Back to Journals » Clinical Epidemiology » Volume 13
Effect of Pharmacological and Neurostimulation Interventions for Cognitive Domains in Patients with Bipolar Disorder: A Systematic Review and Network Meta-Analysis of Randomized Controlled Trials
Authors Chen WY , Liu HC, Cheng YC, Li H, Huang CC , Ding YW, Huang MC, Chiu CC, Tu YK, Kuo PH
Received 25 August 2021
Accepted for publication 20 October 2021
Published 29 October 2021 Volume 2021:13 Pages 1039—1049
DOI https://doi.org/10.2147/CLEP.S335584
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Lars Pedersen
Wen-Yin Chen,1,2 Hsing-Cheng Liu,1,3 Ying-Chih Cheng,2,4 Hua Li,2 Chi-Chieh Huang,2 Yu-Wei Ding,2 Ming-Chyi Huang,1,3 Chih-Chiang Chiu,1,3 Yu-Kang Tu,2,5 Po-Hsiu Kuo2,5,6
1Department of Psychiatry, Taipei City Psychiatric Center, Taipei City Hospital, Taipei, Taiwan; 2Institute of Epidemiology and Preventive Medicine, College of Public Health, National Taiwan University, Taipei, Taiwan; 3Department of Psychiatry, School of Medicine, College of Medicine, Taipei Medical University, Taipei, Taiwan; 4Department of Psychiatry, China Medical University Hsinchu Hospital, China Medical University, Hsinchu, Taiwan; 5Department of Public Health, College of Public Health, National Taiwan University, Taipei, Taiwan; 6Department of Psychiatry, College of Medicine, National Taiwan University, Taipei, Taiwan
Correspondence: Yu-Kang Tu; Po-Hsiu Kuo
Department of Public Health, College of Public Health, National Taiwan University, Room 521, No. 17, Xuzhou Road, Taipei City, 100, Taiwan
Email [email protected]; [email protected]
Introduction: The priority of interventions to alleviate cognitive deficits in patients with bipolar disorder (BD) is inconclusive. We systematically evaluate the efficacy of pharmacological or neurostimulation interventions for cognitive function in BD through a network meta-analysis.
Methods: The PubMed, PsycINFO, Embase, and Cochrane Library databases were searched from database inception to September 30, 2021. Following PRISMA guidelines, all eligible studies were randomized controlled trials of adult bipolar patients that provided detailed cognitive outcomes. Studies were excluded if participants limited to comorbid substance use disorder or the intervention was a psychotherapy. Network meta-analysis comparing different interventions was conducted for 8 cognitive domains. Partially ordered set with Hasse diagram was used to resolve conflicting rankings between outcomes. The study was preregistered on PROSPERO database (CRD42020152044).
Results: Total 21 RCTs including 42 tests for assessing intervention effects on cognition were retrieved. Adjunctive erythropoietin (SMD = 0.61, 95% CI = 0.00– 1.23), Withania somnifera (SMD = 0.58, 95% CI = 0.03– 1.13), and galantamine (SMD = 1.22, 95% CI = 0.10– 2.35) was more beneficial for attention, working memory, and verbal learning in euthymic BD patients than treatment as usual, respectively. Hasse diagram suggested ranking of choice when multiple domains were combined.
Conclusion: Considerable variability in measurements of cognitive domains in BD was observed, and no intervention resulted in superior benefits across all domains. We suggested interventions priority can be tailored according to individual patients’ cognitive deficits. As current findings from relatively small and heterogeneous dataset, future trials with consensus should be applied for building further evidence.
Keywords: cognitive function, systematic review, network meta-analysis, Hasse diagram, partially ordered set
Introduction
According to the Global Burden of Disease Study 2013, bipolar disorder (BD), a major psychiatric condition characterized by early onset and chronicity, results in considerable years lost due to disability.1 The clinical course of BD consists of recurrent episodes of mania and depression interspaced by euthymia, and approximately 40% to 60% of cases are associated with cognitive deficits, even in remission status.2 Cognitive impairment in patients with BD is a susceptibility factor for BD recurrence3 and negatively affects both social functioning and inter-episode recovery.4–6 Therefore, cognitive dysfunction is increasingly recognized as a critical symptom of BD.
In a systematic review from Miskowiak et al, the findings regarding intervention efficacy on cognition in BD were overall disappointing or preliminary. Moreover, there were several major methodological limitations to overcome in future evaluation.7 Conventional pharmacotherapies as treatment as usual (TAU) for BD, which include lithium, anticonvulsants, antidepressants, and atypical antipsychotics, have varying effects on cognitive function. Little evidence supports lithium treatment improves cognitive performance in patients with BD, and some studies included in a literature review reported the opposite.8,9 The effects of anticonvulsants on cognition in BD have also not been extensively studied.9 Some evidence suggests that atypical antipsychotics and antidepressants improve cognitive performance in patients with schizophrenia and major depressive disorder by alleviating psychosis or mood-related symptoms; however, few studies have indicated similar benefits for patients with euthymic BD and more long-term studies are needed to better understand the impact of atypical antipsychotics on BD patients’ neurocognitive functioning.10 Given the stagnant state of research on targeting cognitive function in BD through first-line TAU, studies are beginning to examine the potential of cognitive enhancement agents adjunctive to TAU to prevent or alleviate cognitive deficits. Examples include cholinesterase inhibitors, antioxidants, and neurostimulations (eg, transcranial magnetic stimulation [TMS] and transcranial direct current stimulation [tDCS]), which are used in dementia and chronic inflammatory disease and for neuroplasticity enhancement, respectively. Despite the increasing number of studies investigating novel pharmacological or neurostimulation interventions over the past decade, no robust evidence for therapeutics targeting cognitive impairment in BD has emerged.
