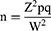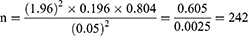Back to Journals » Infection and Drug Resistance » Volume 14
Methicillin and Vancomycin Resistant Staphylococcus aureus and Associated Factors from Surgical Ward Inpatients at Debre Markos Referral Hospital, Northwest Ethiopia
Authors Tefera S, Awoke T , Mekonnen D
Received 11 June 2021
Accepted for publication 26 July 2021
Published 10 August 2021 Volume 2021:14 Pages 3053—3062
DOI https://doi.org/10.2147/IDR.S324042
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Samrawit Tefera,1,2 Tewachew Awoke,1 Daniel Mekonnen1,3
1Department of Medical Laboratory Sciences, College of Medicine and Health Sciences, Bahir Dar University, Bahir Dar, Ethiopia; 2Department of Medical Laboratory, Debre Markos Referral Hospital, Debere Markos, Ethiopia; 3Department of Health Biotechnology, Biotechnology Research Institute, Bahir Dar University, Bahir Dar, Ethiopia
Correspondence: Daniel Mekonnen
Department of Medical Laboratory Sciences, College of Medicine and Health Sciences, Bahir Dar University, Bahir Dar, Ethiopia
Tel +251912990288
Email [email protected]
Introduction: Staphylococcus aureus is a well-known superbug and leading causes of wound infections. The clinical epidemiology of methicillin resistant S. aureus (MRSA) and vancomycin resistant S. aureus (VRSA) is not well documented in Ethiopia. The aim of this study was to determine the proportion of MRSA, VRSA and associated factors from surgical inpatients in Debre Markos Referral Hospital (DMRH), Northwest Ethiopia.
Methods: A facility-based cross-sectional study was conducted from February to April 2020 at DMRH. A structured questionnaire was used to gather demographic and clinical data. Wound swab was collected from inpatients and then inoculated on blood agar and mannitol salt agar. The presence of MRSA and VRSA was determined using the cefoxitin (30 μg) antibiotic disk diffusion and vancomycin E-test methods, respectively. The data were analyzed using SPSS 20. Data were analyzed using bivariate and multivariate logistic regression analysis. A P-value < 0.05 was considered statistically significant.
Results: A total of 242 wound cases were enrolled and the majority of them were males 172 (71.1%). Among the total enrolled cases, S. aureus was identified from 71 (29.3%) of the admitted patients. The proportion of MRSA was 32 (13.22%) and that of VRSA was 4.1%. The proportion of vancomycin intermediate S. aureus (VISA) was gauged at 4.5%. Hospital stay over 72 hrs, wound depth, current antibiotic use, and previous history of wound infection showed statistically significant association with MRSA. On the contrary, VRSA did not showed any significant association against the analyzed variables.
Conclusion: High proportions of S. aureus isolates became MRSA; resistant to all β-lactam antimicrobial agents excluding newer cephalosporin. In addition, the proportion of VRSA/VISA was also high. Multiple variables demonstrated significant associations with MRSA. Hence, intervention measures for MRSA risk groups must be in place. Furthermore, hospital infection control and an antibiotic stewardship program should be strengthened.
