Back to Journals » ClinicoEconomics and Outcomes Research » Volume 13
Cost-Effectiveness of Apixaban versus Other Direct Oral Anticoagulants and Warfarin in the Prevention of Thromboembolic Complications Among Finnish Patients with Non-Valvular Atrial Fibrillation
Authors Hallinen T, Soini E , Asseburg C , Linna M, Eloranta P, Sintonen S, Kosunen M
Received 22 April 2021
Accepted for publication 19 July 2021
Published 13 August 2021 Volume 2021:13 Pages 745—755
DOI https://doi.org/10.2147/CEOR.S317078
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Giorgio Colombo
Taru Hallinen,1 Erkki Soini,1 Christian Asseburg,1 Miika Linna,2 Pia Eloranta,3 Sari Sintonen,3 Mikko Kosunen3
1ESiOR Oy, Kuopio, Finland; 2Aalto University, Department of Industrial Engineering and Management, Espoo, Finland; 3Pfizer Oy, Helsinki, Finland
Correspondence: Taru Hallinen ESiOR Oy, Tulliportinkatu 2 LT4, Kuopio, FI-70100, Finland
Tel +358 50 568 1894
Email [email protected]
Purpose: Direct oral anticoagulant (DOAC) use for the prevention of thromboembolic complications in patients with non-valvular atrial fibrillation (AF) has increased steadily in Finland. DOACs have been shown to be cost-effective in comparison to warfarin, but published evidence of relative cost-effectiveness between DOACs is still scarce and mostly based on indirect comparisons of clinical trial evidence. The aim of this study was to compare the cost-effectiveness of apixaban to dabigatran, rivaroxaban and warfarin in a Finnish setting using real-life evidence where available.
Patients and Methods: A lifetime Markov simulation model used previously in a published Finnish assessment comparing apixaban and warfarin was modified and updated with the relative effectiveness and safety data available from the real-world NAXOS-study and representative Finnish input data for patient characteristics, event risks, mortality, resource use, costs, and quality of life. Apixaban’s cost-effectiveness was assessed from health care payer perspective (using 3% per year discount rate) based on incremental cost-effectiveness ratio (ICER, cost per quality-adjusted life year [QALY] gained), probability of cost-effectiveness (at willingness-to-pay [WTP] of 35,000 euros/QALY), and net monetary benefit (NMB).
Results: Apixaban increased the average modelled quality-adjusted life-expectancy and reduced the average total health care costs of AF patients when compared to warfarin (+0.14 QALYs, − 3691 euros), dabigatran (+0.11 QALYs, − 404 euros), and rivaroxaban (+0.03 QALYs, − 43 euros). The resulting NMB of apixaban versus warfarin, dabigatran and rivaroxaban was 8723, 4168, and 1129 euros, respectively. The respective probabilities of apixaban being cost-effective against each comparator were 100%, 92.7%, and 64.0%.
Conclusion: In this modelling study, apixaban dominated other anticoagulants in the Finnish real-life setting.
Keywords: apixaban, cost-utility, dabigatran, economic evaluation, rivaroxaban, warfarin
Introduction
Atrial Fibrillation (AF) is the most common form of cardiac arrhythmia with increasing prevalence in the aging western populations. Currently lifetime risk for AF is 1 in 3 individuals of European ancestry at index age of 55.1 It causes a significant burden to both primary and specialized healthcare. Approximately 30% of patients with AF have at least one hospitalization annually,1 and in Finland over one third of arrhythmia related hospitalizations are caused by AF.2 If untreated, AF predisposes patients to stroke, thromboembolic complications, heart failure and death, as well as worsens their quality of life.1,2 The single most important prognostic factor for AF patients is well-managed anticoagulation treatment.2
Warfarin was the treatment of choice for anticoagulation treatment of AF patients before the advent of direct oral anticoagulants (DOACs) such as apixaban, dabigatran, rivaroxaban and edoxaban. DOACs have been shown to be superior or non-inferior to warfarin in the prevention of stroke and systemic embolism with similar or lower risk of major bleeds. Especially risk of intracranial bleeds is lower with DOACs.3–6 Accumulating real-life evidence support these findings.7–9
DOACs have been shown to be cost-effective in comparison to warfarin in numerous treatment settings,10–16 including Finland.17,18 As a result, the use of DOACs as the first treatment alternative for the prevention of thromboembolic complications in patients with non-valvular atrial fibrillation (AF) has steadily increased over time.19,20 In Finland, the use of warfarin has drastically decreased, with an almost 50% reduction in the number of users since 2016,21 despite the aging of the population. At the same time, the use of DOACs has more than tripled with almost half of the DOAC-treated patients currently using apixaban.21 Currently available published evidence of relative cost-effectiveness of DOACs is mostly based on indirect comparative evidence from the clinical trials.22–25 Since clinical trials often enroll more homogeneous patient populations differing from those being treated in the real-life setting, it is of interest to investigate the relative cost-effectiveness of DOACs based on the comparative effectiveness findings from real-world studies. Such evidence is useful in assessing how the societal resources can be allocated in a cost-effective manner. Here, we report results from a cost-effectiveness assessment comparing apixaban to two other DOACs (dabigatran, rivaroxaban) and warfarin in the Finnish real-world setting.
