Back to Journals » Nature and Science of Sleep » Volume 13
Artificial Intelligence Analysis of Mandibular Movements Enables Accurate Detection of Phasic Sleep Bruxism in OSA Patients: A Pilot Study
Authors Martinot JB , Le-Dong NN, Cuthbert V, Denison S, Gozal D, Lavigne G, Pépin JL
Received 25 May 2021
Accepted for publication 5 August 2021
Published 23 August 2021 Volume 2021:13 Pages 1449—1459
DOI https://doi.org/10.2147/NSS.S320664
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ahmed BaHammam
Video abstract of "AI analysis of MJM detects SBx in OSA patients" [ID 320664].
Views: 267
Jean-Benoit Martinot,1,2 Nhat-Nam Le-Dong,3 Valérie Cuthbert,1 Stéphane Denison,3 David Gozal,4 Gilles Lavigne,5 Jean-Louis Pépin6
1Sleep Laboratory, CHU Université Catholique de Louvain (UCL) Namur Site Sainte-Elisabeth, Namur, 5000, Belgium; 2Institute of Experimental and Clinical Research, UCL Bruxelles Woluwe, Brussels, 1200, Belgium; 3Sunrise, Namur, 5101, Belgium; 4Department of Child Health and Child Health Research Institute, University of Missouri, Columbia, MO, 65201, USA; 5Faculté de médecine dentaire, Université de Montréal, Montréal, Québec, H3C 3J7, Canada; 6HP2 Laboratory, Inserm U1042, University Grenoble Alpes, Grenoble, 38000, France
Correspondence: Jean-Benoit Martinot
Centre du Sommeil et de la Vigilance, CHU UCL Namur Site Ste Elisabeth, 15, Place Louise Godin, Namur, 5000, Belgium
Tel +32 81 720 411
Fax +32 81 570 754
Email [email protected]
Purpose: Sleep bruxism (SBx) activity is classically identified by capturing masseter and/or temporalis masticatory muscles electromyographic activity (EMG-MMA) during in-laboratory polysomnography (PSG). We aimed to identify stereotypical mandibular jaw movements (MJM) in patients with SBx and to develop rhythmic masticatory muscles activities (RMMA) automatic detection using an artificial intelligence (AI) based approach.
Patients and Methods: This was a prospective, observational study of 67 suspected obstructive sleep apnea (OSA) patients in whom PSG with masseter EMG was performed with simultaneous MJM recordings. The system used to collect MJM consisted of a small hardware device attached on the chin that communicates to a cloud-based infrastructure. An extreme gradient boosting (XGB) multiclass classifier was trained on 79,650 10-second epochs of MJM data from the 39 subjects with a history of SBx targeting 3 labels: RMMA episodes (n=1072), micro-arousals (n=1311), and MJM occurring at the breathing frequency (n=77,267).
Results: Validated on unseen data from 28 patients, the model showed a very good epoch-by-epoch agreement (Kappa = 0.799) and balanced accuracy of 86.6% was found for the MJM events when using RMMA standards. The RMMA episodes were detected with a sensitivity of 84.3%. Class-wise receiver operating characteristic (ROC) curve analysis confirmed the well-balanced performance of the classifier for RMMA (ROC area under the curve: 0.98, 95% confidence interval [CI] 0.97– 0.99). There was good agreement between the MJM analytic model and manual EMG signal scoring of RMMA (median bias − 0.80 events/h, 95% CI − 9.77 to 2.85).
Conclusion: SBx can be reliably identified, quantified, and characterized with MJM when subjected to automated analysis supported by AI technology.
Keywords: masticatory muscular activities, machine learning, jaw movement
Introduction
Recent progress in biosensor technology and digital health are speeding up digitization in sleep medicine.1,2 Artificial intelligence has provided new tools for automated scoring and interpretation of complex biological signals to characterize sleep architecture in various sleep disorders.3–5 These innovations are beginning to offer patients the possibility of monitoring their sleep with simplified devices, in ecological conditions at home. These digital medicine solutions will also enable long-term follow-up and personalized expert guidance coaching.