Keywords: Nosocomial infection, surgical inpatients, MRSA, VRSA, Debre Markos Referral Hospital, Ethiopia
Introduction
Staphylococcus aureus is a notorious pathogen that causes a broad spectrum of clinical conditions. To mention some, it is a leading cause of bacteremia, infective endocarditis, osteoarticular, skin and soft tissue infections (SSTI) and device-related infections.1 Some of the common device-related infections due to S. aureus include urinary catheter-related infection and infection of intravascular devices.2 S. aureus is a cause of both nosocomial and community-acquired infections.3
Methicillin resistant S. aureus (MRSA) is S. aureus that is resistant to early generations of penicillin and penicillinase resistant penicillin (methicillin, oxacillin, cloxacillin, dicloxacillin, nafcillin) antimicrobial agents. Methicillin resistance in Staphylococci is due to conjugated staphylococcal cassette chromosome mec (SCCmec) gene. All SCCmec types contained the mecA gene, which codes for the low-affinity penicillin binding protein 2a (PBP2a).4 MRSA is the predominant S. aureus isolated from infected wounds.1
The current treatment options for MRSA infections include linezolid,5 vancomycin,6 trimethoprim-sulfamethoxazole and doxycycline.7 However, most MRSA strains are evolutionary competent and becoming resistant to several classes of antibiotics including those mentioned above. Hence, S. aureus isolated from wound infections of hospitalized patients are refractory to antibiotic treatment due to biofilms and the presence of persister.4
Beginning the late 1980s, vancomycin became the antibiotic of choice for treatment for patients infected with MRSA in hospital settings.8 However, after a few years of marketing, vancomycin-resistant S. aureus strains emerged from Japan. Then, five years later after the Japanese report, vancomycin-resistant S. aureus (VRSA) strains were reported in the USA.9 S. aureus acquire vancomycin resistance plasmid gene from vancomycin-resistant Enterococcus (VRE) through transposon Tn1546.10 Linezolid, tigecycline, daptomycin, quinupristin, dalfopristin, ceftobiprole, iclaprim, and novel glycopeptides like the dalbavancin, telavancin, and oritavancin are a few choices of antibiotics to treat infections caused by VRSA isolates.11
Globally, the prevalence of VRSA varies from 9.8% to 52.4%.12,13 A systematic review and meta-analysis summarized the prevalence of MRSA and VRSA among S. aureus isolates in Ethiopia. Accordingly, the prevalence of MRSA was extremely variable and ranged from 8.3% to 77.3% (with pooled prevalence of 32.5%).14 Similarly, the prevalence of VRSA ranged from 5.1% to 44.3%.11 There was a previous report regarding the prevalence of MRSA in DMRH;15 however, this study was distant past (eight years ago). The epidemiology of MRSA and VRSA is currently unknown in the study setting. Hence, the objective of the study was to determine the proportion of MRSA and VRSA and associated factors from inpatients at the surgical ward in Debre Markos Referral Hospital (DMRH), Northwest Ethiopia.
Materials and Methods
Study Setting and Context
A facility-based cross-sectional study was conducted among wounded inpatients admitted to the surgical ward at DMRH, Debre Markos, Ethiopia from February to April 2020. The referral hospital provides comprehensive medical services, such as inpatient and outpatient treatment for people coming from the surrounding zones and nearby regional states. Based on our observation, the infection prevention and antibiotic stewardship program is very weak in the study setting. In addition, there is a practice of buying any antibiotics from a pharmacy without prescription. The adherence of people to treatment is very poor due to lack of awareness about drug resistance and related consequences. The choice of antibiotics is neither based on patient's laboratory report nor based on local epidemiological data, rather it is based on best/common practices. Collectively, these practices might be some factors leading to the emergence of drug resistance in the study area.
Variables
While the dependent variables include MRSA and VRSA infection, the independent variables include, age, sex, type of wound (chronic, burn, nonhealing ulcer, operation, trauma, diabetic foot ulcers, and pressure ulcers), length of hospital stay, smoking, recent traditional medical treatment, occupation, HIV, use of antibiotic, duration of operation, types of operation, depth of wound, degree of contamination (clean, contaminated, semi-contaminated, dirty), previous history of hospitalization, and site of infection.
Population and Eligibility
Patients admitted to the surgical ward of DMRH due to wound infection were the study population. Surgical inpatients with active trauma were excluded. Patients who were on treatment with glycopeptides, such as vancomycin were excluded. In addition, inpatients whose wounds were on healing excluded from study.
Sample Size and Sampling Technique
The sample size was calculated based on single population proportion formula by taking a prevalence of 19.6% MRSA among inpatients in DMRH.15 Margin of error was set to 5% and 95% confidence interval (alpha=0.05).
Where: n=sample size; p=prevalence of MRSA among surgical inpatients(19.6%); q=(1–p); z= 1.96, critical value; W=0.05, precision (margin of error)
Thus setting these value in to the formula, the sample size become 242;
Participants were enrolled consecutively until the required sample size was achieved.
