Back to Journals » Infection and Drug Resistance » Volume 13
Oral Prevalence of Candida Species in Patients Undergoing Systemic Glucocorticoid Therapy and the Antifungal Sensitivity of the Isolates
Authors Xiao J, Xu G, de Hoog S , Qiao J, Fang H, Li Y
Received 14 May 2020
Accepted for publication 16 July 2020
Published 29 July 2020 Volume 2020:13 Pages 2601—2607
DOI https://doi.org/10.2147/IDR.S262311
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Jia-ling Xiao,1 Guo-chao Xu,1 Sybren de Hoog,2 Jian-jun Qiao,3 Hong Fang,3 Ya-li Li3
1Department of Stomatology, Zhejiang Hospital, Hangzhou, Zhejiang Province, People’s Republic of China; 2Center of Expertise in Mycology of Radboud University Medical Center/Canisius Wilhelmina Hospital, Nijmegen, The Netherlands; 3Department of Dermatology, The First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou, Zhejiang Province, People’s Republic of China
Correspondence: Ya-li Li
Department of Dermatology, The First Affiliated Hospital, College of Medicine, Zhejiang University, 79# Qingchun Road, Shangcheng District, Hangzhou 310003, People’s Republic of China
Tel +86-571-87235857
Email [email protected]
Background: Candida species are commonly detected as colonizers of the oral cavity; candidiasis or candidemia can develop in patients who are immunocompromised. Use of topical or inhaled glucocorticoids can alter the spectrum of Candida species and can promote oral candidiasis. The present study aims to evaluate the diversity of Candida species in the oral cavity and their susceptibility to antifungal agents in patients undergoing treatment with systemic glucocorticoids (SGCs) compared with non-users.
Methods: We conducted a descriptive, analytical, cross-sectional study that enrolled 120 patients with oral problems who were undergoing treatment with SGCs and who were admitted to the hospital of the First Affiliated Hospital, College of Medicine, Zhejiang University and Zhejiang Hospital, Hangzhou, China, between February 2019 and September 2019. One hundred and twenty age-and sex-matched patients were recruited as the SGC non-user control group. Demographic data included oral complaints and underlying diseases; symptoms of oral candidiasis were identified on physical examination. Candida species were collected using a concentrated oral rinse. Identification of fungal isolates was based on conventional phenotypic methods assisted by DNA sequence analysis of the internal transcribed spacer (ITS) rDNA gene region. Antifungal activities of anidulafungin, amphotericin B, micafungin, caspofungin, 5-flucytosine, posaconazole, voriconazole, itraconazole, and fluconazole were evaluated using the Sensititre YeastOneTM YO10 panel supplemented by the CLSI-M27-A3 protocol.
Results: Fifty-two (43.33%) out of the 120 patients undergoing with SGCs were diagnosed with oral candidiasis, compared with 14 (11.67%) of the non-users (P < 0.05). Likewise, we collected 88 strains from 73.33% of the SGC users compared with only 48 (40%) from non-users (P < 0.05). Candida albicans was detected most frequently in both groups (45.45% vs 66.67%, respectively; P = 0.033); the overall frequency of non-Candida albicans (NCA) strains isolated from patients treated with SGCs were significantly higher than that identified among non-users (51.14% vs 33.33%, respectively; P = 0.046), although there were no significant differences concerning any single species of NCA. Resistance of C. albicans to itraconazole (P = 0.004) and fluconazole (P = 0.001) was significantly higher in patients treated with SGCs than in non-users; however, echinocandins, amphotericin B, voriconazole, and posaconazole were all active against strains from both participant groups with no significant differences detected.
Conclusion: Taken together, our findings indicate that SGC therapy may result in an increased prevalence of oral candidiasis as reflected by the clinical presentations and strains isolated; these findings were also associated with an increased frequency of NCA strains. SGC therapy was also associated with an increased frequency of C. albicans strains that were resistant to both itraconazole and fluconazole. The impact of SGC therapy on Candida species in the oral cavity requires further study.