One of the complexities is the diversity of cognitive impairment in BD, for which the International Society of Bipolar Disorder (ISBD) has defined 8 relevant domains: processing speed, attention (or vigilance), working memory, verbal learning, visual learning, problem-solving, social cognition, and executive function.11 Patients with BD often exhibit varying degrees of impairment across various domains,12 with the domains of executive function and verbal learning reportedly accounting for the most impairments.13,14 Therefore, clinical trials on cognitive function in BD should evaluate intervention effects on multiple domains. Naturally, this would make assessing the efficacy of interventions considerably challenging. To the best of our knowledge, no such systematic comparisons have been conducted to overcome this problem. Furthermore, variation in the tools used to assess these domains in clinical trials creates heterogeneous outcomes, and drawing a generalized conclusion is difficult. In the present study, we evaluated the efficacy of pharmacological and neurostimulation interventions for all 8 cognitive domains outlined by the ISBD.11,15 We first conducted a network meta-analysis (NMA) for direct and indirect comparisons across all available treatments and rank the options by their efficacy in each domain. We then used the partially ordered set to sort out the ranking of treatments when considering potentially conflicting cross-domain results of multiple outcomes.
Methods
Systematic Literature Review
A systematic literature review was performed to look for randomized controlled trials (RCTs) on adult patients with BD that assessed the effect of pharmacological or neurostimulation interventions compared with placebo or TAU on cognitive function. The Embase, PubMed, Cochrane Library, and PsycINFO databases were searched comprehensively from database inception to September 30, 2021. The keywords used were medical subject headings of “Bipolar disorders” combined with “cognition” and filters with RCTs. In this searching process, “cognition” was also replaced with following cognitive domains: “processing speed”, “attention” or “vigilance”, “working memory”, “verbal learning”, “visual learning”, “problem solving”, “social cognition”, and “executive function.” To identify potential additional studies, reference citations were also examined. The review focused on evidence from RCTs because they are regarded as the gold standard trial for evaluating intervention effectiveness and carry a minimal risk of confounding factors. The inclusion criteria were as follows: RCTs of patients with BD aged >18 years that provided detailed descriptions and cognitive measurements of the outcomes. No restrictions on sex or ethnicity were imposed, but studies were excluded if participants limited to BD comorbid with certain substance use disorders or if psychotherapy was the intervention. Duplicate studies were also excluded. Full texts of all included studies were then assessed, and study authors were contacted for additional information if needed. Conference abstracts were not included. The methods and analysis of the present study were followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and prespecified and registered on the PROSPERO database (CRD42020152044).
Data Extraction and Bias Risk Assessment
During data extraction, publications describing the same trial were compiled into a single entry to prevent double counting patients. Extracted data included sample size, age, sex, disease diagnosis (bipolar type I or II), mood phase, intervention duration and protocols, and types and time points of the outcome assessments. Two independent reviewers (WY Chen and YC Cheng) performed both the screening and the data extraction, and any discrepancy between reviewers was resolved by a third independent reviewer. Bias risk assessment was conducted according to the recommendations in the Cochrane Handbook for Systematic Reviews of Interventions.16
Outcome Selection and Network Development
A feasibility assessment was performed to determine possible approaches for conducting NMA. Two approaches were considered: (1) Assessing studies evaluating the same cognitive test and (2) assessing studies evaluating the same cognitive domain, regardless of test. Although it is less prone to validity bias, we could not perform a meaningful NMA by using the first approach because of the large variety and seldom overlapping of cognitive tests used as endpoints among review studies. To overcome these problems, we used the second approach, in which the most commonly applied measurements together with other tests for each domain were evaluated. The priority of cognitive tests selected in each domain is summarized in Supplementary Table S1.
Studies have suggested that episodes of acute depression or mania negatively affect cognitive function in patients with BD.17,18 Considering studies of patients with BD in euthymia with those of patients experiencing mood episodes was not appropriate. To avoid introducing bias into the effects of adjunct interventions, we analyzed studies of patients in the euthymic, depressive, or manic phase separately and provided NMA results for each domain by mood status.
Statistical Analysis
We performed NMA for each domain to simultaneously assess the effects of more than 2 treatments. A NMA synthesizes both direct and indirect evidence over an entire network of treatments within one analysis. Given the expected clinical and methodological heterogeneity among the studies, we used a random effects model but switched to using a fixed effects model if the number of included studies was insufficient to estimate the random effects robustly. The standardized mean difference (SMD) of cognitive measurements in the same domain in changes from baseline to the end of follow-up was the outcome variable. The ranking of treatment formats was estimated according to the surface under the cumulative ranking area (SUCRA), which was based on the estimated random effects models. All analyses were performed using the Network package in Stata, version 14.19
We further used partially ordered set to resolve possible conflicting rankings between cognitive outcomes. The Hasse diagram was used to graphically illustrate the partially ordered set. This statistical method revealed incomparability between treatment rankings or insufficient data for some outcomes.20 We first combined all the outcomes and then deleted them one by one until structured Hasse diagrams, constructed using the hasseDiagram package (version 0.1.3) in R software (version 3.6.1), were formed.