Specimen Collection and Identification
A wound swab was collected using cotton-topped sterile applicator stick. After obtaining the specimen, it was inoculated on a blood agar plate (BAP) and mannitol salt agar (MSA) and then incubated at 35°C for 24 hrs. Presence of bacterial growth was identified using Gram staining followed by catalase and slide/tube coagulase test. Gram-positive cocci, catalase positive, slide/tube coagulase positive and golden yellow colony on MSA was considered as confirmatory test for S. aureus.16
Antimicrobial Susceptibility Testing
From the pure and fresh S. aureus growth, 0.5 McFarland suspensions were prepared using sterile normal saline. Then, the suspension was inoculated on Mueller–Hinton agar (MHA) for modified Kirby–Bauer disk-diffusion susceptibility analysis. The antimicrobial susceptibility test (AST) was carried out following the clinical and laboratory standard institute (CLSI) protocol.17 The MRSA was identified using 30 µg cefoxitin disk diffusion test; which is a surrogate test for oxacillin resistance. After incubation at 35°C for 24 hrs, results were interpreted using CLSI guidelines. Zone diameter of ≤24 mm is a breakpoint for cefoxitin resistance.17 Evidence showed that, MRSA is a requisite for VRSA/VISA.18,19 Hence, we screened VRSA/VISA from MRSA cases only; however, the calculation is among all the enrolled population. Hence, a pure colony of MRSA isolates were taken and inoculated on MHA to look for VRSA. A vancomycin strip of E-test was gripped with a pair of sterile forceps, and placed onto the inoculated agar surface. After incubation at 35–37°C for 18 hrs, the breakpoints of minimum inhibitory concentrations (MIC) were measured. The vancomycin broth MIC ≤2 μg/mL is considered sensitive, 2–4 µg/mL MIC is taken as intermediate and vancomycin broth MIC ≥16 μg/mL is considered resistant to vancomycin.17,20
Quality Assurance
Each wound swab sample was processed based on recommended standard laboratory procedures by strictly following pre-analytical, analytical and postanalytical stages of quality assurance techniques that are incorporated in standard operating procedures (SOPs) of the microbiology laboratory unit. For instance, culture media were prepared aseptically by autoclaving at 121°C for 15 min and; 5% of batch-prepared media were checked for sterility by overnight incubation. In addition, the performance of BAP and MSA were checked for growth of known S. aureus ATCC25923. The performance of the antibiotic disks were evaluated using American Type cell culture (ATCC) controls. As such, S. aureus A TCC25923 (cefoxitin zone 23–29 mm) and S. aureus ATCC 43300 (zone ≤21 mm) were used as control strains to determine the performance of cefoxitin disk diffusion test for MRSA. S. aureus ATCC 29213 MIC of vancomycin broth value 0.5–2.0 µg/mL was used as a control strain to measure the performance of vancomycin.17
Data Analysis
Data were cleaned, double entered and analyzed using Statistical Package for Social Science, version 20 software. To assess the presence of any correlation among independent variables, we did multicollinearity analysis using linear regression analysis. Then, bivariate logistic regression was carried out and variables with a P-value of less than 0.2 were entered in to multivariate logistic regression analysis. A P-value of <0.05 was considered as statistically significant.
Ethical Considerations
The study was approved by Institutional Review Board of Bahir Dar University and a support letter was written to DMRH. Then, the DMRH medical director forwarded the letter to the surgical inpatient team for possible support. Written informed consent was obtained from each participant. For participants under 18 years of age, a parent or legal guardian of the patient provided informed consent and additional assent was also obtained from children between seven and 18 years of age. The purpose and importance of the study was explained to the participants. In addition, absence of link between the study and their surgical service was explained. Participation was entirely voluntary. The confidentiality of study participants were kept and identification codes were deidentified during analysis. In general, this study was conducted in accordance with the Declaration of Helsinki.
Results
Sociodemographic and Clinical Characteristics of Participants
A total of 242 study participants were enrolled. Of which, the majority 172 (71.1%) were males, with a male to female ratio of 1:0.41. The ages of participants ranged from 1–84 years with a mean age of 33 years and median of 30 years. Ninety three (38.4%) of study participants were in the age group of 15–30 years. The majority, 171 (70.7%) were rural dwellers. One hundred thirty nine (57.4%) of samples were collected from traumatized wound (Table 1).