Repetitive jaw muscle activity may lead to development of sleep bruxism (SBx) in some individuals, which is characterized by tooth clenching or grinding and/or mandible bracing or thrusting.6–9 The estimated prevalence of SBx is primarily based on reports by parents or a sleep partner and is highest in childhood, varying from 14% to 20%.10 SBx prevalence then stabilizes at about 8–12% in teenagers and adults, and declines to ~3% in older individuals.7,8,10
Not all individuals with SBx present tooth grinding, but all SBx patients display rhythmic masticatory muscles activities (RMMA) during sleep.7,11,12 Clinical SBx with an RMMA frequency of at least 2 episodes per hour of sleep is frequently found in the general population, as well as in patients with sleep comorbidities such as obstructive sleep apnea (OSA) and insomnia.13–15
RMMA occurs as a result of an involuntary trigeminal motor-neuronal discharge detected through stereotypical and repetitive EMG activities recorded at the surface of the related muscles.6 Conventional in-laboratory type 1 polysomnography (PSG) remains the gold standard for SBx diagnosis in complex or severe cases.16 However, the PSG montage has to be supplemented by the surface EMG electrodes applied over the masseter and/or anterior temporal jaw muscles to record underlying RMMAs (EMG-MMA).17
Until now, the size and discomfort imposed by jaw tracking recording devices have been a major limitation for human sleep studies in a natural home environment.18,19 Recent noninvasive and easy to use technological innovation has made possible the continuous data collection during sleep of mandibular jaw movements (Figure 1). This technological advance supported by artificial intelligence analyses confers the opportunity to collect accurate information on sleep-wake transitions, cortical arousals and respiratory effort in the presence of sleep-related breathing disorders.20 Additionally, preliminary data have demonstrated the feasibility of such technology to assess SBx EMG-MMA.21 Artificial intelligence (AI) methods have been developed to automate and enhance the scoring of sleep mandibular jaw movements (MJM) to characterize SBx episodes, respiratory efforts and micro-arousals.
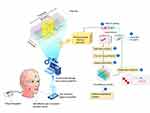 |
Figure 1 Jaw mandibular sensing and overview of data analysis plan. |
The objective of the present study was to test if a machine learning AI automated analysis of MJM recorded with a mandibular sensor provided concordant results with the classical SBx metric, EMG-MMA, in patients undergoing overnight PSG for suspected OSA.
Patients and Methods
Study Subjects and Design
Sixty-seven consecutive patients, clinically suspected of suffering from OSA, were referred for in-laboratory PSG. Among these, 61 SBx subjects were recruited based on positive self-report of awareness of jaw clenching and/or tooth grinding, and/or sleep partner report of tooth grinding, and/or dentist report of high probability of SBx.9 Their sleep was recorded in a dedicated room with a specific EMG montage for collection of jaw muscle activities (masseter) and MJM with the Sunrise device. All recordings were done at a single sleep centre (CHU UCL Namur, Sainte Elisabeth site, Namur, Belgium) between February 2018 and November 2019.
The study has been performed in accordance with the principles stated in the Declaration of Helsinki. Prior to starting the study, ethical approval has been obtained from the local ethics committee (Comité d’Ethique Hospitalo-Facultaire-Universitaire de Liège, Belgium) to confirm the study meets national and international guidelines for research on humans. Each participant provided written informed consent to be part of the study (IRB 00004890 - number B707201523388). Preliminary results of this study were presented at the 2020 virtual Sleep meeting (August 27–30, 2020).21
The study design was a prospective, cross-sectional observational study, whereby 2 subgroups of consecutive suspected OSA patients participated in a training set to develop an AI analytic model, and in a clinical independent validation study.
Sleep Study
In-laboratory type 1 PSG was recorded with a digital acquisition system (Somnoscreen Plus, Somnomedics, Germany). Classical monitored parameters were complemented with bilateral surface masseter electromyograms (see Online Supplement).
PSG data were manually scored by two experienced investigators (V.C. and an experienced sleep technologist; both trained in the same sleep laboratory used the same scoring software [Domino version 3.0.0.1]) and achieved >90% scoring agreement. All sleep stages, EEG arousals, and sleep-related respiratory events were visually scored in accordance with the current American Academy of Sleep Medicine (AASM) criteria.22,23 Hypopneas were scored using the AASM-recommended hypopnea 1A definition, requiring a ≥30% decrease in airflow lasting ≥10 seconds and associated with a ≥3% decrease in oxygen saturation or an EEG arousal.23,24
SBx was diagnosed based on the criteria defined in the third edition of the International Classification of Sleep Disorders (ICSD-3) and the recent version of the AASM manual for the scoring of sleep.13,22
RMMA scoring during polysomnography includes the following classifications: masseter contractions that are phasic (˃3 electromyogram [EMG] bursts lasting 0.25–2.0 s each) and/or tonic (sustained for ˃2 s) and/or a mixture of both.13,25
In this study, we used the definition provided by an international consensus where SBx self-reports and clinical examination are not mandatorily required if EMG-MMA data are recorded.4 According to the AASM manual for the scoring of sleep, SBx was scored if the RMMA was associated with the presence of ≥2 recorded audio-video episodes/sleep period of obvious undisputable grinding. The concomitant bursts of activity at the masseter surface EMG could be phasic, tonic, or mixed according to ICSD-3.13
The RMMA associated to SBx is produced at a typical frequency around 1 Hz. By contrast artifacts due to scratching, swallowing or coughing are not occurring at a predictable frequency. Differences in signal amplitudes are also displayed ie, very large movement in case of swallowing, very sharp when coughing. During the data preparation for model training and labeling, we did remove the epochs including these artifacts (less than 1% of the total amount of the studied epochs).