Materials and Methods
The cost-effectiveness assessment was performed using a lifetime Markov state transition model with 6-week cycles. The model has been previously adapted and modified for cost-effectiveness assessments in numerous countries including Finland,10,11,18,26 and its structure and validation has been described in detail previously.10,11 Here, the model structure is therefore only briefly summarized.
In the current assessment apixaban was compared to rivaroxaban, dabigatran and warfarin based on the relative effectiveness data available from the real-world NAXOS study (Evaluation of Apixaban in Stroke and Systemic Embolism Prevention in Patients With Nonvalvular Atrial Fibrillation),7 and representative Finnish input data for event risks, mortality, resource use, costs, and quality of life. NAXOS-study was chosen to provide relative effectiveness estimates for the comparison, because it is one of the largest real-world studies conducted on DOACs and thus far the largest in Europe. Because the NAXOS-study compared apixaban separately to each of the comparators using propensity score matching,7 the relative cost-effectiveness was likewise assessed pair-wise as apixaban versus comparator, and comparisons between the other three anticoagulants could not be assessed in this setting. As NAXOS did not include comparison between apixaban and edoxaban (due to lack of reimbursement for the latter), edoxaban was excluded from the current analysis.
The primary outcome measures for this analysis were the incremental cost-effectiveness ratio (ICER), given as cost per quality-adjusted life year (QALY) gained and probability of cost-effectiveness based on the societal willingness-to-pay (WTP). In addition, net monetary benefit (NMB = ΔQALY * WTP threshold – Δcosts) was estimated to assess the value of apixaban versus its comparators in monetary terms. In addition, a cost-effectiveness acceptability curve for each comparison was drawn to depict the probability of cost–effectiveness of apixaban versus each comparator at different values of WTP per QALY gained.
In Finland, Health Technology Assessments including cost-effectiveness analyses for new innovative treatments or significant extensions of a therapeutic indication are appraised by separate governmental appraisal committees depending on where the treatment is administered. New hospital-only medicinal products are appraised by the Finnish Medicines Agency (Fimea) whereas self-administered new medicinal products that are used in an outpatient setting are appraised by the Finnish Pharmaceuticals Pricing Board. As for now, there are no publicly announced WTP thresholds in Finland that could be used to determine whether a treatment, new or existing, is considered as cost-effective. When evaluating possible price discount levels for new hospital only products, Fimea has used WTP thresholds ranging from 50,000 €/QALY to 100,000 €/QALY in its recent appraisals.27,28 Since no official Finnish threshold value for cost–effectiveness exists for established outpatient products, we applied the value of 35,000 €/QALY gained as the threshold value, which has been used in Finnish cost-effectiveness assessments in chronic diseases.29 This threshold is in line with the ICER-threshold (20,000–30,000 £/QALY) applied by NICE.30
Model
The modelled cohort consists of 50.6% male and have an average age of 76.3 years at baseline reflecting the current Finnish AF population using anticoagulants.19 The model (Figure 1) captures the health and cost outcomes of the AF cohort starting anticoagulation treatment (AF health state) through transitions to mutually exclusive health states as the result of the following events: ischemic stroke (IS), myocardial infarction (MI), systemic embolism (SE), intracranial hemorrhage (ICH; further classified as either hemorrhagic stroke (HS) or other ICH), other major bleeds (further classified as gastrointestinal [GI] or non-GI bleed) and clinically relevant non-major bleeds. IS, HS, MI and SE events are modelled as permanent health states where the patients reside until death. The remaining health states are modelled as transient states where patients reside for one model cycle.