Keywords: oral candidiasis, identification, antifungal susceptibility, systemic glucocorticoid therapy, Candida, azole resistance
Introduction
Candida species are common asymptomatic colonizers of the oral cavity that may cause opportunistic infections in debilitation host. Symptoms of candidiasis include loss of taste, bleeding, a sense of burning or soreness, and stomatitis; these may result in profound difficulties with eating and/or swallowing. Candidiasis may also lead to invasive infections associated with spread into the systemic circulation; this may result in esophageal, gastrointestinal, respiratory, and urinary tract infections.1 Oral candidiasis is diagnosed frequently in children, the elderly, and patients undergoing systemic corticoid (SC) or immunosuppressive therapy due to eg, organ transplantation.2 Earlier studies revealed that the use of inhaled corticosteroids (ICS) by adults and children with bronchial asthma could have a significant impact on the diversity of yeast species detected as well as on the incidence of candidiasis.3 In females, systemic intake of corticoids increases not only the incidence of vulvovaginal candidiasis (VVC) but also the frequency of infections with non-Candida albicans (NCA) species that are less susceptible to commonly used antifungal drugs.4 Topical or inhaled corticosteroids were both factors that promote an increased risk of oral candidiasis; however, the impact of systemic glucocorticoids (SGCs) therapy on the nature of the fungal species that comprise the normal oral yeast flora has not been determined.5 The present study aimed to compare the diversity and drug susceptibility of Candida species in the oral cavity among individuals undergoing treatment with SGCs compared with non-users as controls.
Methods
Patients’ Recruitment
The study was conducted at the First Affiliated Hospital, College of Medicine, Zhejiang University and Zhejiang Hospital, Hangzhou, China, during the period from February 1 to September 30, 2019. Study participants included patients with oral complaints who were under treatment with SGCs for unrelated underlying diseases for at least 4 weeks before enrollment. Patients who were undergoing treatment with other immunosuppressants or who were under antifungal prophylaxis, as well as those diagnosed with diabetes or undergoing treatment with mineralocorticoids, were excluded from the study. Sex-matched patients of similar age who were not undergoing SGCs treatment were recruited as the control group. Use of the oral rinse samples was approved by the Ethical Committee of the First Affiliated Hospital, College of Medicine, Zhejiang University (reference number 2019–40-1). Written informed consent was obtained from all participants. This research study is in full compliance with the principles of the Declaration of Helsinki.
Collection and Identification of Samples
Samples were collected using a concentrated oral rinse method as previously described with several small and necessary changes.6,7 Briefly, 10 mL of sterile saline was used to rinse the oral cavity; the samples were sent to the microbiological laboratory within 30 min where they were subjected to centrifugation (2300g for 20 min). The supernatants were removed, and the pellets were resuspended in 1 mL saline. A sterile cotton swab was used to sample the suspension, which was spread evenly on Sabouraud glucose agar (SGA) supplemented with chloramphenicol (0.5 g/L). SGA plates were then cultured at 37°C for 4 days; isolates were subsequently inoculated onto CHROMagar Candida (CHROMagar, Paris, France) for detection of mixed cultures. The API 20C system (bioMérieux, Marcy l’Etoile, France) was used for phenotypic identification. Samples that could not be identified by phenotypic analysis were evaluated with molecular identification via sequencing of the conserved ribosomal internal transcribed spacer (ITS) region using primers ITS1 (5′-TCCGTAGGTGAACCTGCGG-3′) and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′) as described in a previous study.8 Methods for extraction and quantification of genomic DNA, as well as for amplification and sequencing were as previously described.9 Amplification by polymerase chain reaction (PCR) was conducted as described using the following cycling parameters: initial at 94 °C for 5 min, followed by 30 cycles of denaturation at 94 °C for 30 s, annealing at 55 °C for 30 s and extension at 72 °C for 30 s, and then a final extension step at 72 °C for 10 min. Sequence data were analyzed using SeqMan Pro (DNAStar, Madison, WI, USA) and species identification was verified by BLAST searches in GenBank (NCBI, Bethesda, MD, USA). Selected isolates were incubated on SGA slants at 37°C for 24–48 h and then maintained in distilled water at 4°C until use.