MJM Recordings and Description of the Movement Sensor (Figure 1)
As indicated above, bilateral masseter surface EMG was added to the PSG montage as the reference method for detecting RMMA. A blinded comparison was done with simultaneous MJM recordings using the MJM sensor system.
MJM was recorded with the Sunrise system (Namur, Belgium), a light-weighted device (3 gr - 42×17 mm) taped with an adhesive to the patient’s chin (in the mentolabial sulcus) (Figure 1) and synchronized with the clock of the PSG acquisition system. The device includes an inertial measurement unit that enables jaw movement sensing, and communicates with a smartphone application for external control. This unit encodes the linear acceleration and rotational velocities of the mandible, measured at the mentolabial sulcus (and therefore MJM) during respiratory and non-respiratory activation of the masseters and the submental muscles.26,27 MJM data are automatically transferred to a cloud-based infrastructure at the end of the night.
The MJM sensor algorithm allows for automatic identification of obstructive and mixed apneas and hypopneas or respiratory effort-related arousals (RERAs) through stereotypical MJM patterns. It also identifies respiratory disturbances, as a period of change in respiratory effort ended by an arousal or an awakening. Respiratory effort is identified through oscillating MJM at the breathing frequency and of increasing amplitude.20 During episodes of SBx, the mandible displays cyclical movements at a frequency and an amplitude that can be readily identified and differentiated from other sleep movements or arousals (closing or opening the mouth), or during periods of altered respiratory effort.30 All episodes of RMMA related to other masticatory muscle activity like chewing, swallowing or facial scratching, or grimacing are discarded from the training set after audio/video examination during PSG.
Data Analysis
An individual data pack was acquired for each patient. This pack consists of: four PSG scoring sequences (sleep stages, micro-arousals, RMMA and respiratory effort-related events) synchronized with 6 channels of MJM raw signals acquired with the Sunrise device and 2 channels of surface masseter EMG signal.
Data pre-processing and preparation, plus model development (training set) and the validation set were performed using Python programming language.29 The data analysis plan included seven key steps (Figure 1):
- Data splitting: Individual data from 61 patients with SBx were randomly split into training (n=39 [65%]) and validation testing (n=22 [35%]) subsets. Six individuals without SBx (true negative subjects) were added to the test subset, making a total of 28 data packs in the final test set.
- MJM signals pre-processing and features generation: Raw MJM sequential data (6 channels) were consecutively segmented into 10-seconds epochs. These segments were passed through a customized processing, labeling and feature generating module. This procedure produces time series from the 6 MJM signals (sliding windows of 10 s each), synchronized with PSG sleep scoring sequences.
- Exploratory analysis: Before training a classification algorithm, we performed an exploratory analysis on a small subset of 3000 segments, randomly sampled from the training set, to verify how many target labels have to be considered for our classification approach, and the performance for each combination including: periods of oscillations of the mandible at the breathing frequency (respiratory mandibular movements), micro-arousals and RMMA events. The data visualization implied K-means clustering and Uniform Manifold Approximation and Projection (UMAP) algorithms.30
- Training set size estimation: Once the classification problem was well determined, we performed an ad-hoc analysis to estimate the necessary size of the training set, which allows an extreme gradient boosting (XGB) classifier to achieve its best performance using the extracted features.
- Machine learning experiment: The learning objective was set as multiclass classification, which aims to classify three target labels: RMMA events, micro-arousals and other MJM at the breathing frequency with different amplitudes regarding the level of RE (BMM). The XGB classifier was adopted as the core algorithm. The model optimization procedure and a leave one out cross-validation at subject level are detailed in the online supplements.31
- The final model was trained on the whole training set using only the most relevant features and the optimized hyperparameter setting.
- Independent model validation: The classifier was independently validated on unseen datasets of 28 participants (including six true negative subjects with RMMA index = 0). The validation aimed to: a) evaluate the overall agreement between model’s epoch-by-epoch prediction and the reference PSG scoring; and b) verify whether the automatic scoring algorithm could provide a reliable estimation of the RMMA hourly index. The epoch-by-epoch agreement evaluation consists of: 1) confusion matrix; 2) overall agreement metrics (Cohen’s Kappa coefficient and balanced accuracy); 3) classwise performance analysis, using precision, recall, F1 score and receiver operating characteristic area under the curve (ROC AUC).
The Bland Altman method was used for quantitative agreement analysis.
Results
Study Population
All sixty-one participants exhibited RMMA associated with grinding sounds and forced displacements of the jaw in a lateral or forward direction during PSG audio-video recordings (eTable 1). Of these, 39 were included in the training set and 22 in the validation study. All were SBx according to ICSD 3 criteria. 53/61 showed on oral inspection the presence of tooth wear consisting of erosion, attrition, or abrasion of the dental enamel, and therefore highly suggestive and confirmatory of SBx. During the period of recruitment 6 other patients with no symptoms and a PSG-verified SBx absence were randomly selected and used to ensure proper representation of a true negative population in our sample (eTable 1).