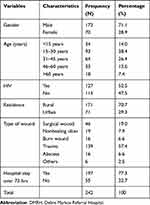 |
Table 1 Sociodemographic and Clinical Characteristics of Participants, DMRH, February to April 2020 |
Proportion of S. aureus, MRSA and VRSA
Among 242 inpatients, S. aureus was isolated from 71 (29.3%) of surgical wound sites. Then, a cefoxitin disk diffusion test, which is a surrogate marker for oxacillin and other penicillinase resistance penicillin, was carried out to determine the proportion of MRSA. The proportion of MRSA became 32/242 (13.2%) and it was gauged at 45.1% when considering S. aureus isolates only. Similarly, the proportion of VRSA was 10/242 (4.1%) and the proportion of vancomycin intermediate S. aureus (VISA) became 11/242 (4.5%). Zooming in, the proportion of VRSA among S. aureus and MRSA isolates were 10/71 (14.1%) and 10/32 (31.25%), respectively (Table 2). Taken together, the proportion of S. aureus, MRSA, VRSA, VISA among the surgical inpatients were 29.3%, 13.2% and 4.1% and 4.5%, respectively.
 |
Table 2 Antibiotic Resistance Pattern of S. aureus Isolates Among Surgical Inpatients at DMRH February to April 2020 (participants=242, S. aureus=71) |
As shown in Figure 1 equal numbers, 16 (6.6%) of MRSA isolates were recovered from both male and female participants. A relatively higher proportion of MRSA 14 (5.9%) were isolated from the age groups of 15–30 years. On the contrary, a relatively, high proportion of VRSA isolates were identified among the age group of 31–45 years, 6 (2.48%). Eighteen (7.44%) MRSA and 7 (2.9%) VRSA isolates were recovered from traumatic wound types. MRSA and VRSA were more frequently isolated among inpatients with deep rather than superficial wounds.
Factors Associated with MRSA and VRSA
Before analyzing the link between dependent and independent variables, we assessed the presence of any positive or negative correlation among independent variables using linear regression model. The tolerance results were between 0.8 and 0.9, variance inflation factors were between 1 and 1.2 and collinearity diagnostic was <0.5. These measures confirmed apparent absence of multicollinearity among independent variables.
Sociodemographic characteristics of study participants were assessed for possible association with MRSA colonization. Gender showed association with MRSA in bivariate analysis (P=0.005). Being a rural resident was found to have a higher number of MRSA isolates compared to an urban resident, 28.2% vs 16.9%. However, the association between residency and infection with MRSA was not statistically significant. Taken together, there was no significantly associated sociodemographic factors with MRSA (Table 3).
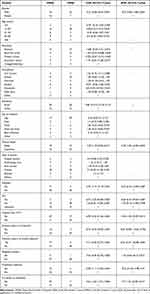 |
Table 3 Clinical and Sociodemographic Analysis for Identification MRSA Predictors from Surgical Inpatients in DMRH, February to April 2020, (N=71) |
Different clinical conditions were assessed for identification of association with MRSA infection. As such, depth of wound (P=0.017), HIV (P=0.027) and diabetes (P=0.031), previous history of antibiotic use (P=0.001), currently being on antibiotics (P=0.004), and traditional medicine use (P=0.034) showed statistically significant association in the bivariate logistic regression analysis (Table 3). To avoid the confounding effect, all variables with P-value of less than 0.2 were subjected to multivariate logistic regression analysis. The output showed that, hospital stay >72 hrs (P=0.014, AOR: 7.402, 95%CI: 1.502–39.49), previous history of wound infection (P=0.001, AOR: 24.33, 95%CI: 3.77–156.89), currently being on antibiotics (P=0.017; AOR: 7.9, 95%CI: 1.44–43.39), and depth of wound (P=0.043, AOR: 4.38, 95%’ CI: 1.049–18.29) still exhibited significant association with MRSA (Table 3).
Similarly, we did both bivariate and multivariate analysis to find any variable that might be a predictor for VRSA. In the initial bivariate analysis deep site wound cases (P=0.036; COR: 10.8, 95%CI: 1.16–100.43) and malignancy (P=0.029; COR: 4, 95%CI: 1.306–150.019) revealed an alliance with VRSA. However, the 95%CI was very wide indicating that the sample size was too small and the result is imprecise for drawing inferential statistics. In addition, similar results were not obtained in the multivariate analysis (table not shown).