Results
Systematic Literature Review
Figure 1 presents the PRISMA flowchart of studies through the systematic review process. Of the 1475 reference citations retrieved initially, 227 remained after duplicates and irrelevant citations were removed. First-stage screening of the citations identified 44 potentially relevant trials based on their abstracts. After the full texts were accessed for more detailed evaluation, 30 remained for extraction. However, detailed measurement data for 9 trials were inaccessible, leaving 21 RCTs for quantitative analysis. The 9 inaccessible trails were investigating adjunctive effect of N-acetylcysteine (NAC),21 melatonin,22 methylene blue,23 pramipexole,24 repetitive TMS (rTMS),25 docosahexaenoic acid (DHA),26 and atorvastatin27 among euthymic BD patients; and adjunctive tDCS,28 olanzapine,29 for depressed BD patients. Across these 21 trials for quantitative analysis, 42 cognitive tests were used to assess intervention effects on the 8 domains. A summary of the tests applied to each domain and details on all 21 RCTs are presented in Supplementary Table S1 and Table S2, respectively.
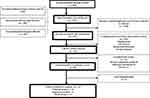 |
Figure 1 Flow diagram of study identification and selection. |
Cognitive Outcomes for Network Development in Euthymic BD
Thirteen of the 21 RCTs reported results related to euthymic BD. Ten out of the 13 trials, comparing for adjunctive interventions, including memantine,30 rTMS,31 lurasidone,32 NAC,33 tDCS,34 quetiapine,35 erythropoietin (EPO),36 Withania somnifera,37 intranasal insulin,38 and galantamine39 to TAU. Three studies, which compared monotherapies of quetiapine or lithium with placebo,40–42 were excluded from the network plot, because of the absence of a common link with other interventions, leaving 10 studies with TAU as the control condition. Except trial for memantine, other 9 studies evaluated processing speed; 9 evaluated verbal learning (adjunctive rTMS, lurasidone, NAC, quetiapine, EPO, Withania somnifera, intranasal insulin, memantine and galantamine); 7 evaluated executive function (adjunctive lurasidone, NAC, tDCS, quetiapine, EPO, intranasal insulin, and galantamine); 6 evaluated working memory (adjunctive rTMS, lurasidone, NAC, quetiapine, EPO, Withania somnifera); 5 evaluated visual learning (adjunctive rTMS, lurasidone, tDCS, memantine and intranasal insulin) and attention (adjunctive rTMS, lurasidone, memantine, quetiapine, EPO); 2 evaluated social cognition (adjunctive rTMS and Withania somnifera), and 1 evaluated problem-solving (adjunctive rTMS). Among the 10 adjunctive trials, the number of patients, mean age, and percentage of male patients in each study ranged between 13 and 175, 28 and 49 years, and 32.4% and 60%, respectively. The timing of cognitive assessment varied between 2 and 24 weeks after baseline assessment. The widely used criteria defining euthymic BD among the studies were Young Maria Rating Score (YMRS) ≦ 14 or Montgomery A° sberg Depression Rating Scale (MADRS) ≦ 18.
Figure 2 shows that positive outcomes were observed for working memory, verbal learning, and attention. The forest plots present the SMD for each study compared with TAU. Out of the interventions used in the 6 studies (209 patients) that evaluated working memory, Withania somnifera was the only one to demonstrate significant benefits in this domain relative to TAU (SMD = 0.58, 95% CI = 0.03–1.13; Figure 2A). Out of the interventions used in the 9 studies (443 patients) that evaluated verbal learning, adjunctive galantamine was the only one to demonstrate benefits in this domain relative to TAU (SMD = 1.22, 95% CI = 0.10–2.35; Figure 2B). Out of the interventions used in the 5 studies (314 patients) that evaluated attention, only adjunctive EPO suggested improvements (SMD = 0.61, 95% CI = 0.00–1.23; Figure 2C) in this domain relative to TAU.
According to the SUCRA results for the remaining domains (processing speed, visual learning, problem-solving, social cognition, and executive function), we revealed the potential benefit of Withania somnifera and EPO in processing speed and executive function, respectively. In addition, rTMS also ranked highly in visual learning domain. However, for these five cognitive domains, none exhibited significant effects compared with TAU (Supplementary Figure S1 and Figure S2).
Through the systematic review, majority of other candidate pharmacological compounds cannot include in quantitative analysis revealed the disappointing results. For example, add-on melatonin did not seem to affect cognition in patients with severe mental illness including BD.22 One study suggested the beneficial effect of methylene blue in residual mood symptoms in BD, but the effects on cognitive symptoms were not significant.23 The trail for pramipexole in BD revealed negative findings in primary cognitive outcomes, and only suggested the potential benefit in subgroup of strictly defined euthymic subjects.24 The trials for atorvastatin and DHA for cognitive deficits in BD also revealed the negative results.26,27 The only promising findings came from the most recently published pilot study for rTMS targeting cognitive function in BD, which indicating a significant group by time interaction in verbal learning domain.25
Cognitive Outcomes for Network Development in Patients with BD Experiencing Depressive or Manic Episodes
Adjunctive creatine monohydrate, high- and low-frequency rTMS, infliximab, tianeptine and mifepristone were the interventions used (compared with TAU) in the 6 studies including patients with BD experiencing depressive episodes.43–48 Across these studies, attention and social cognition were not measured, and only one evaluated problem-solving. Across the 5 domains included in the NMA (Supplementary Figure S3), none of the adjunctive therapies revealed significant benefits compared with TAU. A pilot study assessing effect of adjunctive tDCS on cognition in depressed BD patients revealed no changes in cognitive scores.28 Olanzapine augmentation therapy in a trial among major depressive disorder and BD patients under depression did not improve their cognitive tasks neither.29
Trails of olanzapine and risperidone were the two medications that included patients with BD undergoing acute manic episodes with evaluated cognitive functions.49,50 Perlis et al reported no significant differences in Cognitive Test of Delirium between olanzapine and risperidone in patients with BD undergoing acute manic or mixed episodes.49 Shi et al noted improvements in the cognitive component of the Positive and Negative Syndrome Scale with olanzapine treatment compared with placebo,50 while this improvement was associated with improvement in acute mania state. Because the cognitive measurements were nonspecific, the data were not pooled for analysis.