Exploratory Analysis
Rationale to Formulate a Multiclass Classification Task
Three specific patterns of MJM signals could be visually identified using a 10-seconds sampling window (Figure 2): micro-arousals (n=1311; both respiratory effort induced or spontaneous); BMM with normal (n=71,663) or increased respiratory effort (n=5604); and typical sleep clonic or tonico-clonic RMMA evoking SBx (n=1072). MJM during episodes of RMMA are typically phasic and of large amplitude cyclically repeated at a frequency between 0.5 and 1.5 Hz. Spontaneous micro-arousals appear as brisk MJM of large amplitude, indicating abrupt closure of the mouth characteristic of cortical arousals. Respiratory effort was identified through oscillating MJM at the breathing frequency of increasing amplitude depending on the level of effort (BMM). Data from features extracted from MJM signals after dimensionality reduction provided a clear clustering of three labels allowing separate identification of typical RMMA, micro-arousals and respiratory effort-related MJM (BMM) (eFigure 1).
Training Set Size Estimation
For the chosen algorithm (XGB classifier with logarithmic loss-guided booster), the training curve based on cross-validation indicates that the model would achieve its highest possible performance at a training-set size of around 2000 to 3000 epochs (randomly sampled and balanced for 3 target labels) (eFigure 2), which correspond to a sample of 30 to 39 patients. Thus, our training sample size was adequate, and consequently, the model’s performance was not expected to improve by adding incremental data. The final model was trained on 79,649 10-seconds epochs of MJM data from all 39 subjects, targeting 3 labels: RMMA (n=1072), micro-arousals (n=1311) and other MJM related to respiration (BMM) (n=77,267). Using a learning rate of 0.05, the model reached the optimal status after 87 training steps.
Model Performance
In the validation data set (n=28; 22 SBx plus 6 controls), the model achieved very good agreement (Kappa = 0.799) and high balanced accuracy (86.6%), detecting bruxism episodes with a sensitivity of 84.3%. The class-wise performances of the model’s prediction are summarized in Table 1 and Figure 3. The results issued from the leave one out cross-validation at subject level agreed with the model performance in the validation set of 28 subjects (eTable 2, eFigures 3–8).
 |
Table 1 Class-Wise Performance |
Temporal Agreement Between AI and Manual Scoring
The coincidence of prediction and true events (at a precision of 10 seconds) could also be evaluated by visual inspection. eFigure 7 shows the confusion matrix for the test set, and eFigure 8 shows the temporal agreement and confusion matrix for the model compared with EMG scoring for three patients randomly selected from the validation set.
Quantitative Agreement Evaluation
This consists of evaluating disagreement between the algorithmic analysis and the manual scoring of the EMGs to estimate RMMA hourly index for SBx. Bland-Altman analysis from the data test set showed a low median bias of −0.8 units of EMG-MMA index but an underestimation at the highest values (>15) of the RMMA scale (95% CI +2.85; −9.77) (Figure 4).
Discussion
The present study demonstrates the capability of an automated RMMA recognition system to quantify SBx by measuring stereotypical MJM with a wireless sensor device integrated with a supporting analysis system based on AI. Our findings showed that: 1) MJM activity can accurately detect RMMA episodes with a very good agreement when compared to the gold standard, ie, PSG; and 2) scoring based on this classifier estimates the RMMA index (per hour of sleep) with a median bias of only 0.8 units, with the 95% confidence interval increasing only when the RMMA index exceeds 15/hour of sleep, a value that is infrequently observed in the SBx population.6,12 Thus, the combination of an innovative sensor coupled with an AI algorithm offers a reliable and simple solution for diagnosing and monitoring SBx in an ambulatory setting.