Discussion
S. aureus is a cause of community and hospital acquired infections with high mortality rate. Although it is part of normal human flora, it can cause a wide range of diseases, ranging from relatively mild skin infections to serious systemic diseases. Many of these infections can rapidly become life-threatening unless managed promptly.3
In this study, 242 wound swab samples were processed and the proportion of S. aureus was 29.3%. The proportion of MRSA among the isolates were 45.1% which is above the national pooled prevalence estimate of 32.5%.14 The present finding was comparable to a systematic review and meta-analysis done in Ethiopia in which the pooled prevalence of MRSA was 47%.21 Similar studies22–24 also reported a high proportion of MRSA in different parts of the country indicating that MRSA is becoming a superbug with limited choice of antimicrobial agents in Ethiopia. Moreover, MRSA is an international health challenge as revealed by the high rate of drug resistance reports in different countries such as 72% in Eritrea,25 21% in Turkey,26 82.3% in the Gaza Strip,27 77.9% in Iran27 and 76% tertiary care hospital in Lahore.12
From the total isolate, 10 (14.1%) were VRSA and this figure was in line with a review from Ethiopia where the pooled prevalence of VRSA was 11% (95%CI: 4–20).21 This finding was in conflict with studies elsewhere, 29.4%28 and 21.1%.29 The present VRSA report is in line with studies done elsewhere.12,21,30 Based on a large meta-analysis, the pooled prevalence of VRSA in Asia, Europe, America, and Africa was 1.2%, 1.1%, 3.6% and 2.5%, respectively with high heterogeneity among studies.31
Decreased susceptibility of S. aureus to vancomycin has been reported elsewhere.32 In the present study with cutoff value of MIC of vancomycin broth ≥4 µg/mL E-test; the proportion VRSA and VISA combined reached to 29.6%. With this cutoff value, our finding is much lower compared with reports from other countries.12,30,33
Previous studies suggested that patients with intravenous drug use or cirrhosis were at higher risk for MRSA and VRSA carriage.34 Deep wound infection (P=0.043), previous wound infection (P=0.006), and hospital stay >72 hrs (P=0.001) demonstrated an association with MRSA infection. Unlike our study, a report by Shariati et al (2020) found an association between several variables (hemodialysis dependency, long-term use of vancomycin, hospitalization in ICU, and use of indwelling devices) and VRSA.31 Apparent absence of association between variables and VRSA in the present study might be related with the small sample size used in the present study.
Conclusions
The proportion of MRSA and VRSA were higher in the present study. In addition, multiple variables demonstrated significant association with MRSA; such as previous wound infection, long hospital stay, currently on antibiotics, and long-standing deep chronic wounds. Therefore, prompt prevention and control measures must be in place for MRSA high risk population. Strict adherence to infection prevention methods must be in place. Moreover, periodic surveillance and antibiotics stewardship program should be in place for generating vital AST information which will be used for empirical treatment and further preventions strategy. Further (molecular) epidemiological studies are desirable for understanding the true depth and breadth of MRSA and VRSA problems.
Abbreviations
BDU, Bahir Dar University; CLSI, Clinical and Laboratory Standards Institute; DMRH, Debre Markos Referral Hospital; MHA, Muller–Hinton agar; MIC, minimum inhibitory concentration; MRSA, methicillin resistant S. aureus; MSSA, methicillin sensitive S. aureus; SCCmec, staphylococcal cassette chromosome mec; VISA, vancomycin intermediate S. aureus; VRSA, vancomycin resistant S. aureus; VSSA, vancomycin sensitive S. aureus.
Data Sharing Statement
Almost all data generated and analyzed during in this study were included in the manuscript. But if the spreadsheet dataset is needed, it will be shared upon request by the editor from the corresponding author.
Ethics Approval and Consent to Participate
The study was approved by research and ethical review committee of Department of Medical Laboratory Sciences of BDU. Informed written consent was obtained from each participant before data collection. A parent or legal guardian of the patients under 18 years of age provided informed consent, and this study was conducted in accordance with the Declaration of Helsinki. All the information obtained from the study subjects were coded to maintain confidentially.
Acknowledgments
Authors express deep appreciation to Amhara National Regional Health Bureau, Bahir Dar University, College of Medicine and Health Sciences, Department of Medical Laboratory Sciences and Debre Markos Referral Hospital. Lastly, our countless acknowledgements goes to the study participants.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research received student research grant from Amhara Regional state Health Bureau and Bahir Dar University.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Percival SL, Suleman L, Vuotto C, Donelli G. Healthcare-associated infections, medical devices and biofilms: risk, tolerance and control. J Med Microbiol. 2015;64(4):323–334. doi:10.1099/jmm.0.000032
2. Crnich CJ, Drinka P. Medical device-associated infections in the long-term care setting. Infect Dis Clin. 2012;26(1):143–164.