Antifungal Susceptibility Testing
In vitro susceptibilities for nine common antifungal drugs, including anidulafungin, amphotericin B, micafungin, caspofungin, 5-flucytosine, posaconazole, voriconazole, itraconazole, and fluconazole, were determined using the Sensititre YeastOneTM YO10 panel (Thermo Fisher Scientific, Waltham, USA) according to manufacturer’s recommendations and the Clinical and Laboratory Standards Institute (CLSI) M27-A3 protocol.10 Candida strains were cultured on SGA plates for 24 h. Several colonies of >1 mm diameter were selected and suspended in sterile water and adjusted to the 0.5 McFarland standard at the 530 nm wavelength; this ensures that the concentration in the final inoculum for the 96-well plates was between 1.5× 103 to 8× 103 colony-forming units per milliliter as recommended by the manufacturer. After transfer, the plates were placed in a non-CO2 incubator at 35°C for 24 h. Candida parapsilosis ATCC 22,019 and Candida krusei ATCC 6258 were used as quality controls. Tests were conducted in triplicate on three different days. The concentration in the first blue-colored well was recorded as the minimum inhibitory concentration (MIC). Antifungal susceptibility of Candida species-specific was interpreted by clinical breakpoints (CBPs) based on the CLSI M60;11 when CBPs were not available, epidemiological cutoff values (ECVs) that were used to differentiate wild-type (WT) from non-WT isolates based on the CLSI M59 and the previous study were applied.12,13
Statistical Analysis
The Statistical Product and Service Solutions (SPSS) v21.0 was used for χ2 test to evaluate differences in the proportion of categorized variables. P-values of < 0.05 were considered to be statistically significant.
Results
Two-hundred and forty patients were included in the study; this included 120 patients undergoing treatment with SGCs and 120 non-user controls. There were no differences in mean age or gender composition between the two groups. There were also no statistically significant differences concerning oral complaints and problems, including impacted teeth and residual roots, although the patients undergoing treatment with SGCs were more likely to develop periodontitis and caries (P=0.006 and P=0.026, respectively; Table 1). Patients enrolled in the study had undergone SGCs treatment for at least 4 weeks before evaluation; underlying diagnoses included rheumatoid arthritis, systemic lupus erythematosus, scleroderma, other connective tissue diseases, organ transplantation, inflammatory bowel disease, allergic diseases, cancer, and cutaneous vasculitis, as well as several rare diseases, including adult-onset Still’s disease and immune thrombocytopenia. Of this group, 72 patients were treated with prednisolone oral tablets (2.5–20 mg per day), and 48 patients were treated with methylprednisolone tablets (2–16 mg per day).
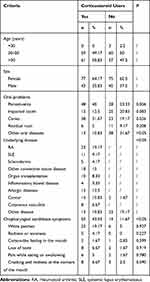 |
Table 1 Demographic Data of Patients Undergoing Treatment with Systemic Glucocorticoids (n=120) and Non-Users (n=120) |
Of the 120 patients undergoing treatment with SGCs, 52 (43.33%) were diagnosed with oral candidiasis based on a clinical sign associated with mycotic stomatitis compared with only 14 (11.67%) of the non-users; this difference was statistically significant (P < 0.05); There were no significant differences concerning the symptoms associated with oropharyngeal candidiasis (Table 1). In clinical samples from those undergoing treatment with SGCs, 82 of 120 (68.33%) tested positive for a single species, whereas three samples included two fungi (multi-fungal); a total of 88 strains (73.33%) were identified. In the control group, 46 of 120 samples (38.33%) included a single fungal species, whereas one was multi-fungal, in a total of 48 strains (40%) were identified (P< 0.05). Multi-fungal specimens included those in which Candida albicans was combined with NCA species. NCA species were identified primarily as C. parapsilosis, Candida glabrata, Candida tropicalis, and C. krusei and occasionally as Candida lusitaniae or Candida guilliermondii, which were both identified in samples from the SGC group. The frequencies of detection of C. albicans were 45.45% vs 66.67% in those treated with SGCs compared with non-user controls (P = 0.033). Similarly, NCA species were more prevalent in samples from those undergoing treatment with SGCs than in those from non-user controls (51.14% vs 33.33%, respectively; P = 0.046), although no significant differences were identified for any single NCA species (Table 2). Four strains of Saccharomyces cerevisiae were identified in samples from both groups; four C. lusitaniae, one C. guilliermondii, and four S. cerevisiae isolates were verified by molecular identification methods.