Partially Ordered Set with Hasse Diagram
Because our NMA contained multiple cognitive outcomes, we used the Hasse diagram for partially ordered set to resolve conflicting treatment rankings between different outcomes. Structured Hasse diagrams were generated only for euthymic BD with combined outcomes across certain domains (Figure 3). Compared with TAU, adjunctive intranasal insulin, NAC, and EPO ranked highly for combined processing speed, verbal learning, and executive function (Figure 3A). Similar rankings were noted when outcomes across 2 domains (among processing speed, verbal learning, and executive function) were considered (Figure 3B–D). Adjunctive galantamine had the lowest ranking for combined outcomes of processing speed and executive function (Figure 3C). Otherwise, adjunctive quetiapine had the lowest ranking compared with TAU, which suggests that add-on second-generation antipsychotics exacerbate cognitive deficits in BD under TAU.
Risk of Bias Assessment
The scores for bias risk for the 21 included RCTs are presented in Supplementary Table S2 and summarized in Figure 4. In general, the studies were of satisfactory quality, with an average of >4 of the 7 assessed categories having low risk of bias. Selective reporting and allocation concealment were the categories with the best and worst scores, respectively. However, in 3 studies35,45,46 the risk of bias was unclear in most categories. As none of our NMA contains treatment loop, there is no need for assessing consistency in the evidence.
 |
Figure 4 Summary of the risk of bias assessment. |
Discussion
To the best of our knowledge, this NMA is the first to compare different interventions for cognitive function in BD and to find a common matrix demonstrating the rankings of interventions for various cognitive outcomes. The topic of cognitive deficits in BD is important, but current manuscript revealed from relatively small and heterogeneous dataset. We applied reasonable methodology, but ultimately conclusions still cannot be draw. According to our findings, we suggested the adjunctive EPO, Withania somnifera, and galantamine benefited attention, working memory, and verbal learning compared with TAU, respectively. Besides, the SUCRA results indicated that rTMS ranked highly for visual learning compared with TAU, but its benefits were not significant. The Hasse diagrams showed that for combined outcomes in processing speed, verbal learning, and executive function, intranasal insulin, NAC, and EPO were ranked highly and adjunctive quetiapine was ranked less compared with TAU. Future homogeneous trials still need to build up the evidence.
Galantamine, which is approved for the treatment of Alzheimer disease, inhibits cholinesterase and potentiates nicotinic neurotransmission. Early case reports51 and a small-scale RCT52 indicated that high-dose galantamine alleviates cognitive impairment in patients with BD and schizophrenia, respectively. These findings provide a rationale for evaluating the therapeutic effect of galantamine on cognition in BD. In our NMA, its benefits were observed significantly only for verbal learning. For Withania somnifera, the extracts of steroidal lactones (glycowithanolides and sitoindosides) may explain its cognitive benefits.53 Animal studies have reported their neuroprotective effects on brain antioxidants and the reversal of memory deficits.54,55 EPO has neuroprotective effect mediated through the activation of anti-inflammatory neural signaling,56 and has been reported to alleviate cognitive decline in patients with schizophrenia and multiple sclerosis.57,58 The only study evaluated EPO in the present review demonstrated that it improved other scores on rapid visual processing and verbal fluency subtests as well as composite scores.36 Future replication studies are required to confirm our findings.
Compared with pharmacological therapy, rTMS is noninvasive and well tolerated. Evidence reports its benefits for cognition in various neuropsychiatric disorders, specifically when high-frequency stimulation is applied over the left dorsolateral prefrontal cortex.59 The study included in our review indicated that high-frequency rTMS improved scores on the spatial span and category fluency tests;31 however, its effects were not significant in the present analysis. We have to emphasized that the effects of rTMS dependent on stimulation parameter and localizations. It is hard to lump rTMS together into several categories for an evaluation of their effect on cognitive function in BD. Future trials of rTMS on alleviating cognitive dysfunction in BD can focus on developing more representative parameters.
The Hasse diagram used in our study is a conservative approach, which maintains the multidimensional characteristics of the underlying decision problem and can reveal the uncertainties, also presents indisputable evidence.20 Specifically, if Hasse diagrams show treatment A to be superior to TAU, thus indicating the superiority of A form all the evidence. Conversely, if outcomes sort treatments in the opposite direction, the sparse diagram also clearly discloses the uncertainty. However, even small, clinically non-significant differences in the outcome values in partially ordered set can lead to variations in rankings. We suggest that this selection approach can be based on patients’ individual characteristics. Finding a treatment that is superior across all domains may be impossible. Instead, interventions can be tailored to specific domains of impairment.