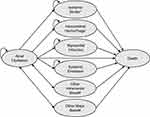 |
Figure 1 Schematic presentation of the model. *Mild, moderate or severe, #transient state (ie, the patients transit back to the previous health state after one cycle and either continue or discontinue anticoagulation treatment). Reproduced from Hallinen T, Soini EJ, Linna M, Saarni SI. Cost-effectiveness of apixaban and warfarin in the prevention of thromboembolic complications among atrial fibrillation patients. Springerplus. 2016;5(1):1354, under the terms of the creative commons attribution 4.0 international license (http://creativecommons.org/licenses/by/4.0/).18 |
In the analysis, apixaban served as the reference product to which the other products were compared. The baseline risk of the modelled events for apixaban treated patients in Finnish real-world setting were derived from the rates reported for warfarin treated AF patients in Finnish real-world studies31–33 and relevant clinical trial evidence where Finnish data is currently lacking (Table 1). The risk of event for treatment comparators were then derived from apixaban rates through the hazard ratios detailed in Table 1. To maintain comparability between the previously conducted clinical trial-based cost-effectiveness analysis for warfarin and apixaban in the Finnish setting, the modelling assumptions and inputs (eg, age-associated increase in the risk of events, severity of IS and HS events, proportion of GI-bleeds among other major bleeds, proportion of HS among ICH, event fatality and post-event mortality risk adjustment factors) were mostly implemented as reported previously by Hallinen et al.18 (Supplementary file, Tables S1–S3).
 |
Table 1 Risk of Modeled Health Events According to Treatment |
For simplicity, the assessment considers only the first line antithrombotic treatment. Patients experiencing HS were assumed to discontinue the first line anticoagulation treatment permanently whereas patients with other ICHs and other major bleeds were assumed to discontinue treatment either permanently (56% and 25% of patients, respectively) or temporarily for 6 weeks (44% and 75%, respectively).10,18 Treatment discontinuations that were not related to modelled events were modelled in accordance with the pivotal clinical trials for each product. The subsequent risk of modelled events for patients residing in AF state at the time of treatment discontinuation are detailed in Table 1. Apart for the cost of treatment-related dyspepsia, other adverse events were not considered in the model.
Treatment Costs and Quality of Life Estimates
The health-related quality of life and cost inputs were modelled in line with the analysis by Hallinen et al18 apart for the health state associated cost inputs which were updated to year 2019 real values (Table 2). The assessment was performed from health care payer perspective with all costs and outcomes discounted at an annual rate of 3% in line with the national guidelines.40
 |
Table 2 Costs and Quality of Life Inputs |
According to Finnish treatment recommendations,39 the routine monitoring of patients using anticoagulants should take place 1–4 times per year. Therefore, the cost of an annual general physician visit and monitoring of basic blood count (incl. thrombocytes) as well as renal and liver function were implemented as routine monitoring costs for all compared treatments. In addition, monitoring of international normalized ratio (INR) was modelled to take place for warfarin treated patients on average 18.5 times a year.41 Drug costs were estimated at Finnish retail prices (excluding value added tax) in February 2021.37 The DOAC costs were estimated based on the reimbursed price of the pack with approximately one-month supply for each product. Similar pack sizes for DOACs were used due to the modelling approach where drug wastage associated with treatment discontinuations are not considered due to the complexity of such modelling.
Sensitivity Analyses
We tested the impact of discounting, modelling timeframe, and warfarin monitoring costs as deterministic sensitivity analyses. In addition, we performed the analyses using the wholesale and retail prices of the largest available pack size for DOACs. These scenarios were conducted to show the impact of the Finnish pharmaceutical pricing scheme on the results of the analyses. In Finland, the retail prices are determined from the wholesale prices using a stepwise scheme that provides the dispensing pharmacies with diminishing retail margins as the wholesale price increases. Thus, the Finnish pricing scheme tends to artificially increase the cost of products with smaller pack sizes relative to those with larger pack sizes.42 Rivaroxaban is currently available in packs containing tablets for 28-day and 98-day treatment whereas dabigatran and apixaban packs provide tablets for a 30-day treatment.
