeutics for adult asthma. Lancet. 2020;395:371–383. doi:10.1016/s0140-6736(19)33005-3
38. Chung Y, Huang TY, Liao YH, Kuo YC. 12-Week Inspiratory Muscle Training Improves Respiratory Muscle Strength in Adult Patients with Stable Asthma: a Randomized Controlled Trial. Int J Environ Res Public Health. 2021;18:3267. doi:10.3390/ijerph18063267
39. Sanz-Santiago V, Diez-Vega I, Santana-Sosa E, et al. Effect of a combined exercise program on physical fitness, lung function, and quality of life in patients with controlled asthma and exercise symptoms: a randomized controlled trial. Pediatr Pulmonol. 2020;55:1608–1616. doi:10.1002/ppul.24798
40. Freitas PD, Silva AG, Ferreira PG, et al. Exercise improves physical activity and comorbidities in obese adults with asthma. Med Sci Sports Exerc. 2018;50.
41. O’Neill C, Dogra S. Low volume high intensity interval training leads to improved asthma control in adults. Journal of Asthma. 2021;58:1256–1260. doi:10.1080/02770903.2020.1766063
42. Winn CON, Mackintosh KA, Eddolls WTB, et al. Effect of high-intensity interval training in adolescents with asthma: the eXercise for Asthma with Commando Joe’s® (X4ACJ) trial. J Sport Health Sci. 2021;10:488–498. doi:10.1016/j.jshs.2019.05.009
43. Evaristo KB, Mendes FAR, Saccomani MG, et al. Effects of Aerobic Training Versus Breathing Exercises on Asthma Control: a Randomized Trial. J Allergy Clin Immunol Pract. 2020;8:2989–2996.e2984. doi:10.1016/j.jaip.2020.06.042
44. Zhang YF, Yang LD. Exercise training as an adjunctive therapy to montelukast in children with mild asthma: a randomized controlled trial. Medicine. 2019;98:e14046. doi:10.1097/md.0000000000014046
45. Jaakkola JJK, Aalto SAM, Hernberg S, Kiihamäki SP, Jaakkola MS. Regular exercise improves asthma control in adults: a randomized controlled trial. Sci Rep. 2019;9:12088. doi:10.1038/s41598-019-48484-8
46. Carew C, Cox DW. Laps or lengths? The effects of different exercise programs on asthma control in children. J Asthma. 2018;55:877–881. doi:10.1080/02770903.2017.1373806
47. Scichilone N, Morici G, Zangla D, et al. Effects of exercise training on airway closure in asthmatics. J Appl Physiol. 2012;113:714–718. doi:10.1152/japplphysiol.00529.2012
48. Abdelbasset WK, Alsubaie SF, Tantawy SA, Abo Elyazed TI, Kamel DM. Evaluating pulmonary function, aerobic capacity, and pediatric quality of life following a 10-week aerobic exercise training in school-aged asthmatics: a randomized controlled trial. Patient Prefer Adherence. 2018;12:1015–1023. doi:10.2147/PPA.S159622
49. Arandelović M, Stanković I, Nikolić M. Swimming and persons with mild persistant asthma. ScientificWorldJournal. 2007;7:1182–1188. doi:10.1100/tsw.2007.221
50. Wang JS, Hung WP. The effects of a swimming intervention for children with asthma. Respirology. 2009;14:838–842. doi:10.1111/j.1440-1843.2009.01567.x
51. Boyd A, Yang Ct Fau - Estell K, Estell K, Fau - Ms CT. Feasibility of exercising adults with asthma: a randomized pilot study. Allergy Asthma Clin Immunol. 2012;8:5654.