Despite being a prevalent and important issue, SBx remains underrecognized in the clinical setting due to the lack of simple to use and noninvasive monitoring, and the need for visual, labor-intensive and time-consuming scoring even when using diagnostic home sleep testing (HST) tools.32,33 Undiagnosed and untreated SBx can have multiple adverse consequences.4 The most frequent complaints are alterations in sleep quality, tensional headaches, masticatory muscle pain or fatigue, and temporomandibular disorders, all of which have a significant negative impact on quality of life.34 Sleep comorbidities are frequent in SBx patients, and include OSA, insomnia, periodic limb movement disorder of sleep, gastroesophageal reflux, and in rare cases REM behavior disorder and sleep-associated epilepsy.6,14,15,35–37
RMMA events are associated with sleep arousals, and are characteristically accompanied by a surge of sympathetic cardiac activity. This association is temporally related in 50–80% of RMMA events in otherwise healthy children and adults.11,14,37
Audio-video recordings during PSG are used to exclude atypical non-SBx-related orofacial activity and movement disorders, and to confirm the presence and the specificity of typical grinding sounds.7,16,38–40 However long waiting lists, access inequalities, overall costs, and patient acceptability issues, make the PSG as a less than optimal or cost-effective approach for numerous SBx patients, which has prompted the emergence of HST options, such as a single EMG recordings as a viable alternative to PSG.31,32,41–43 Similarly, MJM are under corticobulbar motor control, and can be tracked in animals and humans during sleep.18,19,44–46 However, all these methodologies have significant limitations, and suffer from lack of standardization for quantitative SBx assessments, particularly in the presence of sleep comorbidities.7,9,33,44
The majority of current alternatives to PSG use one or more sensors to capture EMG activities of the jaw muscles, but some have also used interdental pressure and accelerometers, all of which present different levels of discomfort.47–51 Furthermore, none of these technologies has been sufficiently validated to date to allow implementation in clinical practice and endorsement by professional evidence-based guidelines. In addition, current diagnostic tools are contingent on expert human resources and suffer from interobserver reproducibility. Application of decision tree algorithms applied to EMG signals has only been undertaken recently, and not yet translated into clinical practice.52
The rationale of our study was based on previous observations that periods of teeth grinding could be characterized by specific behaviors of the mandible.28 The adoption of the MJM signal provides an alternative to masseter surface EMG for detecting RMMA. During episodes of SBx, the mandible displays cyclical movements at a frequency and an amplitude that can be readily distinguished from other mandibular sleep movements occurring concurrently with arousals (closing or opening the mouth) or typically reflecting normal or abnormal ventilatory patterns.53 Thus, the MJM signal has the potential to collect information not only about RMMA but also its consequences (micro-arousal) as well as potential underlying precipitating factors (eg, OSA).53 However, it remains to be demonstrated whether both entities are coincidental, causally related, linked to arousal reactivity, or relate to a physiological state, which involves the triggering of one or the other.54,55
Detected SBx episodes were analyzed in time series of MJM where variations in morphology can identify related cortical arousal and possible changes in respiratory effort (eg, increased respiratory effort during episodes of OSA or RERA before the SBx episode and/or periods of decreased respiratory effort during central apnea-hypopnea after a SBx episode). Using this technology, time series of clustered MJM remain editable for manual review and analysis in expert centers. However, the automated detection of the stereotypical MJM based on AI could prevent human error or drift, as observed during visual and manual PSG scoring.56
MJM recordings over several nights have potential future use in physiological, epidemiological and clinical studies of SBx, thereby amplifying our understanding of this condition, and optimizing its treatment.
Limitations
The large amplitude of movements during RMMA could potentially be confused with micro-arousal amplitudes; therefore, some misclassifications of these events might occur when an RMMA episode ends with a micro-arousal. While our OSA study population was typical of those referred to a sleep laboratory, generalization of data to SBx real life and in absence of OSA requires additional studies. Finally, the AI algorithms will require external validation, especially in other sleep cohorts.
In this pilot study, we set the trained machine learning algorithms with fragments of MJM in comparison to RMMA collected from masseter EMG. We recognize that our algorithms may have underestimate the occurrence of sustained-tonic contractions, a less dominant but co-occurring type of EMG activities in presence of SBx. Most sleep bruxism EMG events associate to phasic pattern, tonic and mixed contractions; these in otherwise healthy SBx individuals and in the ones with painful temporomandibular disorders whereas tonic phenotype could be prominent.57,58
Conclusion
This study shows that automated analysis of MJM recorded with an inertial unit integrated with a machine learning-derived algorithmic set can identify and quantify SBx episodes. The study shows very good agreement with PSG. Informative sleep MJM provides a unique opportunity to easily detect RMMA and confirm a clinical diagnosis of SBx in conjunction with clinical history, and more importantly when OSA is a comorbid condition.
Role of the Sponsors
The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Data Sharing Statement
The data sets generated during the current study are available from the corresponding author based upon reasonable request.
Acknowledgments
The authors wish to thank Ms. Liesbeth Ory and Ms. Ravzat Ashurlaeva for their secretarial assistance and support. English language editing assistance was provided by Nicola Ryan, independent medical writer, funded by Sunrise.
Author Contributions
All authors helped revise the manuscript and approved it for submission. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
J-L.P. is supported by the French National Research Agency in the framework of the “Investissements d’avenir” program (ANR-15-IDEX-02) and the “e-health and integrated care and trajectories medicine and MIAI artificial intelligence” chairs of excellence from the Grenoble Alpes University Foundation. This work has been partially supported by MIAI @ Grenoble Alpes, (ANR-19-P3IA-0003). G.L. hold a Canada Research Chair in pain, sleep and trauma. D.G. is supported in part by National Institutes of Health grants HL130984, AG 061824, and HL140548 and a University of Missouri Tier 2 grant.