3. Bishara J, Goldberg E, Leibovici L, et al. Healthcare-associated vs hospital-acquired Staphylococcus aureus bacteremia. Int J Infect Dis. 2012;16(6):e457–e63.
4. DeLeo FR, Otto M, Kreiswirth BN, Chambers HF. Community-associated meticillin-resistant Staphylococcus aureus. Lancet. 2010;375(9725):1557–1568. doi:10.1016/S0140-6736(09)61999-1
5. Weigelt J, Kaafarani HM, Itani KM, Swanson RN. Linezolid eradicates MRSA better than vancomycin from surgical-site infections. Am J Surg. 2004;188(6):760–766. doi:10.1016/j.amjsurg.2004.08.045
6. Wilcox M, Al-Obeid S, Gales A, et al. Reporting elevated vancomycin minimum inhibitory concentration in methicillin-resistant Staphylococcus aureus: consensus by an International Working Group. Future Microbiol. 2019;14(4):345–352. doi:10.2217/fmb-2018-0346
7. Lambert M. IDSA guidelines on the treatment of MRSA infections in adults and children. Am FAM Physician. 2011;84(4):455–463.
8. Tomasz A. The staphylococcal cell wall. In: Vincent A. Fischetti, Richard P. Novick, Joseph J. Ferretti, Daniel A. Portnoy, Julian I. Rood, editors. Gram-Positive Pathogens. New York: Wiley Online Library; 2006;443–455. doi:10.1128/9781555816513.ch36
9. D’Agata EM, Webb GF, Horn MA, Moellering RC, Ruan S. Modeling the invasion of community-acquired methicillin-resistant Staphylococcus aureus into hospitals. Clin Infect Dis. 2009;48(3):274–284. doi:10.1086/595844
10. Zhu W, Murray PR, Huskins WC, et al. Dissemination of an Enterococcus Inc18-Like vanA plasmid associated with vancomycin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2010;54(10):4314–4320. doi:10.1128/AAC.00185-10
11. Anagaw B, Shiferaw Y, Anagaw B, et al. Frequency of methicillin-resistant Staphylococcus aureus isolates from clinical specimens in Gondar University Hospital, Northwest Ethiopia. Asian J Med Sci. 2013;5(3):59–64. doi:10.19026/ajms.5.5343
12. Liaqat F, Sheikh AA, Nazir J, et al. Isolation identification and control of vancomycin resistant Staphylococcus aureus. Pak J Pharm Sci. 2015;28(3):997–1004.
13. Alani HA, Hassawi DS, Flayih MT. Patterns of antibiotic resistance in Staphylococcus aureus isolates and detection the heteroresistance to vancomycin by population analysis method. JUAPS. 2017;11(3):26–33.
14. Eshetie S, Tarekegn F, Moges F, et al. Methicillin resistant Staphylococcus aureus in Ethiopia: a meta-analysis. BMC Infect Dis. 2016;16(1):689. doi:10.1186/s12879-016-2014-0
15. Kahsay A, Mihret A, Abebe T, Andualem T. Isolation and antimicrobial susceptibility pattern of Staphylococcus aureus in patients with surgical site infection at Debre Markos Referral Hospital, Amhara Region, Ethiopia. Arch Public Health. 2014;72(1):16. doi:10.1186/2049-3258-72-16
16. Karmakar A, Dua P, Ghosh C. Biochemical and molecular analysis of Staphylococcus aureus clinical isolates from hospitalized patients. Can J Infect Dis Med Microbiol. 2016;2016. doi:10.1155/2016/9041636
17. Kejela T, Bacha K. Prevalence and antibiotic susceptibility pattern of methicillin-resistant Staphylococcus aureus (MRSA) among primary school children and prisoners in Jimma Town, Southwest Ethiopia. Ann Clin Microbiol Antimicrob. 2013;12(1):11. doi:10.1186/1476-0711-12-11
18. Thati V, Shivannavar CT, Gaddad SM. Vancomycin resistance among methicillin resistant Staphylococcus aureus isolates from intensive care units of tertiary care hospitals in Hyderabad. Indian J Med Res. 2011;134(5):704. doi:10.4103/0971-5916.91001
19. Centers for Disease Control and Prevention. Laboratory detection of vancomycin-intermediate/resistant Staphylococcus aureus (VISA/VRSA). USA: CDC; 2010.
20. Clinical and Laboratory Standards Institute. Performance standard for antimicrobial susceptibility testing. M100ED29. USA: CLIS; 2019.