52. Gonçalves RC, Nunes MPT, Cukier A, Stelmach R, Martins M, Carvalho C. Effects of an aerobic physical training program on psychosocial characteristics, quality-of-life, symptoms and exhaled nitric oxide in individuals with moderate or severe persistent asthma. Braz J Phys Ther. 2008;12:127–135. doi:10.1590/S1413-35552008000200009
53. Turner S, Eastwood P, Cook A, Jenkins S. Improvements in symptoms and quality of life following exercise training in older adults with moderate/severe persistent asthma. Respiration. 2011;81:302–310. doi:10.1159/000315142
54. Franca-Pinto A, Mendes FA, de Carvalho-Pinto RM, et al. Aerobic training decreases bronchial hyperresponsiveness and systemic inflammation in patients with moderate or severe asthma: a randomised controlled trial. Thorax. 2015;70:732–739. doi:10.1136/thoraxjnl-2014-206070
55. Mendes FA, Almeida FM, Cukier A, et al. Effects of aerobic training on airway inflammation in asthmatic patients. Med Sci Sports Exerc. 2011;43:197–203. doi:10.1249/MSS.0b013e3181ed0ea3
56. Wicher IB, Ribeiro MA, Marmo DB, et al. Effects of swimming on spirometric parameters and bronchial hyperresponsiveness in children and adolescents with moderate persistent atopic asthma. J Pediatr. 2010;86:384–390. doi:10.2223/jped.2022
57. Counil FP, Varray A, Matecki S, et al. Training of aerobic and anaerobic fitness in children with asthma. J Pediatr. 2003;142:179–184. doi:10.1067/mpd.2003.83
58. Mendes FA, Gonçalves RC, Nunes MP, et al. Effects of aerobic training on psychosocial morbidity and symptoms in patients with asthma: a randomized clinical trial. Chest. 2010;138:331–337. doi:10.1378/chest.09-2389
59. Refaat A, Gawish M. Effect of physical training on health-related quality of life in patients with moderate and severe asthma. Egypt J Chest Dis Tuberc. 2015;64:761–766. doi:10.1016/j.ejcdt.2015.07.004
60. Toennesen LL, Meteran H, Hostrup M, et al. Effects of Exercise and Diet in Nonobese Asthma Patients—A Randomized Controlled Trial. J Allergy Clin Immunol Pract. 2018;6:803–811. doi:10.1016/j.jaip.2017.09.028
61. Francisco CO, Bhatawadekar SA, Babineau J, Reid WD, Yadollahi A. Effects of physical exercise training on nocturnal symptoms in asthma: systematic review. PLoS One. 2018;13:e0204953. doi:10.1371/journal.pone.0204953
62. Adeniyi FB, Young T. Weight loss interventions for chronic asthma. Cochrane Database Syst Rev. 2012;Cd009339. doi:10.1002/14651858.CD009339.pub2
63. O’Sullivan S, Roquet A, Dahlén B, et al. Evidence for mast cell activation during exercise-induced bronchoconstriction. Eur Respir J. 1998;12:345–350. doi:10.1183/09031936.98.12020345
64. Perry C, Pick M, Bdolach N, et al. Endurance exercise diverts the balance between Th17 cells and regulatory T cells. PLoS One. 2013;8:e74722. doi:10.1371/journal.pone.0074722
65. Eijkemans M, Mommers M, Draaisma JM, Thijs C, Prins MH. Physical activity and asthma: a systematic review and meta-analysis. PLoS One. 2012;7:e50775. doi:10.1371/journal.pone.0050775
66. Ford ES. Does exercise reduce inflammation? Physical activity and C-reactive protein among U.S. adults. Epidemiology. 2002;13:561–568. doi:10.1097/00001648-200209000-00012