Disclosure
Dr. Martinot reported being a non-remunerated scientific advisor to Sunrise and being an investigator in pharmacy trials for Jazz Pharmaceuticals and Theranexus. Dr. Nhat-Nam Le-Dong reports being a full-time employee at Sunrise during the conduct of the study. Dr. Le-Dong and Mr. Denison reported receiving as employees personal fees from Sunrise. Dr. Gozal is the recipient of an investigator-initiated grant from Jazz Pharmaceuticals, and serves as scientific consultant to Nanit, Dr. Lullaby, ClairLabs, and Sleep Foundation. Dr. Pépin reports grants from Air Liquide Foundation, Mutualia, Resmed, and Vitalaire, and personal fees from Agiradom, AstraZeneca, Fisher and Paykel, Philips, Boehringer Ingelheim, Jazz pharmaceutical, Night Balance, and Sefam, outside the submitted work; and reported being a scientific advisor to Sunrise; receiving grants and/or personal fees from ResMed, Philips, Fisher & Paykel, Sefam, AstraZeneca, AGIR à dom, Elevie, VitalAire, Boehringer Ingelheim, Jazz Pharmaceuticals, and Itamar Medical Ltd; and receiving research support for clinical studies from Mutualia and Air Liquide Foundation. There are no other disclosures to report in relation to this work. There was no funding or other financial support to this research from Sunrise company.
Non-financial disclosure: None.
References
1. Pépin JL, Bailly S, Tamisier R. Big data in sleep apnoea: opportunities and challenges. Respirology. 2020;25:486–494. doi:10.1111/resp.13669
2. Sharma M, Patel V, Acharya UR. Automated identification of insomnia using optimal bi-orthogonal wavelet transform technique with single-channel EEG signals.”. Knowl-Based Syst. 2021;224:107078. doi:10.1016/j.knosys.2021.107078
3. Arnal PJ, Thorey V, Debellemaniere E, et al. The Dreem Headband compared to polysomnography for electroencephalographic signal acquisition and sleep staging. Sleep. 2020;43(11):zsaa097. doi:10.1093/sleep/zsaa097
4. Stephansen JB, Olesen AN, Olsen M, et al. Neural network analysis of sleep stages enables efficient diagnosis of narcolepsy. Nat Commun. 2018;9:5229. doi:10.1038/s41467-018-07229-3
5. Goldstein CA, Berry RB, Kent D, et al. Artificial intelligence in sleep medicine: an American Academy of Sleep Medicine position statement. J Clin Sleep Med. 2020;16:605–607. doi:10.5664/jcsm.8288
6. Carra MC, Huynh N, Lavigne G. Sleep bruxism: a comprehensive overview for the dental clinician interested in sleep medicine. Dent Clin North Am. 2012;56:387–413. doi:10.1016/j.cden.2012.01.003
7. Mayer P, Heinzer R, Lavigne G. Sleep bruxism in respiratory medicine practice. Chest. 2016;149:262–271. doi:10.1378/chest.15-0822
8. Rompré PH, Daigle-Landry D, Guitard F, Montplaisir JY, Lavigne GJ. Identification of a sleep bruxism subgroup with a higher risk of pain. J Dent Res. 2007;86:837–842. doi:10.1177/154405910708600906
9. Lobbezoo F, Ahlberg J, Raphael KG, et al. Manfredini International consensus on the assessment of bruxism: report of a work in progress. J Oral Rehabil. 2018;45:837–844. doi:10.1111/joor.12663
10. Carra MC, Huynh N, Morton P, et al. Prevalence and risk factors of sleep bruxism and wake-time tooth clenching in a 7- to 17-yr-old population. Eur J Oral Sci. 2011;119:386–394. doi:10.1111/j.1600-0722.2011.00846.x
11. Manfredini D, Fabbri A, Peretta R, Guarda-Nardini L, Lobbezoo F. Influence of psychological symptoms on home-recorded sleep-time masticatory muscle activity in healthy subjects. J Oral Rehabil. 2011;38:902–911. doi:10.1111/j.1365-2842.2011.02226.x
12. Manfredini D, Ahlberg J, Wetselaar P, Svensson P, Lobbezoo F. The bruxism construct: from cut-off points to a continuum spectrum. J Oral Rehabil. 2019;46:991–997. doi:10.1111/joor.12833