A probabilistic sensitivity analysis (PSA) was performed to explore the impact of parameter uncertainty on the outcomes of the assessment. The values of the key input parameters were varied based on their probability distributions over 2000 simulations and a cost–effectiveness plane was drawn of the findings to illustrate the observed differences in costs and effects between apixaban and its comparators. Probabilities of cost-effectiveness were separately reported for strong dominance (<0 €/QALY) and cost-effectiveness (<35,000 €/QALY).
Results
Base Case Analysis
Apixaban increased life-expectancy and quality-adjusted life-expectancy compared to warfarin, dabigatran, and rivaroxaban (Table 3). The discounted gain in life-years and QALYs with apixaban was 0.05 and 0.03 years compared to rivaroxaban, and 0.13 and 0.11 when compared to dabigatran, respectively. Highest total treatment costs over patients’ lifetime were seen in the warfarin group (22,033 euros) followed by dabigatran (18,746 euros), rivaroxaban (18,385 euros), and apixaban (18,342 euros). Apixaban dominated both warfarin and other DOACs. With WTP of 35,000 euros per QALY gained, NMB of apixaban was 8723, 4168, and 1129 euros per patient when compared to warfarin, dabigatran, and rivaroxaban, respectively.
 |
Table 3 Results of the Cost-Effectiveness Analysis |
Sensitivity Analyses
The cost-effectiveness plane illustrating differences between apixaban and its comparators over 2000 model simulations are shown in Figure 2. Based on these simulations, the average total treatment costs were 18,384 euros for apixaban, 18,552 euros for rivaroxaban, 19,074 euros for dabigatran and 22,237 euros for warfarin during patient’s lifetime. The respective average QALYs were 6.09 for apixaban, 6.06 for rivaroxaban, 5.98 for dabigatran and 5.95 for warfarin. Apixaban dominated warfarin in 99.8% of the simulations and was cost-effective in all. As WTP increases from 35,000 to 100,000 euros/QALY, the probability of apixaban being cost-effective increases from 64% to 79% and from 92% to 99% versus rivaroxaban and dabigatran (Figure 3), respectively.
 |
Figure 3 The cost-effectiveness acceptability curve for apixaban versus warfarin, dabigatran and rivaroxaban. |
Apixaban remained cost-effective versus warfarin and other DOACs in all conducted deterministic sensitivity analyses (Table 4). Cost-savings associated with apixaban ranged from 824 to 4257 euros when compared to warfarin and from 270 to 507 euros when compared to dabigatran. When compared to rivaroxaban the cost difference ranged from −109 to 279 euros. Apixaban was associated with additional costs only in the scenario where drug costs were based on the largest pack size and retail prices where the distorting impact of the Finnish Pharmaceutical Pricing Scheme used for determining retail prices is visible.
 |
Table 4 Results of the Deterministic Sensitivity Analyses |
Discussion
Apixaban improved the outcomes of Finnish AF patients, when compared to warfarin, dabigatran, and rivaroxaban in our real-world based analysis. At the current Finnish drug prices, apixaban was also a less costly treatment alternative than warfarin despite the higher drug acquisition costs. Apixaban dominated warfarin, dabigatran, and rivaroxaban as it was both less costly and more effective. Apixaban’s incremental NMB was positive in all comparisons indicating that the cost to derive the benefits associated with apixaban treatment is less than what the society is willing to pay (here 35,000 euros/QALY gained). Our analysis confirmed the importance of even slight differences in relative efficacy between the different DOACs and suggests that, overall, apixaban has slightly better overall efficacy than rivaroxaban and dabigatran. While it also appears to have slightly higher overall drug costs during patients’ lifetime due to improved survival, the analysis demonstrated that the investment “pays itself back” through reduced event costs.