67. Camargo Hizume-Kunzler D, Greiffo FR, Fortkamp B, et al. Aerobic Exercise Decreases Lung Inflammation by IgE Decrement in an OVA Mice Model. Int J Sports Med. 2017;38:473–480. doi:10.1055/s-0042-121638
68. Prossegger J, Huber D, Grafetstätter C, et al. Winter Exercise Reduces Allergic Airway Inflammation: a Randomized Controlled Study. Int J Environ Res Public Health. 2019;16(11):2040. doi:10.3390/ijerph16112040
69. Moraes-Ferreira R, Brandao-Rangel MAR, Gibson-Alves TG, et al. Physical Training Reduces Chronic Airway Inflammation and Mediators of Remodeling in Asthma. Oxid Med Cell Longev. 2022;2022:5037553. doi:10.1155/2022/5037553
70. O’Donnell AE. Bronchiectasis - A Clinical Review. N Engl J Med. 2022;387:533–545. doi:10.1056/NEJMra2202819
71. de Camargo AA, Boldorini JC, Holland AE, et al. Determinants of Peripheral Muscle Strength and Activity in Daily Life in People With Bronchiectasis. Phys Ther. 2018;98:153–161. doi:10.1093/ptj/pzx123
72. Polverino E, Goeminne PC, McDonnell MJ, et al. European Respiratory Society guidelines for the management of adult bronchiectasis. Eur Respir J. 2017;50(3):1700629. doi:10.1183/13993003.00629-2017
73. Kelly C, Grundy S, Lynes D, et al. Self-management for bronchiectasis. Cochrane Database Syst Rev. 2018;2:Cd012528. doi:10.1002/14651858.CD012528.pub2
74. Cedeño de Jesús S, Almadana Pacheco V, Valido Morales A, Muñíz Rodríguez AM, Ayerbe García R, Arnedillo-Muñoz A. Exercise Capacity and Physical Activity in Non-Cystic Fibrosis Bronchiectasis after a Pulmonary Rehabilitation Home-Based Programme: a Randomised Controlled Trial. INT J ENVIRON HEAL R. 2022;19:11039.
75. José A, Holland AE, Selman JPR, et al. Home-based pulmonary rehabilitation in people with bronchiectasis: a randomised controlled trial. ERJ Open Res. 2021;7:00021–2021. doi:10.1183/23120541.00021-2021
76. Van Zeller M, Mota PC, Amorim A, et al. Pulmonary rehabilitation in patients with bronchiectasis: pulmonary function, arterial blood gases, and the 6-minute walk test. J Cardiopulm Rehabil. 2012;32:278–283. doi:10.1097/HCR.0b013e3182631314
77. Pehlivan E, Niksarlıoğlu EY, Balcı A, Kılıç L. The Effect of Pulmonary Rehabilitation on the Physical Activity Level and General Clinical Status of Patients with Bronchiectasis. Turk Thorac J. 2019;20:30–35. doi:10.5152/TurkThoracJ.2018.18093
78. Zanini A, Aiello M, Adamo D, et al. Effects of Pulmonary Rehabilitation in Patients with Non-Cystic Fibrosis Bronchiectasis: a Retrospective Analysis of Clinical and Functional Predictors of Efficacy. Respiration. 2015;89:525–533. doi:10.1159/000380771
79. Araújo AS, Figueiredo MR, Lomonaco I, Lundgren F, Mesquita R, Pereira EDB. Effects of Pulmonary Rehabilitation on Systemic Inflammation and Exercise Capacity in Bronchiectasis: a Randomized Controlled Trial. Lung. 2022;200:409–417. doi:10.1007/s00408-022-00540-3
80. Deniz S, Şahin H, Erbaycu AE. Efficacy of pulmonary rehabilitation on patients with non-cystic bronchiectasis according to disease severity. Tuberk Toraks. 2021;69:449–457. doi:10.5578/tt.20219602
81. Patel S, Cole AD, Nolan CM, et al. Pulmonary rehabilitation in bronchiectasis: a propensity-matched study. Eur Respir J. 2019;53:1801264. doi:10.1183/13993003.01264-2018