21. Hasan R, Acharjee M, Noor R. Prevalence of vancomycin resistant Staphylococcus aureus (VRSA) in methicillin resistant S. aureus (MRSA) strains isolated from burn wound infections. Tzu Chi Med J. 2016;28(2):49–53. doi:10.1016/j.tcmj.2016.03.002
22. Dilnessa T, Bitew A. Antimicrobial susceptibility pattern of Staphylococcus aureus with emphasize on methicilin resistance with patients postoperative and wound infections at Yekatit 12 Hospital Medical College in Ethiopia. Adv Clin Exp Med. 2016;4(1):7–12. doi:10.11648/j.ajcem.20160401.12
23. Mama M, Aklilu A, Misgna K, Tadesse M, Alemayehu E. Methicillin- and inducible clindamycin-resistant Staphylococcus aureus among patients with wound infection attending Arba Minch Hospital, South Ethiopia. Int J Microbiol. 2019;2019. doi:10.1155/2019/2965490
24. Tadesse S, Alemayehu H, Tenna A, et al. Antimicrobial resistance profile of Staphylococcus aureus isolated from patients with infection at Tikur Anbessa Specialized Hospital, Addis Ababa, Ethiopia. BMC Pharmacol Toxicol. 2018;19(1):24. doi:10.1186/s40360-018-0210-9
25. Garoy EY, Gebreab YB, Achila OO, et al. Methicillin-resistant Staphylococcus aureus MRSA): prevalence and antimicrobial sensitivity pattern among patients—a multicenter study in Asmara, Eritrea. Can J Infect Dis Med Microbiol. 2019;2019. doi:10.1155/2019/8321834
26. Rağbetli C, Parlak M, Bayram Y, Guducuoglu H, Ceylan N. Evaluation of antimicrobial resistance in Staphylococcus aureus isolates by years. Interdiscip Perspect Infect Dis. 2016;2016. doi:10.1155/2016/9171395
27. Emaneini M, Beigverdi R, van Leeuwen WB, et al. Prevalence of methicillin-resistant Staphylococcus aureus isolated from burn patients in Iran: a systematic review and meta-analysis. J Glob Antimicrob Resist. 2018;12:202–206. doi:10.1016/j.jgar.2017.10.015
28. Dilnessa T, Bitew A. Prevalence and antimicrobial susceptibility pattern of methicillin resistant Staphylococcus aureus isolated from clinical samples at Yekatit 12 Hospital Medical College, Addis Ababa, Ethiopia. BMC Infect Dis. 2016;16(1):398. doi:10.1186/s12879-016-1742-5
29. Godebo G, Kibru G, Tassew H. Multidrug-resistant bacterial isolates in infected wounds at Jimma University Specialized Hospital, Ethiopia. Ann Clin Microbiol Antimicrob. 2013;12(1):17. doi:10.1186/1476-0711-12-17
30. Arshad F, Javed I, Mushtaq S, Anwer S. Emergence of vancomycin intermediate Staphylococcus aureus (VISA) in a Tertiary Care Hospital in Lahore: an alarming situation. Intern J Pathol. 2015;13(3):89–94.
31. Shariati A, Dadashi M, Moghadam MT, et al. Global prevalence and distribution of vancomycin resistant, vancomycin intermediate and heterogeneously vancomycin intermediate Staphylococcus aureus clinical isolates: a systematic review and meta-analysis. Sci Rep. 2020;10(1):1–16.
32. Shibabaw A, Abebe T, Mihret A. Nasal carriage rate of methicillin resistant Staphylococcus aureus among Dessie Referral Hospital health care workers; Dessie, Northeast Ethiopia. Antimicrob Resist Infect Control. 2013;2(1):25. doi:10.1186/2047-2994-2-25
33. Kelley PG, Gao W, Ward PB, Howden BP. Daptomycin non-susceptibility in vancomycin-intermediate Staphylococcus aureus (VISA) and heterogeneous-VISA (hVISA): implications for therapy after vancomycin treatment failure. J Antimicrob Chemother. 2011;66(5):1057–1060. doi:10.1093/jac/dkr066
34. Stacey HJ, Clements CS, Welburn SC, Jones JD. The prevalence of methicillin-resistant Staphylococcus aureus among diabetic patients: a meta-analysis. Acta Diabetol. 2019;56:907–921.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.