 |
Table 2 Frequency of Yeast Strains Isolated from the Oral Cavities of Patients Undergoing Treatment with Systemic Glucocorticoids (n=88) vs Non-Users (n=48) |
In vitro susceptibility testing of nine antifungal agents against 132 Candida strains showed revealed an average 90% MIC90 values for isolates from those undergoing treatment with SGCs vs non-user controls were as follows: for anidulafungin, 0.5 vs 0.125 μg/mL; for amphotericin B, 1 vs 0.5 μg/mL; for micafungin, 0.25 vs 0.125 μg/mL; for caspofungin, 0.5 vs 0.125 μg/mL; for 5-flucytosine, 2 vs 0.25 μg/mL; for posaconazole, 0.5 vs 0.06 μg/mL; for voriconazole, 1 vs 0.25 μg/mL; for itraconazole, 2 vs 1 μg/mL; and for fluconazole, 64 vs 64 μg/mL. The echinocandins, amphotericin B, voriconazole, and posaconazole were all active antifungal agents with low MICs that were effective against 90% of the strains isolated from study participants in both groups; however, C. albicans strains isolated from patients undergoing treatment with SGCs were more resistant to itraconazole (P = 0.004) and fluconazole (P = 0.001) than those from non-users (Table 3). Except for the case of fluconazole, the other eight antifungals exhibited low MIC values (≤1 μg/mL) for the four S. cerevisiae strains; the MIC for fluconazole was no greater than 8 μg/mL.
 |
Table 3 Antifungal Sensitivity of Candida Spp. Isolated from Patients Undergoing Treatment with Systemic Glucocorticoids (n=85) vs Non-Users (n=47) |
Discussion
In the present study, we identify multiple strains of Candida from the oral cavities of adults undergoing treatment with SGCs and from non-user controls; we established antifungal susceptibilities characteristic of the strains from both groups. First, our findings confirmed that SGC therapy resulted in an increased risk for oral candidiasis. We also found that the NCA strains were isolated at greater frequency from those undergoing treatment with SGC than those from non-users (51.14% vs 33.33%). Additionally, the ratios of itraconazole- and fluconazole-resistant C. albicans strains were significantly higher among those undergoing treatment with SGCs than those among the non-user controls.
Oral candidiasis is promoted by numerous disorders and most notably associated with those that impair host immunity. Use of prednisone and/or ICS are known to increase the risk of developing oral candidiasis.2,3 To the best of our knowledge, no previously published studies have focused on fungal pathogens in the oral cavity and sensitivities to antifungal drugs, specifically among those undergoing SGC treatment. Renal transplant recipients, patients with diabetics, and those with pemphigus vulgaris frequently develop oral lesions; those patients have a higher prevalence of Candida species in their oral cavities than do immunocompetent control subjects.14–16 The underlying diseases associated with SGC use in our target patient cohort were all associated with immunoreactivity and immunosuppression. Among those, SGC use was necessitated by underlying conditions including autoimmune disorders, organ transplantation, or cancer; those findings may explain in part the high frequencies of Candida detected. Additionally, some diseases, including Sjögren’s syndrome and scleroderma, are themselves associated with an increased risk for oral candidiasis.17 In the present study, we also found that, compare with non-users, patients treated with SGCs have significantly more problems that are associated with periodontitis and caries; this finding is consistent with those reported the previous studies.18,19
Candida albicans is usually the most prominent of the fungal colonizers found in the oral cavity; indeed, 30% to 45% of healthy individuals are carriers of this organism.5 NCA species, including C. glabrata, C. guilliermondii, C. krusei, C. parapsilosis, C. pseudotropicalis, C. stellatoidea, and C. tropicalis, are also detected frequently in the oral cavity.20,21 In the present study, the prevalence of NCA was higher among the patients undergoing treatment with SGCs; this may be related in part to SGC-mediated immunosuppression of the host, although similar results were obtained from patients diagnosed with diabetics, pemphigus vulgaris, or vulvovaginal candidiasis.12–14
The first-line therapy for oral candidiasis is topical antifungal treatment; however, previous studies indicated that one in four patients were provided with systemic antifungal therapy as the first-line treatment after initiation of ICS therapy, which was contrary to current guidelines.22,23 In the present investigation, we found that most of the commonly used antifungal drugs were effective against Candida species in vitro, except for itraconazole and fluconazole. Only a few of the isolates were resistant to echinocandins; this included a strain of C. guilliermondii isolated from a patient undergoing treatment with SGCs with MICs of 16, 8, and 8 μg/mL for anidulafungin, micafungin, and caspofungin, respectively. Likewise, two strains of C. parapsilosis, one from an SGC-treated patient and the other from a non-user exhibited MICs of 8 μg/mL for both anidulafungin and caspofungin. Similarly, there were no significant differences between the responses to amphotericin B and 5-flucytosine among WT or non-WT strains from both study groups.