82. Dos Santos DO, de Souza HCD, Baddini-Martinez JA, Ramos EMC, Gastaldi AC. Effects of exercise on secretion transport, inflammation, and quality of life in patients with noncystic fibrosis bronchiectasis: protocol for a randomized controlled trial. Medicine. 2018;97:e9768. doi:10.1097/md.0000000000009768
83. La Rovere MT, Mortara A, Sandrone G, Lombardi F. Autonomic nervous system adaptations to short-term exercise training. Chest. 1992;101:299s–303s. doi:10.1378/chest.101.5_supplement.299s
84. Guan WJ, Gao YH, Xu G, et al. Effect of airway Pseudomonas aeruginosa isolation and infection on steady-state bronchiectasis in Guangzhou, China. J Thorac Dis. 2015;7:625–636. doi:10.3978/j.issn.2072-1439.2015.04.04
85. Zheng L, Lam WK, Tipoe GL, et al. Overexpression of matrix metalloproteinase-8 and −9 in bronchiectatic airways in vivo. Eur Respir J. 2002;20:170–176. doi:10.1183/09031936.02.00282402
86. Fahy JV, Schuster A, Ueki I, Boushey HA, Nadel JA. Mucus hypersecretion in bronchiectasis. The role of neutrophil proteases. Am Rev Respir Dis. 1992;146:1430–1433. doi:10.1164/ajrccm/146.6.1430
87. Reynolds CJ, Quigley K, Cheng X, et al. Lung Defense through IL-8 Carries a Cost of Chronic Lung Remodeling and Impaired Function. Am J Respir Cell Mol Biol. 2018;59:557–571. doi:10.1165/rcmb.2018-0007OC
88. Alizaei Yousefabadi H, Niyazi A, Alaee S, Fathi M, Mohammad Rahimi GR. Anti-Inflammatory Effects of Exercise on Metabolic Syndrome Patients: a Systematic Review and Meta-Analysis. Biol Res Nurs. 2021;23:280–292. doi:10.1177/1099800420958068
89. Shi Y, Liu T, Nieman DC, et al. Aerobic Exercise Attenuates Acute Lung Injury Through NET Inhibition. Front Immunol. 2020;11:409. doi:10.3389/fimmu.2020.00409
90. Dong J, Chen P, Wang R, Yu D, Zhang Y, Xiao W. NADPH oxidase: a target for the modulation of the excessive oxidase damage induced by overtraining in rat neutrophils. Int J Biol Sci. 2011;7:881–891. doi:10.7150/ijbs.7.881
91. Kozu R, Shingai K, Hanada M, et al. Respiratory Impairment, Limited Activity, and Pulmonary Rehabilitation in Patients with Interstitial Lung Disease. Phys Ther Res. 2021;24:9–16. doi:10.1298/ptr.R0012
92. Ryu JH, Daniels CE, Hartman TE, Yi ES. Diagnosis of Interstitial Lung Diseases. Mayo Clinic Proceedings. 2007;82:976–986. doi:10.4065/82.8.976
93. De Vries J, Drent M. Quality of life and health status in interstitial lung diseases. Curr Opin Pulm Med. 2006;12(5):354–358. doi:10.1097/01.mcp.0000239553.93443.d8
94. Betancourt-Peña J, Rivera JA, Orozco LM, Torres-del Castillo N, Benadives-Córdoba V. Impacto de la rehabilitación pulmonar en pacientes con enfermedad pulmonar restrictiva. Fisioterapia. 2022;44(6):327–335. doi:10.1016/j.ft.2022.01.001
95. Ferreira G, Feuerman M, Spiegler P. Results of an 8-week, Outpatient Pulmonary Rehabilitation Program on Patients With and Without Chronic Obstructive Pulmonary Disease. J Cardiopulm Rehabil. 2006;26(1):54–60. doi:10.1097/00008483-200601000-00011
96. Holland AE, Hill CJ, Conron M, Munro P, McDonald CF. Short term improvement in exercise capacity and symptoms following exercise training in interstitial lung disease. Thorax. 2008;63:549–554. doi:10.1136/thx.2007.088070