As shown in Figure 3, when multiple domains were considered, intranasal insulin, NAC, and EPO had higher rankings, whereas quetiapine had a low ranking. Converging lines of evidence indicate that insulin has activity in the brain.60 Reduced cerebrospinal fluid insulin levels have been reported in patients with Alzheimer disease,61 and trials for cognitive impaired patients have reported that intranasal insulin demonstrated improvements on memory.62,63 Evidence is emerging for the effectiveness of NAC in treating various psychiatric disorders. Its effectiveness may be due to its glutamatergic modulation and antioxidative activities.64–67 A study noted improvements in working memory in patients with psychosis (including schizophrenia and BD) under NAC treatment;21 however, the present analysis did not exert significant cognitive benefits of NAC among BD patients. Whether the benefits of NAC are limited to psychotic BD or whether larger sample sizes are necessary to reveal significant effects warrants further investigation. Second-generation antipsychotic treatments for cognitive dysfunction in BD, which mainly work to reduce mood symptoms, may also have direct adverse cognitive effects mediated by their anticholinergic, extrapyramidal, or sedative effects.68,69 In the present study, quetiapine had a low ranking in the Hasse diagram, which was consistent with the exacerbated cognition in trial conducted by Rakofsky et al.35 Because of conflicting results on their efficacies between outcomes, the Hasse diagram rankings for adjunctive Withania somnifera, galantamine, memantine or neurostimulation indicated sparse representation.
This study has several limitations. First, all the included studies were compared with TAU, which consisted of various psychotropic medications; the uneven effects of TAU medications could not be assessed.69 Second, the small number of studies and small sample sizes prevented us from exploring potentially important factors, such as subtypes or cognitive stages of BD. Therefore, we could not determine the robustness of our findings across different categories of BD. Third, because all the studies came from a single trial of the intervention, we adopted the fixed effects model. Consequently, the uncertainty of the effect sizes, such as the confidence interval, may be underestimated, and the generalizability of our results needs further validation. In addition, these effect sizes are based on single, generally small studies, and are thus subject to effect size inflation that can occur with smaller studies. Furthermore, because of the limited number of common measurements across studies, the outcome selection strategy was arbitrary. As various cognitive tests were used as endpoints, collapsing them into domain-specific groups introduced some inevitable validity bias. Future establishment of consensus for cognitive measurements in patients with BD is warranted. Finally, the reviewed studies included in our analysis had different lengths of follow-up, and mostly investigated immediate or short-term intervention effects. Those differences amongst studies could indicate as a source of heterogeneity. Future studies with long-term follow-up are necessary to determine whether such effects are sustained over time. As cognitive outcomes in BD is an emerging important issue, we believe that newly published trials will keep presenting new findings in the coming years. The on-going trials may impact on the current findings of the NMA, especially the ranking of choices in each domain. Therefore, we suggest that updating search and re-analyzing new data should be done regularly in the future to renew the knowledge in this field.
Conclusion
No single intervention demonstrated improvements on cognitive function across all domains; therefore, interventions tailored to each patient’s specific cognitive deficits is preferable. As current review with the relatively small and heterogeneous dataset, future trials with consensus to explore strategies to alleviate cognitive impairment in BD should be given clinical priority.
Acknowledgments
We thank several authors who released data in a format that enabled their inclusion in the meta-analysis.
Funding
This work was supported by the Ministry of Science and Technology (MOST 110-2314-B-532-007). The funder played no role in the study design,collection, analysis or interpretation of the data, writing the manuscript, or the decision to submit the paper for publication.
Disclosure
The Authors declare that there is no conflict of interest.
References
1. Ferrari AJ, Stockings E, Khoo JP, et al. The prevalence and burden of bipolar disorder: findings from the Global Burden of Disease Study 2013. Bipolar Disord. 2016;18(5):440–450. doi:10.1111/bdi.12423
2. Bora E, Hidiroglu C, Ozerdem A, et al. Executive dysfunction and cognitive subgroups in a large sample of euthymic patients with bipolar disorder. Eur Neuropsychopharmacol. 2016;26(8):1338–1347. doi:10.1016/j.euroneuro.2016.04.002
3. Robinson LJ, Ferrier IN. Evolution of cognitive impairment in bipolar disorder: a systematic review of cross-sectional evidence. Bipolar Disord. 2006;8(2):103–116. doi:10.1111/j.1399-5618.2006.00277.x
4. Atre-Vaidya N, Taylor MA, Seidenberg M, Reed R, Perrine A, Glick-Oberwise F. Cognitive deficits, psychopathology, and psychosocial functioning in bipolar mood disorder. Neuropsychiatry Neuropsychol Behav Neurol. 1998;11(3):120–126.