Azole resistance among Candida species is currently a critical problem in clinical settings; numerous studies exploring the mechanisms underlying acquired resistance have been published. Our results revealed that C. albicans from those undergoing treatment with SGCs had increased rates of resistance; this may be due to genetic modifications within the fungal cell promoted by corticosteroid-induced stress.24 The NCA strains are of particular concern with respect to azole resistance.25 Although the proportion of NCA isolates clearly increased in the SGC treatment group, the numbers of non-susceptible strains for each species remained low; as such, this was unlikely to have an impact on differential drugs sensitivity when comparing the two groups.
Among the limitations of the present study, this work features a relatively small number of patients that were recruited from a single institutional setting. We also did not conduct subgroup analysis and as such as are unable to associate any of our findings with specific underlying diseases.
In conclusion, SGC therapy may result in an increase in the incidence of oral candidiasis together with an increase in the frequency of NCA strains; treatment with SGCs may also promote itraconazole and/or fluconazole resistance among C. albicans species. Our findings provide evidence suggesting that SGC therapy not only influences colonization and infection with Candida spp., but also affects species diversity and azole sensitivity.
Acknowledgments
This work was supported by the National Natural Science Foundation of China [81701982 to Y.L.L.] and the Zhejiang Provincial Natural Science Foundation [LY20H110001 to Y.L.L.].
Disclosure
The authors declare that they have no relevant conflicts of interest.
References
1. Badiee P, Kordbacheh P, Alborzi A, et al. Fungal infections in solid organ recipients. Exp Clin Transplant. 2005;3(2):385–389.
2. Singh A, Verma R, Murari A, Agrawal A. Oral candidiasis: an overview. J Oral Maxillofac Pathol. 2014;18(Suppl 1):S81–S855. doi:10.4103/0973-029X.141325
3. Rachelefsky GS, Liao Y, Faruqi R. Impact of inhaled corticosteroid-induced oropharyngeal adverse events: results from a meta-analysis. Ann Allergy Asthma Immunol. 2007;98(3):225–238. doi:10.1016/S1081-1206(10)60711-9
4. Farhan MA, Moharram AM, Salah T, Shaaban OM. Types of yeasts that cause vulvovaginal candidiasis in chronic users of corticosteroids. Med Myco. 2019;57(6):681–687. doi:10.1093/mmy/myy117
5. Patil S, Rao RS, Majumdar B, et al. Clinical appearance of oral Candida infection and therapeutic strategies. Front Microbiol. 2015;6:1391. doi:10.3389/fmicb.2015.01391
6. Samaranayake LP, MacFarlane TW, Lamey PJ, et al. A comparison of oral rinse and imprint sampling techniques for the detection of yeast, coliform and Staphylococcus aureus carriage in the oral cavity. J Oral Pathol. 1986;15(7):386–388. doi:10.1111/j.1600-0714.1986.tb00646.x
7. Tooyama H, Matsumoto T, Hayashi K, et al. Candida concentrations determined following concentrated oral rinse culture reflect clinical oral signs. BMC Oral Health. 2015;15:150. doi:10.1186/s12903-015-0138-z
8. Leaw SN, Chang HC, Sun HF, et al. Identification of medically important yeast species by sequence analysis of the internal transcribed spacer regions. J Clin Microbiol. 2006;44(3):693–699. doi:10.1128/JCM.44.3.693-699.2006
9. Li Y, Xiao J, De Hoog GS, et al. Biodiversity and human-pathogenicity of Phialophora verrucosa and relatives in Chaetothyriales. Mol Phylogen Evol Fungi. 2017;38:1–19. doi:10.3767/003158517X692779
10. Clinical and Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts Approved Standard-Third Edition. CLSI Document M27-A3. Wayne, PA: Clinical and Laboratory Standards Institute; 2008.