The results of our analysis were insensitive to changes in most modelling assumptions and the probability of apixaban being cost-effective was high in all comparisons. However, due to the Finnish pharmaceutical pricing scheme that provides the dispensing pharmacies with a lower relative retail margin for larger pack sizes, apixaban was slightly more expensive than rivaroxaban in a scenario using the retail price of the largest available rivaroxaban pack as the acquisition cost for rivaroxaban. In this scenario, apixaban remained nevertheless a cost-effective treatment alternative when compared to rivaroxaban.
In our analysis, we applied the relative risk estimates for the primary efficacy and safety outcomes from the NAXOS study (Evaluation of Apixaban in Stroke and Systemic Embolism Prevention in Patients With Nonvalvular Atrial Fibrillation).7 NAXOS was a French real-world study including altogether 321,501 patients initiating VKA (35.0%), apixaban (27.2%), rivaroxaban (31.1%) or dabigatran (6.6%) with the aim to compare the safety, effectiveness, and mortality of the alternative anticoagulants in oral anticoagulant-naive patients with nonvalvular atrial fibrillation. Even though the NAXOS study was conducted in France we chose it for the current analyses, since it had a highly representative sample of AF patients in France and it is one of the largest real-world studies ever conducted in Europe. Even though large real-world studies have been conducted in the United States,8,9 there are remarkable differences in the organization of health care between the United States and European countries. The modelled life-expectancy of AF patients in our analyses is well in line with what would be expected for the population in Finland. The remaining life-expectancy for Finns aged 76 years is currently 12 years,43 and the mortality risk in AF is approximately two-fold compared to the general population.44
In cost-effectiveness assessments the compared treatments are assumed to be perfect substitutes which means that they would be interchangeable. However, in real-world setting the treatments may nevertheless be prescribed to slightly different kind of patients. In Finland19 and elsewhere45 DOAC-treated patients have been younger and with less comorbidities than patients treated with warfarin. In Finland, patients using apixaban and warfarin appear to be at increased risk of thromboembolic complications (based on CHA2DS2-VASc) when compared to users of dabigatran and rivaroxaban.19 In addition, apixaban is more frequently prescribed to women and patients with prior history of myocardial infarction.19 Even factors not related to patient’s disease characteristics may influence treatment choices in real life. Current evidence suggests that there may be inequalities in access to DOACs as DOAC-treated patients tend to have higher education and income levels.45,46 In our NAXOS-study based analysis, the relative treatment effects were obtained from analyses where apixaban was separately compared to each of the comparators using propensity score matching.7 Because of this, the current analysis should not be interpreted to provide evidence of the relative cost-effectiveness between the other three anticoagulants. Furthermore, it should be kept in mind that the results may not be generalizable to other countries since country-specific differences (eg drug prices, costs of other health care resources) influence the results.
Our assessment was conducted from a health care payer perspective. Therefore, the analysis excluded productivity losses as well as time and travelling costs. In the elderly patient population, the inclusion of productivity losses would have been unlikely to have a significant impact on the results of the analysis. However, the total costs associated with warfarin treatment may have been underestimated due to the exclusion of time and travelling costs which have been estimated to form 26.6% of total therapy costs for warfarin in Finland.47
Conclusion
Apixaban use increased the life-expectancy and quality-adjusted life-expectancy of Finnish AF-patients when compared to warfarin, dabigatran, and rivaroxaban in a real-world setting. Apixaban dominated warfarin, dabigatran and rivaroxaban as these gains were obtained with lower total health care costs.
Disclosure
TH and ES are partners and employees, and CA is an employee of ESiOR Oy, which was commissioned by Pfizer Oy to perform this study. ESiOR has carried out commissioned studies and health-economic analyses for several other pharmaceutical companies, food industry companies, device companies, research groups, health care organizations, and hospitals. ML is an employee of Aalto University. PE, SS and MK are employees of Pfizer Oy. This study was sponsored by Pfizer and Bristol Myers Squibb. Medical Writing support was provided by Taru Hallinen at ESiOR Oy and was funded by Pfizer and Bristol Myers Squibb. The authors report no other conflicts of interest in this work.
References
1. Hindricks G, Potpara T, Dagres N, et al; ESC Scientific Document Group. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2021;42(5):373–498.