97. Keyser RE, Christensen EJ, Chin LM, et al. Changes in fatigability following intense aerobic exercise training in patients with interstitial lung disease. Respir Med. 2015;109:517–525. doi:10.1016/j.rmed.2015.01.021
98. Dowman L, Hill CJ, May A, Holland AE. Pulmonary rehabilitation for interstitial lung disease. Cochrane Database Syst Rev. 2021;2:Cd006322. doi:10.1002/14651858.CD006322.pub4
99. Jarosch I, Schneeberger T, Gloeckl R, et al. Short-Term Effects of Comprehensive Pulmonary Rehabilitation and its Maintenance in Patients with Idiopathic Pulmonary Fibrosis: a Randomized Controlled Trial. J Clin Med. 2020;9(5):1567. doi:10.3390/jcm9051567
100. Sciriha A, Lungaro-Mifsud S, Fsadni P, Scerri J, Montefort S. Pulmonary Rehabilitation in patients with Interstitial Lung Disease: the effects of a 12-week programme. Respir Med. 2019;146:49–56. doi:10.1016/j.rmed.2018.11.007
101. Perez-Bogerd S, Wuyts W, Barbier V, et al. Short and long-term effects of pulmonary rehabilitation in interstitial lung diseases: a randomised controlled trial. Respir Res. 2018;19:182. doi:10.1186/s12931-018-0884-y
102. Naz I, Ozalevli S, Ozkan S, Sahin H. Efficacy of a Structured Exercise Program for Improving Functional Capacity and Quality of Life in Patients With Stage 3 and 4 Sarcoidosis: a RANDOMIZED CONTROLLED TRIAL. J Cardiopulm Rehabil Prev. 2018;38:124–130. doi:10.1097/hcr.0000000000000307
103. Dowman LM, McDonald CF, Hill CJ, et al. The evidence of benefits of exercise training in interstitial lung disease: a randomised controlled trial. Thorax. 2017;72:610–619. doi:10.1136/thoraxjnl-2016-208638
104. Tonelli R, Cocconcelli E, Lanini B, et al. Effectiveness of pulmonary rehabilitation in patients with interstitial lung disease of different etiology: a multicenter prospective study. BMC Pulm Med. 2017;17:130. doi:10.1186/s12890-017-0476-5
105. Essam H, Abdel Wahab NH, Younis G, El-Sayed E, Shafiek H. Effects of different exercise training programs on the functional performance in fibrosing interstitial lung diseases: a randomized trial. PLoS One. 2022;17:e0268589. doi:10.1371/journal.pone.0268589
106. Brunetti G, Malovini A, Maniscalco M, et al. Pulmonary rehabilitation in patients with interstitial lung diseases: correlates of success. Respir Med. 2021;185:106473. doi:10.1016/j.rmed.2021.106473
107. Shimba A, Ikuta K. Control of immunity by glucocorticoids in health and disease. Semin Immunopathol. 2020;42:669–680. doi:10.1007/s00281-020-00827-8
108. Nieman DC, Wentz LM. The compelling link between physical activity and the body’s defense system. J Sport Health Sci. 2019;8:201–217. doi:10.1016/j.jshs.2018.09.009
109. Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209–249. doi:10.3322/caac.21660
110. Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization Classification of Lung Tumors: impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol. 2015;10:1243–1260. doi:10.1097/jto.0000000000000630
111. Bade BC, Dela Cruz CS. Lung Cancer 2020: epidemiology, Etiology, and Prevention. Clin Chest Med. 2020;41:1–24. doi:10.1016/j.ccm.2019.10.001