11. Clinical and Laboratory Standards Institute. Performance Standards for Antifungal Susceptibility Testing of Yeasts.
12. Clinical and Laboratory Standards Institute. Epidemiological Cutoff Values for Antifungal Susceptibility Testing.
13. Pfaller MA, Diekema DJ. Progress in antifungal susceptibility testing of Candida spp. by use of Clinical and Laboratory Standards Institute broth microdilution methods, 2010 to 2012. J Clin Microbiol. 2012;50(9):2846–2856. doi:10.1128/JCM.00937-12
14. Pakshir K, Ghasemi N, Zomorodian K, et al. Identification and antifungal activity profile of Candida species isolated from patients with pemphigus vulgaris with oral lesions. Acta Dermatovenerol Croat. 2019;27(3):137–141.
15. Al-Attas SA, Amro SO. Candidal colonization, strain diversity, and antifungal susceptibility among adult diabetic patients. Ann Saudi Med. 2010;30(2):101–108. doi:10.4103/0256-4947.60514.
16. Keerthi M, Reddy GS, Shekar PC, et al. A study on isolation, identification, and antifungal susceptibility of various oral candidal species in renal transplant patients. J NTR Univ Health Sci. 2015;4(3):170–175. doi:10.4103/2277-8632.165399
17. Yan Z, Young AL, Hua H, Xu Y. Multiple oral Candida infections in patients with Sjögren’s syndrome-prevalence and clinical and drug susceptibility profiles. J Rheumatol. 2011;38(11):2428–2431. doi:10.3899/jrheum.100819
18. Genco RJ, Borgnakke WS. Risk factors for periodontal disease. Periodontology. 2000;62(1):59–94. doi:10.1111/j.1600-0757.2012.00457.x
19. Hassanpour K, Tehrani H, Goudarzian M, et al. Comparison of the frequency of dental caries in asthmatic children under treatment with inhaled corticosteroids and healthy children in Sabzevar in 2017–2018. Electron J Gen Med. 2019;16(2):em119. doi:10.29333/ejgm/93478
20. Terézhalmy GT, Huber MA. Oropharyngeal candidiasis: etiology, epidemiology, clinical manifestations, diagnosis, and treatment. Crest Oral-B at dentalcare. Contin Educ Course. 2011;1–16.
21. Dangi YS, Soni ML, Namdeo KP. Oral candidiasis: a review. Int J Pharm Pharm Sci. 2010;2(4):36–41.
22. Henriksen DP, Davidsen JR, Christiansen A, et al. Inhaled corticosteroids and systemic or topical antifungal therapy: a symmetry analysis. Ann Am Thorac Soc. 2017;14(6):1045–1047. doi:10.1513/AnnalsATS.201612-1043LE
23. Pappas PG, Kauffman CA, Andes DR, et al. Clinical practice guideline for the management of candidiasis: 2016 update by the infectious diseases society of America. Clin Infect Dis. 2016;62(4):e1–50. doi:10.1093/cid/civ933
24. Črešnar B, Žakelj-Mavrič M. Aspects of the steroid response in fungi. Chem Biol Interact. 2009;178(1–3):303–309. doi:10.1016/j.cbi.2008.11.002
25. Pfaller MA, Diekema DJ, Gibbs DL, et al. Results from the ARTEMIS DISK global antifungal surveillance study, 1997 to 2007: a 10.5-year analysis of susceptibilities of Candida species to fluconazole and voriconazole as determined by CLSI standardized disk diffusion. J Clin Microbiol. 2010;48(4):1366–1377. doi:10.1128/JCM.02117–09
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