5. Duarte W, Becerra R, Cruise K. The relationship between neurocognitive functioning and occupational functioning in bipolar disorder: a literature review. Eur j Psychol. 2016;12(4):659–678. doi:10.5964/ejop.v12i4.909
6. Cipriani G, Danti S, Carlesi C, Cammisuli DM, Di Fiorino M. Bipolar disorder and cognitive dysfunction: a complex link. J Nerv Ment Dis. 2017;205(10):743–756. doi:10.1097/NMD.0000000000000720
7. Miskowiak KW, Carvalho AF, Vieta E, Kessing LV. Cognitive enhancement treatments for bipolar disorder: a systematic review and methodological recommendations. Eur Neuropsychopharmacol. 2016;26(10):1541–1561. doi:10.1016/j.euroneuro.2016.08.011
8. Paterson A, Parker G. Lithium and cognition in those with bipolar disorder. Int Clin Psychopharmacol. 2017;32(2):57–62. doi:10.1097/YIC.0000000000000152
9. Dias VV, Balanzá-Martinez V, Soeiro-de-souza MG, et al. Pharmacological approaches in bipolar disorders and the impact on cognition: a critical overview. Acta Psychiatr Scand. 2012;126(5):315–331. doi:10.1111/j.1600-0447.2012.01910.x
10. Harvey PD, Hassman H, Mao L, Gharabawi GM, Mahmoud RA, Engelhart LM. Cognitive functioning and acute sedative effects of risperidone and quetiapine in patients with stable bipolar I disorder: a randomized, double-blind, crossover study. J Clin Psychiatry. 2007;68(8):1186–1194. doi:10.4088/JCP.v68n0804
11. Yatham LN, Torres IJ, Malhi GS, et al. The international society for bipolar disorders-battery for assessment of neurocognition (ISBD-BANC). Bipolar Disord. 2010;12(4):351–363. doi:10.1111/j.1399-5618.2010.00830.x
12. Van Rheenen TE, Lewandowski KE, Bauer IE, et al. Current understandings of the trajectory and emerging correlates of cognitive impairment in bipolar disorder: an overview of evidence. Bipolar Disord. 2020;22(1):13–27. doi:10.1111/bdi.12821
13. Robinson LJ, Thompson JM, Gallagher P, et al. A meta-analysis of cognitive deficits in euthymic patients with bipolar disorder. J Affect Disord. 2006;93(1–3):105–115. doi:10.1016/j.jad.2006.02.016
14. Bourne C, Aydemir O, Balanza-Martinez V, et al. Neuropsychological testing of cognitive impairment in euthymic bipolar disorder: an individual patient data meta-analysis. Acta Psychiatr Scand. 2013;128(3):149–162. doi:10.1111/acps.12133
15. Miskowiak KW, Burdick KE, Martinez-Aran A, et al. Assessing and addressing cognitive impairment in bipolar disorder: the International Society for Bipolar Disorders Targeting Cognition Task Force recommendations for clinicians. Bipolar Disord. 2018;20(3):184–194. doi:10.1111/bdi.12595
16. Higgins JPT, Chandler J, Cumpston M, Li T, Page MJ, Welch VA. Cochrane handbook for systematic reviews of interventions version 6.0 (updated July 2019). 2019.
17. Vrabie M, Marinescu V, Talaşman A, Tăutu O, Drima E, Micluţia I. Cognitive impairment in manic bipolar patients: important, understated, significant aspects. Ann Gen Psychiatry. 2015;14:41. doi:10.1186/s12991-015-0080-0
18. Păunescu R, Micluţia I. Outcome of cognitive performances in bipolar euthymic patients after a depressive episode: a longitudinal naturalistic study. Ann Gen Psychiatry. 2015;14:32. doi:10.1186/s12991-015-0070-2
19. White IR. Network meta-analysis. Stata J. 2015;15(4):951–985. doi:10.1177/1536867X1501500403
20. Rucker G, Schwarzer G. Resolve conflicting rankings of outcomes in network meta-analysis: partial ordering of treatments. Res Synth Methods. 2017;8(4):526–536. doi:10.1002/jrsm.1270
21. Rapado-Castro M, Dodd S, Bush AI, et al. Cognitive effects of adjunctive N-acetyl cysteine in psychosis. Psychol Med. 2017;47(5):866–876. doi:10.1017/S0033291716002932
22. Baandrup L, Fagerlund B, Glenthoj B. Neurocognitive performance, subjective well-being, and psychosocial functioning after benzodiazepine withdrawal in patients with schizophrenia or bipolar disorder: a randomized clinical trial of add-on melatonin versus placebo. Eur Arch Psychiatry Clin Neurosci. 2017;267(2):163–171. doi:10.1007/s00406-016-0711-8
23. Alda M, McKinnon M, Blagdon R, et al. Methylene blue treatment for residual symptoms of bipolar disorder: randomised crossover study. Br J Psychiatr. 2017;210(1):54–60. doi:10.1192/bjp.bp.115.173930
24. Burdick KE, Braga RJ, Nnadi CU, Shaya Y, Stearns WH, Malhotra AK. Placebo-controlled adjunctive trial of pramipexole in patients with bipolar disorder: targeting cognitive dysfunction. J Clin Psychiatry. 2012;73(1):103–112. doi:10.4088/JCP.11m07299
25. McIntyre RS, Lee Y, Rodrigues NB, et al. Repetitive transcranial magnetic stimulation for cognitive function in adults with bipolar disorder: a pilot study. J Affect Disord. 2021;293:73–77. doi:10.1016/j.jad.2021.05.075
26. Ciappolino V, DelVecchio G, Prunas C, et al. The effect of DHA supplementation on cognition in patients with bipolar disorder: an exploratory randomized control trial. Nutrients. 2020;12(3):708. doi:10.3390/nu12030708
27. Fotso Soh J, Almadani A, Beaulieu S, et al. The effect of atorvastatin on cognition and mood in bipolar disorder and unipolar depression patients: a secondary analysis of a randomized controlled trial. J Affect Disord. 2020;262:149–154. doi:10.1016/j.jad.2019.11.013