13. American Academy of Sleep Medicine. International Classification of Sleep Disorders.
14. Maluly M, Andersen ML, Dal-Fabbro C, et al. Polysomnographic study of the prevalence of sleep bruxism in a population sample. J Dent Res. 2013;92:97S–103S. doi:10.1177/0022034513484328
15. da Costa Lopes AJ, Cunha TCA, Monteiro MCM, Serra-Negra JM, Cabral LC, Júnior PCS. Is there an association between sleep bruxism and obstructive sleep apnea syndrome? A systematic review. Sleep Breath. 2020;24:913–921. doi:10.1007/s11325-019-01919-y
16. Stuginski-Barbosa J, Porporatti AL, Costa YM, Svensson P, Conti PC. Agreement of the International Classification of Sleep Disorders Criteria with polysomnography for sleep bruxism diagnosis: a preliminary study. J Prosthet Dent. 2017;117:61–66. doi:10.1016/j.prosdent.2016.01.035
17. Martynowicz H, Gac P, Brzecka A, et al. The relationship between sleep bruxism and obstructive sleep apnea based on polysomnographic findings. J Clin Med. 2019;8:1653. doi:10.3390/jcm8101653
18. Akamatsu Y, Minagi S, Sato T. A new method for recording mandibular position during nocturnal bruxism. J Oral Rehabil. 1996;23:622–626. doi:10.1111/j.1365-2842.1996.tb00901.x
19. Minagi S, Akamatsu Y, Matsunaga T, Sato T. Relationship between mandibular position and the coordination of masseter muscle activity during sleep in humans. J Oral Rehabil. 1998;25:902–907. doi:10.1046/j.1365-2842.1998.00335.x
20. Martinot JB, Le-Dong NN, Cuthbert V, et al. Mandibular movements as accurate reporters of respiratory effort during sleep: validation against diaphragmatic electromyography. Front Neurol. 2017;8:353. doi:10.3389/fneur.2017.00353
21. Martinot JB, Le-Dong NN, Cuthbert V, Denison S, Pepin JL. Mandibular movement monitoring with artificial intelligence analysis for the diagnosis of sleep bruxism. Sleep. 2020;43:A301–A302. doi:10.1093/sleep/zsaa056.788
22. Berry RB, Brooks R, Gamaldo C, et al. AASM scoring manual updates for 2017 (Version 2.4). J Clin Sleep Med. 2017;13(5):665–666. doi:10.5664/jcsm.6576
23. Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM manual for the scoring of sleep and associated events. Deliberations of the sleep apnea definitions task force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8:597–619. doi:10.5664/jcsm.2172
24. Berry RB, Brooks R, Gamaldo C, et al. AASM scoring manual updates for 2017 (Version 2.4). J Clin Sleep Med. 2017;13:665–666. doi:10.5664/jcsm.6576
25. Yoshida Y, Suganuma T, Takaba M, et al. Association between patterns of jaw motor activity during sleep and clinical signs and symptoms of sleep bruxism. J Sleep Res. 2017;26(4):415–421. doi:10.1111/jsr.12481
26. Hollowell DE, Suratt PM. Mandible position and activation of submental and masseter muscles during sleep. J Appl Physiol. 1991;71:2267–2273. doi:10.1152/jappl.1991.71.6.2267
27. Martinot JB, Le-Dong NN, Cuthbert V, et al. The key role of the mandible in modulating airflow amplitude during sleep. Respir Physiol Neurobiol. 2020;279:103447. doi:10.1016/j.resp.2020.103447
28. Martinot JB, Borel JC, Le-Dong NN, et al. Bruxism relieved under CPAP treatment in a patient with OSA syndrome. Chest. 2020;157:e59–e62. doi:10.1016/j.chest.2019.07.032
29. Python Core Team. Python: a dynamic open-source programming language. Python Softward Foundation; 2019. Available from: https://www.python.org/.
30. McInnes L, Healy J, Melville J. UMAP: Uniform manifold approximation and projection for dimension reduction. arXiv:1802.03426; 2018. Available from: https://arxiv.org/abs/1802.03426.
31. Chen T, Guestrin C. XGBoost: A Scalable Tree Boosting System. 22nd SIGKDD Conference on Knowledge Discovery and Data Mining. 2016;785–794. Available from: https://pypi.org/project/xgboost/. doi:10.1145/2939672.2939785
32. Stuginski-Barbosa J, Porporatti AL, Costa YM, Svensson P, Conti PC. Diagnostic validity of the use of a portable single-channel electromyography device for sleep bruxism. Sleep Breath. 2016;20:695–702. doi:10.1007/s11325-015-1283-y
33. Manfredini D, Ahlberg J, Castroflorio T, Poggio CE, Guarda-Nardini L, Lobbezoo F. Diagnostic accuracy of portable instrumental devices to measure sleep bruxism: a systematic literature review of polysomnographic studies. J Oral Rehabil. 2014;41:836–842. doi:10.1111/joor.12207
34. Hublin C, Kaprio J, Partinen M, Koskenvuo M. Sleep bruxism based on self-report in a nationwide twin cohort. J Sleep Res. 1998;7:61–67. doi:10.1046/j.1365-2869.1998.00091.x
35. Tan MWY, Yap AU, Chua AP, Wong JCM, Parot MVJ, Tan KBC. Prevalence of sleep bruxism and its association with obstructive sleep apnea in adult patients: a retrospective polysomnographic investigation. J Oral Facial Pain Headache. 2019;33:269–277. doi:10.11607/ofph.2068
36. Maluly M, Dal Fabbro C, Andersen ML, Herrero Babiloni A, Lavigne GJ, Tufik S. Sleep bruxism and its associations with insomnia and OSA in the general population of Sao Paulo. Sleep Med. 2020;75:141–148. doi:10.1016/j.sleep.2020.06.016
37. Abe S, Gagnon JF, Montplaisir JY, et al. Sleep bruxism and oromandibular myoclonus in rapid eye movement sleep behavior disorder: a preliminary report. Sleep Med. 2013;14:1024–1030. doi:10.1016/j.sleep.2013.04.021
38. Bonnet M, Carley D, Carskadon M, et al. EEG arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep. 1992;15:173–184.