2. Working group set up by the Finnish Medical Society Duodecim and the Finnish Cardiac Society. Atrial fibrillation. Current care guidelines. Helsinki: The Finnish Medical Society Duodecim; 2017. (referred February 5, 2021). Available from: www.kaypahoito.fi.
3. Granger C, Alexander J, McMurray J, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981–992. doi:10.1056/NEJMoa1107039
4. Patel M, Mahaffey K, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883–891. doi:10.1056/NEJMoa1009638
5. Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139–1151. doi:10.1056/NEJMoa0905561
6. Giugliano RP, Ruff CT, Braunwald E, et al; ENGAGE AF-TIMI 48 Investigators. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369(22):2093–2104. doi:10.1056/NEJMoa1310907
7. Van Ganse E, Danchin N, Mahé I, et al. Comparative safety and effectiveness of oral anticoagulants in nonvalvular atrial fibrillation: the NAXOS Study. Stroke. 2020;51(7):2066–2075. doi:10.1161/STROKEAHA.120.028825
8. Lip GYH, Keshishian A, Li X, et al. Effectiveness and safety of oral anticoagulants among nonvalvular atrial fibrillation patients. Stroke. 2018;49(12):2933–2944. doi:10.1161/STROKEAHA.118.020232
9. Graham DJ, Baro E, Zhang R, et al. Comparative stroke, bleeding, and mortality risks in older medicare patients treated with oral anticoagulants for nonvalvular atrial fibrillation. Am J Med. 2019;132(5):596–604.e11. doi:10.1016/j.amjmed.2018.12.023
10. Dorian P, Kongnakorn T, Phatak H, et al. Cost-effectiveness of apixaban vs. current standard of care for stroke prevention in patients with atrial fibrillation. Eur Heart J. 2014;35(28):1897–1906. doi:10.1093/eurheartj/ehu006
11. Lip GY, Kongnakorn T, Phatak H, et al. Cost-effectiveness of apixaban versus other new oral anticoagulants for stroke prevention in atrial fibrillation. Clin Ther. 2014;36(2):192–210.e20. doi:10.1016/j.clinthera.2013.12.011
12. Wouters H, Thijs V, Annemans L. Cost-effectiveness of dabigatran etexilate in the prevention of stroke and systemic embolism in patients with atrial fibrillation in Belgium. J Med Econ. 2013;16(3):407–414. doi:10.3111/13696998.2013.766200
13. Kansal AR, Sorensen SV, Gani R, et al. Cost-effectiveness of dabigatran etexilate for the prevention of stroke and systemic embolism in UK patients with atrial fibrillation. Heart. 2012;98(7):573–578. doi:10.1136/heartjnl-2011-300646
14. Mensch A, Stock S, Stollenwerk B, Müller D. Cost effectiveness of rivaroxaban for stroke prevention in German patients with atrial fibrillation. Pharmacoeconomics. 2015;33(3):271–283. doi:10.1007/s40273-014-0236-9
15. Kleintjens J, Li X, Simoens S, et al. Cost-effectiveness of rivaroxaban versus warfarin for stroke prevention in atrial fibrillation in the Belgian healthcare setting. Pharmacoeconomics. 2013;31(10):909–918. doi:10.1007/s40273-013-0087-9
16. Rognoni C, Marchetti M, Quaglini S, Liberato NL. Edoxaban versus warfarin for stroke prevention in non-valvular atrial fibrillation: a cost-effectiveness analysis. J Thromb Thrombolysis. 2015;39(2):149–154. doi:10.1007/s11239-014-1104-3
17. Hallinen T, Soini E, Tuovinen M. Dabigatraanin kustannusvaikuttavuus eteisvärinäpotilaiden aivohalvausten ehkäisyssä [Cost-effectiveness of dabigatran compared to warfarin in the prevention of stroke and systemic embolism in Finnish AF patients]. DOSIS. 2012;28(2):130–144. Finnish.