28. Pereira BDS, Tortella G, Lafer B, et al. The bipolar depression electrical treatment trial (better): design, rationale, and objectives of a randomized, sham-controlled trial and data from the pilot study phase. Neural Plast. 2015;2015:1–10. doi:10.1155/2015/684025
29. Lazowski LK, Townsend B, Hawken ER, Jokic R, Du Toit R, Milev R. Sleep architecture and cognitive changes in olanzapine-treated patients with depression: a double blind randomized placebo controlled trial. BMC Psychiatr. 2014;14:202. doi:10.1186/1471-244X-14-202
30. Lu RB, Wang TY, Lee SY, et al. Add-on memantine may improve cognitive functions and attenuate inflammation in middle- to old-aged bipolar II disorder patients. J Affect Disord. 2021;279:229–238. doi:10.1016/j.jad.2020.10.003
31. Yang LL, Zhao D, Kong LL, et al. High-frequency repetitive transcranial magnetic stimulation (rTMS) improves neurocognitive function in bipolar disorder. J Affect Disord. 2019;246:851–856. doi:10.1016/j.jad.2018.12.102
32. Yatham LN, Mackala S, Basivireddy J, et al. Lurasidone versus treatment as usual for cognitive impairment in euthymic patients with bipolar I disorder: a randomised, open-label, pilot study. Lancet Psychiatr. 2017;4(3):208–217. doi:10.1016/S2215-0366(17)30046-9
33. Dean OM, Bush AI, Copolov DL, et al. Effects of N‐acetyl cysteine on cognitive function in bipolar disorder. Psychiatry Clin Neurosci. 2012;66(6):514–517. doi:10.1111/j.1440-1819.2012.02392.x
34. Bersani FS, Minichino A, Bernabei L, et al. Prefronto-cerebellar tDCS enhances neurocognition in euthymic bipolar patients. Findings from a placebo-controlled neuropsychological and psychophysiological investigation. J Affect Disord. 2017;209:262–269. doi:10.1016/j.jad.2016.11.037
35. Rakofsky JJ, Dunlop BW, Beyer JL, et al. Cognitive effects of quetiapine XR in patients with euthymic bipolar disorder. J Clin Psychopharmacol. 2014;34(3):383–385. doi:10.1097/JCP.0000000000000078
36. Miskowiak KW, Ehrenreich H, Christensen EM, Kessing LV, Vinberg M. Recombinant human erythropoietin to target cognitive dysfunction in bipolar disorder: a double-blind, randomized, placebo-controlled phase 2 trial. J Clin Psychiatry. 2014;75(12):1347–1355. doi:10.4088/JCP.13m08839
37. Chengappa KN, Bowie CR, Schlicht PJ, Fleet D, Brar JS, Jindal R. Randomized placebo-controlled adjunctive study of an extract of withania somnifera for cognitive dysfunction in bipolar disorder. J Clin Psychiatry. 2013;74(11):1076–1083. doi:10.4088/JCP.13m08413
38. McIntyre RS, Soczynska JK, Woldeyohannes HO, et al. A randomized, double-blind, controlled trial evaluating the effect of intranasal insulin on neurocognitive function in euthymic patients with bipolar disorder. Bipolar Disord. 2012;14(7):697–706. doi:10.1111/bdi.12006
39. Ghaemi SN, Gilmer WS, Dunn RT, et al. A double-blind, placebo-controlled pilot study of galantamine to improve cognitive dysfunction in minimally symptomatic bipolar disorder. J Clin Psychopharmacol. 2009;29(3):291–295. doi:10.1097/JCP.0b013e3181a497d7
40. Daglas R, Cotton SM, Allott K, et al. A single-blind, randomised controlled trial on the effects of lithium and quetiapine monotherapy on the trajectory of cognitive functioning in first episode mania: a 12-month follow-up study. Eur Psychiatr. 2016;31:
41. Kocsis JH, Shaw ED, Stokes PE, et al. Neuropsychologic effects of lithium discontinuation. J Clin Psychopharmacol. 1993;13(4):268–275. doi:10.1097/00004714-199308000-00007
42. Shaw ED, Stokes PE, Mann JJ, Manevitz AZ. Effects of lithium carbonate on the memory and motor speed of bipolar outpatients. J Abnorm Psychol. 1987;96(1):64–69. doi:10.1037/0021-843X.96.1.64
43. Toniolo R, Fernandes F, Silva M, Dias R, Lafer B. Cognitive effects of creatine monohydrate adjunctive therapy in patients with bipolar depression. Nutr Neurosci. 2018;21:S6.
44. Myczkowski ML, Fernandes A, Moreno M, et al. Cognitive outcomes of TMS treatment in bipolar depression: safety data from a randomized controlled trial. J Affect Disord. 2018;235:20–26. doi:10.1016/j.jad.2018.04.022
45. Hu SH, Lai JB, Xu DR, et al. Efficacy of repetitive transcranial magnetic stimulation with quetiapine in treating bipolar II depression: a randomized, double-blinded, control study. Sci Rep. 2016;6:30537. doi:10.1038/srep30537
46. Young AH, Gallagher P, Watson S, Del-Estal D, Owen BM, Ferrier IN. Improvements in neurocognitive function and mood following adjunctive treatment with mifepristone (RU-486) in bipolar disorder. Neuropsychopharmacology. 2004;29(8):1538–1545. doi:10.1038/sj.npp.1300471
47. Kauer-Sant’Anna M, Frey BN, Fijtman A, et al. Adjunctive tianeptine treatment for bipolar disorder: a 24-week randomized, placebo-controlled, maintenance trial. J Psychopharmacol. 2019;33(4):502–510. doi:10.1177/0269881119826602
48. Mansur RB, Subramaniapillai M, Lee Y, et al. Effects of infliximab on brain neurochemistry of adults with bipolar depression. J Affect Disord. 2021;281:61–66. doi:10.1016/j.jad.2020.11.128
49. Perlis RH, Baker RW, Zarate CA, et al. Olanzapine versus risperidone in the treatment of manic or mixed States in bipolar I disorder: a randomized, double-blind trial. J Clin Psychiatr. 2006;67(11):1747–1753.