39. Carra MC, Huynh N, Lavigne GJ. Diagnostic accuracy of sleep bruxism scoring in absence of audio-video recording: a pilot study. Sleep Breath. 2015;19:183–190. doi:10.1007/s11325-014-0986-9
40. Dutra KM, Pereira FJ
41. Mainieri VC, Saueressig AC, Pattussi MP, Fagondes SC, Grossi ML. Validation of the Bitestrip versus polysomnography in the diagnosis of patients with a clinical history of sleep bruxism. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;113:612–617. doi:10.1016/j.oooo.2011.10.008
42. Saczuk K, Lapinska B, Wilmont P, Pawlak L, Lukomska-Szymanska M. The Bruxoff device as a screening method for sleep bruxism in dental practice. J Clin Med. 2019;8(7):930. doi:10.3390/jcm8070930
43. Shochat T, Gavish A, Arons E, et al. Validation of the BiteStrip screener for sleep bruxism. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104(3):e32–9. doi:10.1016/j.tripleo.2007.03.009
44. Lavigne GJ, Kato T, Kolta A, Sessle BJ. Neurobiological mechanisms involved in sleep bruxism. Crit Rev Oral Biol Med. 2003;14:30–46. doi:10.1177/154411130301400104
45. Yamada Y, Uchida K, Sato T. Mandibular movement trajectories and masticatory muscle activities in the rabbit in the sleep and wake states. Dent Jpn (Tokyo). 1990;27:35–39.
46. Yao D, Lavigne GJ, Lee JC, Adachi K, Sessle BJ. Jaw-opening reflex and corticobulbar motor excitability changes during quiet sleep in non-human primates. Sleep. 2013;36:269–280. doi:10.5665/sleep.2388
47. Kim J, McAuliffe P, O’Connell B, Diamond D, Lau K. Development of wireless bruxism monitoring device based on pressure-sensitive polymer composite. Sens Actuators A Phys. 2010;163:486–492. doi:10.1016/j.sna.2010.08.033
48. Kim JH, McAuliffe P, O’Connell B, Diamond D, Lau KT Development of a wireless autonomous bruxism monitoring device.
49. Kostka PS, Tkacz EJ. Multi-sources data analysis with sympatho-vagal balance estimation toward early bruxism episodes detection. Annu Int Conf IEEE Eng Med Biol Soc. 2015;2015:6010–6013.
50. Morgado P, Lantada A, Alvarez A, et al. Instrumented splint for the diagnosis of bruxism.
51. Yoshimi H, Sasaguri K, Tamaki K, Sato S. Identification of the occurrence and pattern of masseter muscle activities during sleep using EMG and accelerometer systems. Head Face Med. 2009;5:7. doi:10.1186/1746-160X-5-7
52. Heyat MBB, Lai D, Khan FI, Zhang Y. Sleep bruxism detection using decision tree method by the combination of C4-P4 and C4-A1 channels of scalp EEG. IEEE Access. 2019;7:102542–102553. doi:10.1109/ACCESS.2019.2928020
53. Pépin JL, Letesson C, Le-Dong NN, et al. Assessment of mandibular movement monitoring with machine learning analysis for the diagnosis of obstructive sleep apnea. JAMA Netw Open. 2020;3:e1919657. doi:10.1001/jamanetworkopen.2019.19657
54. Oksenberg A, Arons E. Sleep bruxism related to obstructive sleep apnea: the effect of continuous positive airway pressure. Sleep Med. 2002;3:513–515. doi:10.1016/S1389-9457(02)00130-2
55. Winck M, Drummond M, Viana P, Pinho JC, Winck JC. Sleep bruxism associated with obstructive sleep apnoea syndrome - A pilot study using a new portable device. Rev Port Pneumol. 2017;23:22–26.
56. Rosenberg RS, Van Hout S. The American Academy of sleep medicine inter-scorer reliability program: sleep stage scoring. J Clin Sleep Med. 2013;9(01):81–87. doi:10.5664/jcsm.2350
57. Yoshizawa S, Suganuma T, Takaba M, et al. Phasic jaw motor episodes in healthy subjects with or without clinical signs and symptoms of sleep bruxism: a pilot study. Sleep Breath. 2014;18:187–193. doi:10.1007/s11325-013-0868-6
58. Smardz J, Martynowicz H, Michalek-Zrabkowska M, et al. Sleep bruxism and occurrence of temporomandibular disorders-related pain: a polysomnographic study. Front Neurol. 2019;10:168. doi:10.3389/fneur.2019.00168
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