112. Collins LG, Haines C, Perkel R, Enck RE. Lung cancer: diagnosis and management. Am Fam Physician. 2007;75:56–63.
113. Su XL, Wang JW, Che H, et al. Clinical application and mechanism of traditional Chinese medicine in treatment of lung cancer. Chin Med J. 2020;133:2987–2997. doi:10.1097/cm9.0000000000001141
114. Vinod SK, Hau E. Radiotherapy treatment for lung cancer: current status and future directions. Respirology. 2020;25 Suppl 2:61–71. doi:10.1111/resp.13870
115. Zhang L, Bing S, Dong M, Lu X, Xiong Y. Targeting ion channels for the treatment of lung cancer. Biochim Biophys Acta Rev Cancer. 2021;1876:188629. doi:10.1016/j.bbcan.2021.188629
116. Brunelli A, Kim AW, Berger KI, Addrizzo-Harris DJ. Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143:e166S–e190S. doi:10.1378/chest.12-2395
117. Win T, Jackson A, Sharples L, et al. Relationship between pulmonary function and lung cancer surgical outcome. Eur Respir J. 2005;25:594–599. doi:10.1183/09031936.05.00077504
118. Cesario A, Ferri L, Galetta D, et al. Pre-operative pulmonary rehabilitation and surgery for lung cancer. Lung Cancer. 2007;57:118–119. doi:10.1016/j.lungcan.2007.03.022
119. Weiner P, Man A, Weiner M, et al. The effect of incentive spirometry and inspiratory muscle training on pulmonary function after lung resection. J Thorac Cardiov Sur. 1997;113:552–557. doi:10.1016/S0022-5223(97)70370-2
120. Sanchez-Lorente D, Navarro-Ripoll R, Guzman R, et al. Prehabilitation in thoracic surgery. J Thorac Dis. 2018;10:S2593–s2600. doi:10.21037/jtd.2018.08.18
121. Salhi B, Huysse W, Van Maele G, Surmont VF, Derom E, van Meerbeeck JP. The effect of radical treatment and rehabilitation on muscle mass and strength: a randomized trial in stages I-III lung cancer patients. Lung Cancer. 2014;84:56–61. doi:10.1016/j.lungcan.2014.01.011
122. Vanderbyl BL, Mayer MJ, Nash C, et al. A comparison of the effects of medical Qigong and standard exercise therapy on symptoms and quality of life in patients with advanced cancer. Supportive Care Cancer. 2017;25:1749–1758. doi:10.1007/s00520-017-3579-x
123. Chen HM, Tsai CM, Wu YC, Lin KC, Lin CC. Randomised controlled trial on the effectiveness of home-based walking exercise on anxiety, depression and cancer-related symptoms in patients with lung cancer. Brit J Cancer. 2015;112:438–445. doi:10.1038/bjc.2014.612
124. Huang HP, Wen FH, Yang TY, et al. The effect of a 12-week home-based walking program on reducing fatigue in women with breast cancer undergoing chemotherapy: a randomized controlled study. Int J Nurs Stud. 2019;99:103376. doi:10.1016/j.ijnurstu.2019.06.007
125. Morano MT, Araújo AS, Nascimento FB, et al. Preoperative pulmonary rehabilitation versus chest physical therapy in patients undergoing lung cancer resection: a pilot randomized controlled trial. Arch Phys Med Rehabil. 2013;94:53–58. doi:10.1016/j.apmr.2012.08.206
126. Pehlivan E, Turna A, Gurses A, Gurses HN. The effects of preoperative short-term intense physical therapy in lung cancer patients: a randomized controlled trial. Ann Thorac Cardiovasc Surg. 2011;17:461–468. doi:10.5761/atcs.oa.11.01663
127. Brocki BC, Andreasen J, Nielsen LR, Nekrasas V, Gorst-Rasmussen A, Westerdahl E. Short and long-term effects of supervised versus unsupervised exercise training on health-related quality of life and functional outcomes following lung cancer surgery - a randomized controlled trial. Lung Cancer. 2014;83:102–108. doi:10.1016/j.lungcan.2013.10.015
128. Henke CC, Cabri J, Fricke L, et al. Strength and endurance training in the treatment of lung cancer patients in stages IIIA/IIIB/IV. Support Care Cancer. 2014;22:95–101. doi:10.1007/s00520-013-1925-1