50. Shi L, Schuh LM, Trzepacz PT, Huang LX, Namjoshi MA, Tohen M. Improvement of positive and negative syndrome scale cognitive score associated with olanzapine treatment of acute mania. Curr Med Res Opin. 2004;20(9):1371–1376. doi:10.1185/030079904125004493
51. Schrauwen E, Ghaemi SN. Galantamine treatment of cognitive impairment in bipolar disorder: four cases. Bipolar Disord. 2006;8(2):196–199. doi:10.1111/j.1399-5618.2006.00311.x
52. Buchanan RW, Conley RR, Dickinson D, et al. Galantamine for the treatment of cognitive impairments in people with schizophrenia. Am J Psychiatry. 2008;165(1):82–89. doi:10.1176/appi.ajp.2007.07050724
53. Kulkarni SK, Dhir A. Withania somnifera: an Indian ginseng. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(5):1093–1105. doi:10.1016/j.pnpbp.2007.09.011
54. Jain S, Shukla SD, Sharma K, Bhatnagar M. Neuroprotective effects of Withania somnifera Dunn. in hippocampal sub-regions of female albino rat. Phytother Res. 2001;15(6):544–548. doi:10.1002/ptr.802
55. Kuboyama T, Tohda C, Komatsu K. Neuritic regeneration and synaptic reconstruction induced by withanolide A. Br J Pharmacol. 2005;144(7):961–971. doi:10.1038/sj.bjp.0706122
56. Sargin D, Friedrichs H, El-Kordi A, Ehrenreich H. Erythropoietin as neuroprotective and neuroregenerative treatment strategy: comprehensive overview of 12 years of preclinical and clinical research. Best Pract Res Clin Anaesthesiol. 2010;24(4):573–594. doi:10.1016/j.bpa.2010.10.005
57. Ehrenreich H, Fischer B, Norra C, et al. Exploring recombinant human erythropoietin in chronic progressive multiple sclerosis. Brain. 2007;130(Pt 10):2577–2588. doi:10.1093/brain/awm203
58. Ehrenreich H, Hinze-Selch D, Stawicki S, et al. Improvement of cognitive functions in chronic schizophrenic patients by recombinant human erythropoietin. Mol Psychiatry. 2007;12(2):206–220. doi:10.1038/sj.mp.4001907
59. Kim TD, Hong G, Kim J, Yoon S. Cognitive enhancement in neurological and psychiatric disorders using transcranial magnetic stimulation (TMS): a review of modalities, potential mechanisms and future implications. Exp Neurobiol. 2019;28(1):1–16. doi:10.5607/en.2019.28.1.1
60. Craft S, Watson GS. Insulin and neurodegenerative disease: shared and specific mechanisms. Lancet Neurol. 2004;3(3):169–178. doi:10.1016/S1474-4422(04)00681-7
61. Craft S. Insulin resistance and Alzheimer’s disease pathogenesis: potential mechanisms and implications for treatment. Curr Alzheimer Res. 2007;4(2):147–152. doi:10.2174/156720507780362137
62. Reger MA, Watson GS, Frey WH, et al. Effects of intranasal insulin on cognition in memory-impaired older adults: modulation by APOE genotype. Neurobiol Aging. 2006;27(3):451–458. doi:10.1016/j.neurobiolaging.2005.03.016
63. Reger MA, Watson GS, Green PS, et al. Intranasal insulin administration dose-dependently modulates verbal memory and plasma amyloid-beta in memory-impaired older adults. J Alzheimers Dis. 2008;13(3):323–331. doi:10.3233/JAD-2008-13309
64. Dean O, Giorlando F, Berk M. N-acetylcysteine in psychiatry: current therapeutic evidence and potential mechanisms of action. J Psychiatr Neurosci. 2011;36(2):78–86. doi:10.1503/jpn.100057
65. Berk M, Copolov D, Dean O, et al. N-acetyl cysteine as a glutathione precursor for schizophrenia–a double-blind, randomized, placebo-controlled trial. Biol Psychiatry. 2008;64(5):361–368. doi:10.1016/j.biopsych.2008.03.004
66. Berk M, Copolov DL, Dean O, et al. N-acetyl cysteine for depressive symptoms in bipolar disorder–a double-blind randomized placebo-controlled trial. Biol Psychiatry. 2008;64(6):468–475. doi:10.1016/j.biopsych.2008.04.022
67. Holmay MJ, Terpstra M, Coles LD, et al. N-acetylcysteine boosts brain and blood glutathione in Gaucher and Parkinson diseases. Clin Neuropharmacol. 2013;36(4):103–106. doi:10.1097/WNF.0b013e31829ae713
68. Vieta E. The influence of medications on neurocognition in bipolar disorder. Acta Psychiatr Scand. 2009;120(6):414–415. doi:10.1111/j.1600-0447.2009.01503.x
69. Xu N, Huggon B, Saunders KEA. Cognitive impairment in patients with bipolar disorder: impact of pharmacological treatment. CNS Drugs. 2020;34(1):29–46. doi:10.1007/s40263-019-00688-2
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