18. Hallinen T, Soini EJ, Linna M, Saarni SI. Cost-effectiveness of apixaban and warfarin in the prevention of thromboembolic complications among atrial fibrillation patients. Springerplus. 2016;5(1):1354. doi:10.1186/s40064-016-3024-5
19. Hellman T, Salo H, Kulathinal S, Eloranta P, Airaksinen J, Laine J. Oraalisten antikoagulanttien käyttö eteisvärinäpotilailla vuosina 2015–2017-rekisteritutkimus [Use of oral anticoagulants among patients with atrial fibrillation in 2015–2017 – a register study]. Suom Laakaril. 2020;75(47):2531–2535. Finnish.
20. Guelker JE, Ilousis D, Kröger K, Santosa F, Kowall B, Stang A. Increasing use of anticoagulants in Germany and its impact on hospitalization for gastrointestinal bleeding. Thromb Res. 2019;181:135–140. doi:10.1016/j.thromres.2019.07.009
21. Kelasto statistical database [homepage on the Internet]. Reimbursements of medicine expenses: number of recipients and prescription data. The Social Insurance Institution of Finland, 2021. Available from: https://www.kela.fi/kelasto.
22. López-López JA, Sterne JAC, Thom HHZ, et al. Oral anticoagulants for prevention of stroke in atrial fibrillation: systematic review, network meta-analysis, and cost effectiveness analysis. BMJ. 2017;359:j5058. doi:10.1136/bmj.j5058
23. Harrington AR, Armstrong EP, Nolan PE
24. Thom HHZ, Hollingworth W, Sofat R, et al. Directly acting oral anticoagulants for the prevention of stroke in atrial fibrillation in England and Wales: cost-effectiveness model and value of information analysis. MDM Policy Pract. 2019;4(2):2381468319866828.
25. Wisløff T, Hagen G, Klemp M. Economic evaluation of warfarin, dabigatran, rivaroxaban, and apixaban for stroke prevention in atrial fibrillation. Pharmacoeconomics. 2014;32(6):601–612. doi:10.1007/s40273-014-0152-z
26. de Jong LA, Groeneveld J, Stevanovic J, et al. Cost-effectiveness of apixaban compared to other anticoagulants in patients with atrial fibrillation in the real-world and trial settings. PLoS One. 2019;14(9):e0222658. doi:10.1371/journal.pone.0222658
27. Rannanheimo P, Hyvärinen A, Kiviniemi V. Atetsolitsumabi yhdessä nab-paklitakselin kanssa kolmoisnegatiivisen rintasyövän hoidossa [Atezolizumab in combination with nab-paclitaxel in the treatment of triple-negative breast cancer]. Finnish Medicines Agency Fimea. Fimea kehittää, arvioi ja informoi -julkaisusarja 9/2019. ISBN 978-952-7299-07-4. Finnish.
28. Wikman E, Rannanheimo P Isatuksimabi yhdessä pomalidomidin ja deksametasonin kanssa uusiutuneen multippelin myelooman hoidossa [Isatuximab together with pomalidomide and dexamethasone in the treatment of relapsed multiple myeloma]. Finnish Medicines Agency Fimea. Fimea kehittää, arvioi ja informoi -julkaisusarja 3/2020. ISBN 978-952-7299-10-4. Finnish.
29. Joensuu JT, Huoponen S, Aaltonen KJ, Konttinen YT, Nordström D, Blom M. The cost-effectiveness of biologics for the treatment of rheumatoid arthritis: a systematic review. PLoS One. 2015;10(3):e0119683. doi:10.1371/journal.pone.0119683
30. National Institute for Health and Care Excellence. Guide to the methods of technology appraisal 2013. Process and methods [PMG9]; 2013. Available from: https://www.nice.org.uk/article/PMG9/chapter/Foreword.
31. Linna M, Keto J, Piuhola J, Vesalainen R, Hällberg V, Laine J. Eteisvärinäpotilaan sosiaali- ja terveydenhuoltopalvelujen käyttö komplikaation jälkeen [Use of social and health care services by patients with complications of atrial fibrillation]. Suom Laakaril. 2017;35:1856–1862. Finnish.