129. Jastrzębski D, Maksymiak M, Kostorz S, et al. Pulmonary rehabilitation in advanced lung cancer patients during chemotherapy. Adv Exp Med Biol. 2015;861:57–64. doi:10.1007/5584_2015_134
130. Machado P, Pimenta S, Garcia AL, et al. Home-Based Preoperative Exercise Training for Lung Cancer Patients Undergoing Surgery: a Feasibility Trial. J Clin Med. 2023;12(8):2971. doi:10.3390/jcm12082971
131. Mikkelsen MK, Lund CM, Vinther A, et al. Effects of a 12-Week Multimodal Exercise Intervention Among Older Patients with Advanced Cancer: results from a Randomized Controlled Trial. Oncologist. 2022;27:67–78. doi:10.1002/onco.13970
132. Scott JM, Thomas SM, Herndon JE, et al. Effects and tolerability of exercise therapy modality on cardiorespiratory fitness in lung cancer: a randomized controlled trial. J Cachexia Sarcopenia Muscle. 2021;12:1456–1465. doi:10.1002/jcsm.12828
133. Messaggi-Sartor M, Marco E, Martínez-Téllez E, et al. Combined aerobic exercise and high-intensity respiratory muscle training in patients surgically treated for non-small cell lung cancer: a pilot randomized clinical trial. Eur J Phys Rehabil Med. 2019;55:113–122. doi:10.23736/s1973-9087.18.05156-0
134. Cavalheri V, Jenkins S, Cecins N, et al. Exercise training for people following curative intent treatment for non-small cell lung cancer: a randomized controlled trial. Br J Phys Therapy. 2017;21:58–68. doi:10.1016/j.bjpt.2016.12.005
135. Quist M, Adamsen L, Rørth M, Laursen JH, Christensen KB, Langer SW. The Impact of a Multidimensional Exercise Intervention on Physical and Functional Capacity, Anxiety, and Depression in Patients With Advanced-Stage Lung Cancer Undergoing Chemotherapy. Integr Cancer Ther. 2015;14:341–349. doi:10.1177/1534735415572887
136. Lei J, Yang J, Dong L, et al. An exercise prescription for patients with lung cancer improves the quality of life, depression, and anxiety. Front Public Health. 2022;10:1050471. doi:10.3389/fpubh.2022.1050471
137. Bhatia C, Kayser B. Preoperative high-intensity interval training is effective and safe in deconditioned patients with lung cancer: a randomized clinical trial. J Rehabil Med. 2019;51:712–718. doi:10.2340/16501977-2592
138. Kennedy LB, Salama AKS. A review of cancer immunotherapy toxicity. CA Cancer J Clin. 2020;70:86–104. doi:10.3322/caac.21596
139. Estruel-Amades S, Camps-Bossacoma MA-O, Massot-Cladera MA-O, Pérez-Cano FA-O, Castell MA-O. Alterations in the innate immune system due to exhausting exercise in intensively trained rats. Sci Rep. 2020;10:967. doi:10.1038/s41598-020-57783-4
140. Pedersen L, Idorn M, Olofsson GH, et al. Voluntary Running Suppresses Tumor Growth through Epinephrine- and IL-6-Dependent NK Cell Mobilization and Redistribution. Cell Metab. 2016;23:554–562. doi:10.1016/j.cmet.2016.01.011
141. Kruijsen-Jaarsma M, Révész D, Bierings MB, Buffart LM, Takken T. Effects of exercise on immune function in patients with cancer: a systematic review. Exerc Immunol Rev. 2013;19:120–143.
142. Di Vito C, Mikulak J, Zaghi E, Pesce S, Marcenaro E, Mavilio D. NK cells to cure cancer. Semin Immunol. 2019;41:101272. doi:10.1016/j.smim.2019.03.004
143. Liu J, Chen P, Wang R, Yuan Y, Wang X, Li C. Effect of Tai Chi on mononuclear cell functions in patients with non-small cell lung cancer. BMC Complement Altern Med. 2015;15:3. doi:10.1186/s12906-015-0517-7
144. Wang R, Liu J, Fau - Chen P, Chen P, Fau - Yu D, Yu D. Regular tai chi exercise decreases the percentage of type 2 cytokine-producing cells in postsurgical non-small cell lung cancer survivors. Cancer Nurs. 2013;36:E27–E34. doi:10.1097/NCC.0b013e318268f7d5
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