32. Penttilä T, Lehto M, Niiranen J, et al. Differences in the risk of stroke, bleeding events, and mortality between female and male patients with atrial fibrillation during warfarin therapy. Eur Heart J Cardiovasc Pharmacother. 2019;5(1):29–36. doi:10.1093/ehjcvp/pvy026
33. Raatikainen MJP, Penttilä T, Korhonen P, Mehtälä J, Lassila R, Lehto M. The quality of warfarin therapy and CHA2DS2-VASc score associate with the incidence of myocardial infarction and cardiovascular outcome in patients with atrial fibrillation: data from the nationwide FinWAF registry. Eur Heart J Cardiovasc Pharmacother. 2018;4(4):211–219. doi:10.1093/ehjcvp/pvy009
34. Lip GY, Mitchell SA, Liu X, et al. Relative efficacy and safety of non-vitamin K oral anticoagulants for non-valvular atrial fibrillation: network meta-analysis comparing apixaban, dabigatran, rivaroxaban and edoxaban in three patient subgroups. Int J Cardiol. 2016;204:88–94. doi:10.1016/j.ijcard.2015.11.084
35. Kapiainen S, Väisänen A, Haula T. Terveyden- ja sosiaalihuollon yksikkökustannuksen Suomessa vuonna 2011 [Health and social care unit costs in Finland in 2011]. THL Raportti 3/2014. Juvenes Print, Suomen Yliopistopaino Oy. Tampere. Finnish.
36. T12 laboratoriotoimialue hinnasto [Tariffs for the laboratory division]. Turku: Hospital District of Southwest Finland; 2021. Finnish.
37. Official public tariff of reimbursable authorized medicinal products in Finland [homepage on the Internet]. Helsinki: Pharmaceuticals Pricing Board; 2021. Available from: https://www.hila.fi/luettelot/korvattavat-myyntiluvalliset-laakevalmisteet/.
38. Official Statistics of Finland (OSF). Price index of public expenditure [e-publication]. Helsinki: statistics Finland. Available from: http://www.stat.fi/til/jmhi/index_en.html.
39. Lassila R. Suorat oraaliset antikoagulantit [Direct oral anticoagulants]. Lääkärin käsikirja; [updated January 8, 2021]. Available from: https://www.terveysportti.fi/apps/ltk/article/ykt01922/search/suorat%20oraaliset%20antikoagulantit.
40. Lääkkeiden hintalautakunta. Preparing a health economic evaluation to be attached to the application for reimbursement status and wholesale price for a medicinal product. Application instructions, health economic evaluation; December 17, 2019. Available from: https://www.hila.fi/content/uploads/2020/01/Instructions_TTS_2019.pdf.
41. Lehto M, Niiranen J, Korhonen P, et al. Quality of warfarin therapy and risk of stroke, bleeding, and mortality among patients with atrial fibrillation: results from the nationwide FinWAF registry. Pharmacoepidemiol Drug Saf. 2017;26(6):657–665. doi:10.1002/pds.4194
42. Hallinen T, Soini E. The impact of the pharmaceutical pricing system on cost-effectiveness results: finnish analysis. Open Pharmacoeconomics Health Econ J. 2011;3:6–10. doi:10.2174/1876824501103010006
43. Official Statistics of Finland (OSF): Deaths [e-publication]. ISSN=1798-2545. Helsinki: statistics Finland; referred 26 May 2021. Available from: http://www.stat.fi/til/kuol/meta_en.html.
44. Benjamin EJ, Wolf PA, D’Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98(10):946–952. doi:10.1161/01.CIR.98.10.946
45. Zhu J, Alexander GC, Nazarian S, Segal JB, Wu AW. Trends and variation in oral anticoagulant choice in patients with atrial fibrillation, 2010–2017. Pharmacotherapy. 2018;38(9):907–920. doi:10.1002/phar.2158
46. Gurusamy VK, Brobert G, Vora P, Friberg L. Sociodemographic factors and choice of oral anticoagulant in patients with non-valvular atrial fibrillation in Sweden: a population-based cross-sectional study using data from national registers. BMC Cardiovasc Disord. 2019;19(1):43. doi:10.1186/s12872-019-1029-z
47. Leminen A, Pyykönen M, Tynkkynen J, Tykkyläinen M, Laatikainen T. Modeling patients’ time, travel, and monitoring costs in anticoagulation management: societal savings achievable with the shift from warfarin to direct oral anticoagulants. BMC Health Serv Res. 2019;19(1):901. doi:10.1186/s12913-019-4711-z
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

